418 (15)
Metallurgical Analysis ofthe Dress Accessories
somewhal purer (aboul 55% gold) and had been mainly debased with silver although it still con-tained about 15% copper. Both nos. 1612 and 1613 were in two parts and in both cases the analytical results were consistent with the two parts being of the same composition.
Number 1610 consists of three parts; the hoop, bezel and collet. The hoop is significantly purer (about 75%) gold than either the bezel or collet. The analyses of the bezel and the collet were not significantly different. from each other (55-60% gold, debased with about equal amounts of copper and silver).
Two other objects, gold fmger ring no. 1614 and the coating of silver-gilt rnirror case no. 1718, were analysed qualitatively by XRF. Comparison with similar analyses of the gold finger rings that were also analysed by SEM suggest that the gold of both was relatively fine. The metal of the former is probably about 75-85% gold, whilst the coating on the latter is almost pure gold.
Nonę of the silver objects was analysed quanti-391
tatively but the seven silver objects analysed by XRF were of varying fineness. Comparison with silver objects of known composition havc allowed some estimate of fineness to be attached to the silver dress accessories analysed. Three objects were relatively pure silver (ie over 90% silver): probable buckie no. 211, brooch no. 1334 and the pin of brooch no. 1337. Dress pin ąo. 1488 is heavily debased, with a silver eon tent of about 50%, whilst the pin of brooch no. 1339 and the frame of no. 1337 were even less fine, with silver contents of about 40%. In the debased silver objects the main other element present was copper, though they also contained smali levels of zinc, which suggests the silver was debased by adding brass to the metal.
Gold and silver were also used to coat the surface of a smali number of objects, mostly brooches, buckles and strap-ends. Ninę objects coated with precious metals were analysed; two of these were clearly mercury-gilded, but mer-cury was not definitely detected in the other
Variations through time
analyses
19 16
20 112
15
215
72
An attempt was madę to identify possible changes in alloy usage from the mid twelfth century to the mid fifteerith century. The number of analyses for each ceramic phase and the metals represented are as
|
follows: ceramic |
no. of |
copper brass |
gunmetal |
bronze |
lead |
tin |
pewter |
gold |
silver | ||
|
phase 6 (1150-1200) |
analyses 24 |
9 |
4 |
5 |
1 |
3 |
1 | ||||
|
7 (1200-1230) |
26 |
5 |
3 |
4 |
4 |
- |
5 |
4 |
- |
1 | |
|
8 (1230-1260) |
33 |
3 |
11 |
4 |
2 |
- |
2 |
10 |
- |
- | |
|
9 (1270-1350) |
147 |
13 |
55 |
40 |
14 |
- |
8 |
12 |
- |
- | |
|
10 (1330-1380) |
16 |
- |
8 |
3 |
1 |
- |
3 |
1 |
- |
- | |
|
10/11(1330-1400) |
4 |
- |
2 |
1 |
- |
- |
- |
- |
1 |
- | |
|
11 (1350-1400) |
275 |
15 |
125 |
46 |
29 |
2 |
22 |
19 |
3 |
5 | |
|
12 (1400-1450) |
113 |
3 |
51 |
12 |
6 |
- |
27 |
9 |
- |
- | |
|
unstratified |
26 |
1 |
7 |
1 |
2 |
1 |
5 |
5 |
1 |
- | |
|
unphased |
9 |
2 |
3 |
1 |
- |
- |
1 |
1 |
1 |
- | |
|
total |
649 |
51 |
269 |
117 |
59 |
3 |
73 |
64 |
6 |
7 | |
|
Element ratios |
for the copper |
alloys analysed |
ceramic |
no. of |
proportion of |
copper alloys | |||||
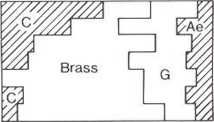
from each ceramic phase are shown in figs 263 to 269 plotted on ternary diagrams. The proportion of the individual copper alloys in each phase is shown at the right (C = copper, G = gunmetal, Ae = bronze):
phase
6
7
8 9
10
11
12
Wyszukiwarka
Podobne podstrony:
427 (11) 400 Dress AccessoriesAcknowledgements In a work of tliis size and comp!exity the principal
419 (10) 392 Dress Accessories cases, though it is likely to have been present. Brass mount no. 935
422 (13) Metallurgical Analysis of the Dress Accessories site form which include a forked spacer san
333 (43) 306 Dress Accessories colours represented (nos. 1493, 1498, 1514 and 1524), were analysed b
420 (12) 393Metallurgical Analysis of the Dress Accessories Lead Lead Fig 266 Copper alloys c. 1270-
369 (15) 342 Dress AccessońesPurses (Identification of leather by Glynis Edwards, AMD Purses and pou
414 (12) 387Metallurgical analysis of the dress accessories MIKĘ HEYWORTHIntroduction A sełection of
416 (14) 389Metallurgical Analysis of the Dress Accessories Lead Lead Fig 258 Ali copper alloys. Eac
Transient Stability AnalysLs ofthe IEEE 9-Hus Electric Power SystemAbstract It k widel> accepted
286 (34) 258 Dress Accessońes 166 Pentagonal, hexagonal and six-lobed brooches -odginał shapes resto
292 (37) 264 Dress Accessories 1365 171 Yiolet brooches (drawings 1:1, photograph 2:1) (?)flower bud
296 (37) 268 Dress Accessońes Brooches with circular frames Leaf 1374 BC72 2037 (83) 11 fig 174 Poss
298 (39) 270 Dress Accessories Table 5 Brooches - metals used 1 1 Copper i i 2 I I I -1-1—[— 1 1 1
302 (41) 274 Dress Accessońes buttons of the former kind, found in Lund in Sweden). Composite sheeti
więcej podobnych podstron