8013826877
NEWBORN SCREENING OUALITY ASSURANCE PROGRAM CYSTIC FIBROSIS VARIANT DETECTION SURVEY
GUARTER 4 - NOYEMBER 2018
LAB 1268
DATA YERIFICATION
|
i Specimen ] Nuntber |
Alicie 1 1, .j-7 'J .1 |
. jjble 2.1 |
Clinical Assessment |
|
418C1 |
F508del (c.1521_1523delCTT) |
F508del (c.1521J523deiCTT) |
j 2 |
|
416C2 ł |
No variants deteeted |
No variants deteeted |
■1! |
|
1 418C3 |
F508del (c.l521_1523delCTT) |
No variants deteeted |
2 |
|
418C4 |
3849+1Okb C>T (c.3717+12191C>T) |
G542X (c.1624G>T) |
2 i' |
|
418C5 |
2183AA>G (c.2051_2052delAAinsG) |
No variants deteeted |
2 |
NSOAP ReYiewer^s Comments
100% satisfactory based on the reported variant panel.
Please notę that specimen 418C3 contains a CF-causing variantthat is not deteeted using your reported variant panel
Resuits have been

mwycznej Title
ed by:.
■ « _
- ~r

IM
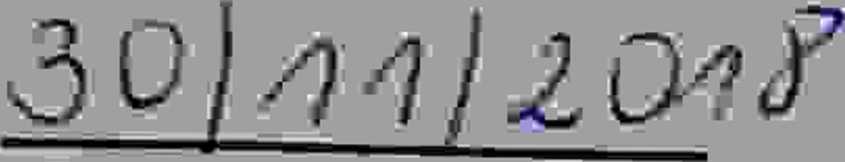
talm&ofA
lahorataryjr.B,
Datę
if you have any questions about your resuits, please contact Suzanne K. Cordovado by email at SCordovado@cdc.0ov by telephone at 770-488^4048 or the Newbom Screening Quality Assurai Program at NSQAPDMT@cdc.gov,
Wyszukiwarka
Podobne podstrony:
NEWBORN SCREENING OUALITY ASSURANCE PROGRAM CYSTIC FIBROSIS MUTATION DETECTION SURVEY OUARTER 3
NEWBORN SCREENING OUALITY ASSURANCE PROGRAM CYSTIC FIBROSIS MUTATION DETECTION SURVEY OUARTER 3
NEWBORN SCREENING OUALITY ASSURANCE PROGRAM CYSTIC FIBROSIS MUTATION DETECTION SURVEY OUARTER 2
NEWBORN SCREENING OUALITY ASSURANCE PROGRAM CYSTIC FIBROSIS MUTATION DETECTION SURVEY OUARTER 2 - MA
NEWBORN SCREENING OUALITY ASSURANCE PROGRAM CYSTIC FIBROSIS VAR1ANT DETECTION SURVEY OUARTER 4 - NOV
NEWBORN SCREENING QUALITY ASSURANCE PROGRAM CYSTIC FIBROSIS MUTATION DETECTION SURVEY OUARTER 4 - NO
f21 18 LEE Selection Screen Formatting Example Program Edit Goto System Help l/
f21 20 LEE Selection Screen Formatting Example Program Edit Goto System Help H P zj <śi o &
f21 24 LEE Selection Screen Formatting Example Program Edit Goto System Help l/
f21 26 Selection Screen Formatting Example Program Edit Goto System Help J_!El
f21 28 LEE Selection Screen Formatting Example Program Edit Goto System Help J_!El
Slajd44 (9) MUKOWISCYDOZA W populacji I Inheritance of Cystic Fibrosis (CF) Często
Slajd45 (9) MUKOWISCYDOZAzwłóknienie torbielowate (cystic fibrosis, CF) ■ Mukowisc
59689 str367 Mukowiscydoza (cystic fibrosis CF, zwyrodnienie torbielowate trzustki) Choroba jest spo
C22Z3Certificate of Achievement EQAS External Ouality Assurance Services WOJEWÓDZKI SPECJALISTYCZNY
Certificate of Participation In The 2018 External Quality Assessment Scheme For Cystic Fibrosis
więcej podobnych podstron