26693
|
Alkany węglowodory nasycone | |
Alkanes, also known as paraffins, are Chemical compounds that consist only of the elements carbon (C) and hydrogen (H) (i.e., hydrocarbons), wherein these atoms are linked together exclusively by single bonds (i.e., they are saturated compounds) without any cyclic structure (i.e. loops).
Saturated hydrocarbons can be linear (generał formula CnH2n+2) wherein the carbon atoms are joined in a snake-like structure, branched (generał formula CnH2n+2. n>3) wherein the carbon backbone splits off in one or morę directions.
Cyclic (generał formula CnH*,, n>2) wherein the carbon backbone is linked so as to form a loop. According to the definition by IUPAC, the former two are alkanes, whereas the third group is called cycloalkanes.
An alkyl group is a functional group or side-chain that, like an alkane, consists solely of singly-bonded carbon and hydrogen atoms, for example a methyl or ethyl group.
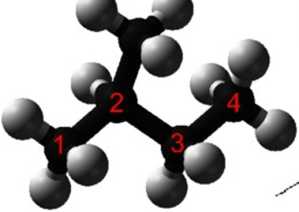
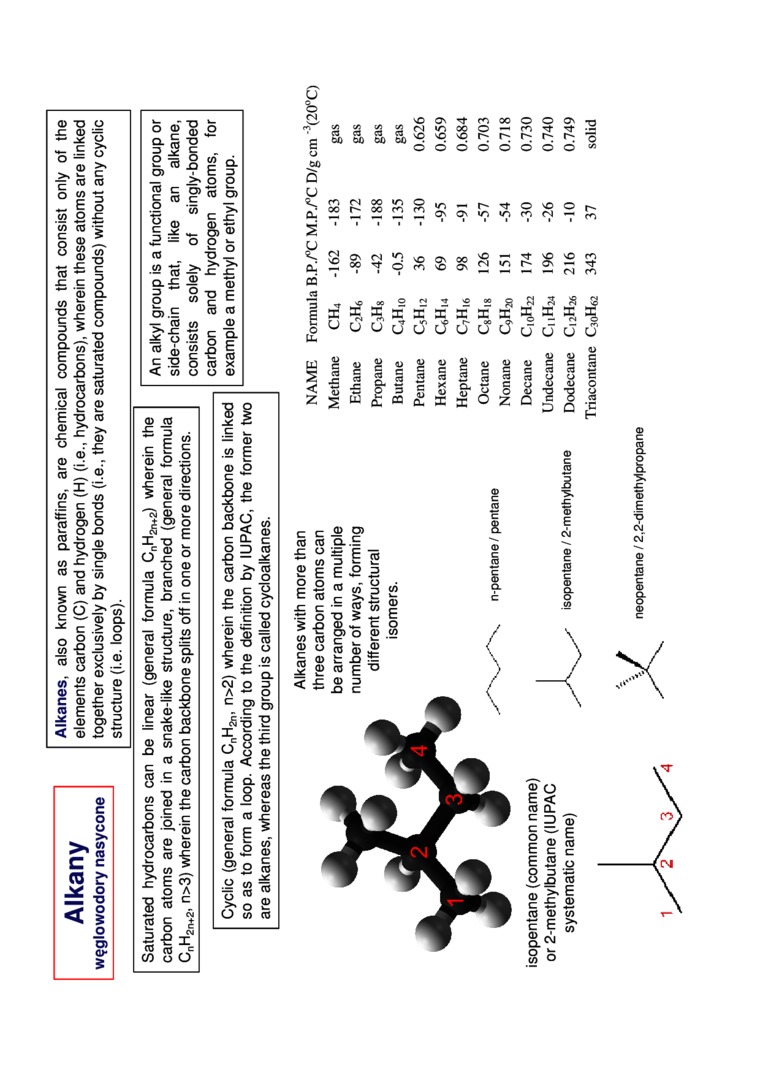
isopentane (common name) or 2-methylbutane (IUPAC systematic name)
Alkanes with morę than three carbon atoms can be arranged in a multiple number of ways, forming different structural isomers.
n-pentane / pentane
NAME Formula B.P./°C M.P./°C D/g cm ’3(20°C)
isopentane / 2-methyltxjtane
|
Methane |
CH4 |
-162 |
-183 |
gas |
|
Ethane |
c2h6 |
-89 |
-172 |
gas |
|
Propane |
c3h8 |
-42 |
-188 |
gas |
|
Butane |
C4Hj0 |
-0.5 |
-135 |
gas |
|
Pentane |
C5Hi2 |
36 |
-130 |
0.626 |
|
Hexane |
CfiHu |
69 |
-95 |
0.659 |
|
Heptane |
c7h,6 |
98 |
-91 |
0.684 |
|
Octane |
c8hI8 |
126 |
-57 |
0.703 |
|
Nonane |
CęHjO |
151 |
-54 |
0.718 |
|
Decane |
C10H22 |
174 |
-30 |
0.730 |
|
Undecane |
C,,H24 |
1% |
-26 |
0.740 |
|
Dodecane |
CI2H26 |
216 |
-10 |
0.749 |
|
Triacontane C30H62 |
343 |
37 |
solid | |
neopentane / 2.2-dimethylpropane
Wyszukiwarka
Podobne podstrony:
OUILLING ... This ancient craft, dating back to the I5th cen tury, is also known as paper fifigrce.
mysąid— The MySQL Server mysqld, also known as MySQL Server, is the main program that does most of t
Total Physical Rcsponse (TPR) This method is also known as Leaming Another Language Tlirougli Action
The merge Storage Engine The MERGE storage engine, also known as the MRG_MyISAM engine, is a collect
patlefta Also Known As Motivation Applicability Structure Partie
patlefta Intent Also Known As
Boldface names refer to Ruby psions, also known as the “Rhon.” Ali Rhon psions who arc also members
judge on the planet Lyshriol (also known as Skyfall). His spcctacular voice is legendary among his p
chain11 Queen s Chain (also known as Sąuare Chain & Box Chain) Notę: A set of rings or a set of
popcorn story Read the passage and answer the guestions. Last Q 2/4 Popcorn, also known as popping c
TME&7 THE IMPERIAL GUARD The Elitę Corps of the Shi ar Imperial Guard, also known as the Superguardi
TME 7 SCARLET WlTCH Wanda Maximoif is the daughter of Erik Magnus Lehsherr also known as thc mutant
więcej podobnych podstron