3582498432
nhic and igneous layers based on the foliowi,,. Step 2: Calculate the ages of *he metainorphic and
Information. 150.000 atoms of uranium-235 and
. A minerał samplefrom rock mn.Bhas!:
50.000 atoms of lead-20 . atoms of uraniUm-235 and
. A minerał sample from rock »»" c
20.000 atoms of 'e®^° ' G ,ms 100,000 atoms of potassium-40 and
• A minerał sample from rocK
100.000 atoms of argon-40.
c alculate how many half-lives have passed
Use Figurę 9.8. if necessary. to he f >01' d on ,hc remaining amount of a parent
sińce rock units B, C, and G were each tortnea,
isotope compared to the original amount of parent isotope.
Once you know how many half-lives have past sińce the sample crystałlized, use the half-life table in Figurę 9.9 to calculate an absolute datę ' fr‘r 1
Recall that the original amount of parent isotope can be calculated by add.ng together the amount of parent isotope plus the amount of daughter isotope, making t e assump> ton a ne,t er has escaped the sample. Also notę that if less time has passed than a u a i e, you must calculate what portion of a half-life has passed to make the calculations.
11. The igneous rock in rock unit B was formed_
12. The igneous rock in rock unit C was formed_
13. The metamorphic rock in rock unit G was formed
Step 3: Index fossils have been found in the sedimentary layers! Use Figurę 9.11 to help
you to identify the periods in which layers A, D, E, and F were formed as well as their age in mya based on the discovery of the following fossils:
14. The following fossils were found in Layer A:
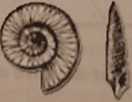
Figurę 9.13
Layer A was formed_mya during the__Period
15. The following fossils were found in Layer D:
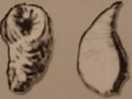
Figurę 9.14
Period.
Layer D was formed__mya during the _
Wyszukiwarka
Podobne podstrony:
based on the work of M. Klimaszewski [2]. The results of investigations of L. S t a r k e 1 [3] and
Hefalump (2) #1134-49 LUMPY AND ROO © Disney Based on the “Winnie the Pooh” works by A.A. Milne and
13 NOTICES BIBLlOGRAPHlQUES 793 The dictłonary of authors is based on the Idea of prescuting in
Summaries 199 Małgorzata Olczak Creativity of the university teacher (based on the idea of inno
14 Łukasz Sułkowski nagement processes are based on the principles of the interpretive approach, the
NEOLITYCZNE GÓRNICTWO NA JAŃSKIEJ GÓRZE 45 by L.Fober and G.Wcisgerber (1980, 32) on the basis of ob
19958 w25$ ABOVE ‘Siena’s Chamberlain and clerk at work on the cover of the Biccherna records
258Sylwester Dziki: THE POLISH ACADEMIC AND SPECIALIST PERIODICAL PRESS (ON THE BASIS OF THE SITUATI
What can TPM do for YOU? TPM is based on the ideas of optimizing equipment effectiveness, achieving
Ryszard Broi, Andrzej Sztando, Strategie construction of local economy innovation based on the examp
Clóśslf icatidn of natural and artif icial lakes on the basis of the presence of caddisflies (a
COURSE AIM: The aim of the course is to familiarize students problem-solving methodology based on th
censorship, (6) io8 Blackout all was lost... Double Indemnity was based on the principal of M...
shallow depth and is based on the same operating principle as the BAT/GEON. * DMT
więcej podobnych podstron