92666
1850
I). Du et aL/Procent Biochemiary 4112006) 1849-1853
and ma i mai ns a high rcaction ratę 112]. Addilionally. aft er transcstcrilication. thc wastc ABE that conlains lipasc can be regardcd as immobili/ed lipasc that can he reuscd in thc next cycle reaclion. which will reduce thc calalyst cost.
For thc rcutilizalion of ABE. we cxamined thc rcpcatcd production of FAMĘ: using ABE as a reaclion medium without any organie solvent. Two processes containing ABE were considcrcd for thc rcpcatcd FAMĘ production. and lipasc inactivation and thc productivity thc FAMĘ in each process is discusscd.
2. Materials and melhods
2.1. Materials
Lipascs powders (QLM) from Alcaligenes sp. wen: purchascd from Mcito Sangyo (Kagoya. Japan). The methanol and rapeseed «il used for methanolysis were analsiical grade and were purehased from Wako Pure Chemical Industries Ltd. (Osaka. Japan). Ali ołher Chemicals used were of arudytical reagent grade. Mi/usuwa Industrial Chemicals Lid. (Tokyo. Japan) prmidcd wadę ABE.
2.2. Methanolysis of ra/>esccd oil
The initial conditions were adopied from a presious study [3|. The reaclion mixlurcs consisicd of2g rapeseed oil. 3g ABE and 0.06g lipasc (3% (w/w based on oil weighl)) under the conditions of 37 C and 200 rounds per minutę <rpm> in a red proca I -haker i Bio Shuker. Takasaki Scicnlilic Instrument* Co.. Japan). The nw>lar ralio of oil lo methanol was varied from 1:1 to 1:8.
To study thc effcct of ABE in the solvcnt-frcc system, the initial FAMĘ production ratę was insestigated under a lipase concenłration of 10%. on the basis of ABE and rapeseed oil weighl. Because the reaclion conditions. including ABE. lipasc. methanol and rapeseed oil concentrations. arc known at tirne zero. the initial FAMĘ production ratę and the spccitic FAMĘ ptoduc-łion ratę were detined as.
f d/*l
Initial FAMEptoduclion ratę |mM/min) = | — j (I)
(dP/dl) .
Specific FAMĘ production ratę (mmol/min/g lipase) -——'■— (2)
Co
where P. C0 a ml t de notę concentrations of produced FAMĘ and used lipase. and reaclion time. respectisely.
2.3. Rcpcatcd production of FAMĘ frtmt nipeseed oil in reaclor
Two kinds of rcpcatcd production of FAMĘ were performed. One was a rcpcatcd batch process in which the produced FAMĘ was separated from the reaclion mix(ure and then ncw rapeseed oil and methanol were added to thc rcactor for thc ncxt cycle rcaction. The other is a rapeseed oil-fcd batch process. In c»cry cycle rcaction. rapeseed oil and methanol were added al regular intersal* for the production of FAMĘ. The produced FAMĘ was harsested at the finał cycle reaclion. The starting reactants consisted of 234 g of ABE. I56g of rapeseed oil. 23 g of methanol (thc mol ar ratio of melhamd and rapeseed oil 4:1 h and 39 g of lipase. respectisely. The reaclion was carried out in a 2-1 reaclor miting at 200 rpm using a stirrer (RZR 2020, licidolph. Kelheim. Germany) and at a rcaction temperalure of 37 C. For the second cycle. rapeseed oil (I56g) and methanol (23 g) were added to (he reaclor. and the reaclion was repeated under the same rcaction conditions as the fint cycle.
2.4. Analytical melhods
Lipase actisity (lUAnlk detined as the amountoflipa.se that liberated I plof free thiol groups per minutę, was mcasured using a Lipase Kil S (Dainippon
Pharmaceutical. Osaka). Glycerol was measured using Kit F (R-Biophami AG. Damtstadt. Germany). Fivc hundred microłilers of thc sample after methano-lysis was extracted and centrifuged at 7155 x g in a microcentrifuge. A 25-pg aliquot of supematant was dissolsed in 1.5 g of chloroform, and 2-pl aliquot of the solution was injeeted onto a gas chromatograph (GC-I4B. Shimad/u. Kyoto) co u pled with a glass column (3 mm x 2 mm) packed with 5% Advans DS on 8£VIOO meshes Chromosorb W (Shimad/u. Kyoto) and a (lamę ioni/ed detector. Nilnigen gas as a carricr flowed at a ratę of 50 ml/min. and the prcssure of the used hydrogen gas and air were 0.6 and 0.5 kg/cm'. respectis ely. The detcctorand injcction port tempenitures were 240 C.and an isolhermal column temperaturę was maintaincd al 190 C. Ali GC measurcments were performed in triplicate and the concenłration of FAMĘ was determined from thc mcthyl ester fatty acids of rapeseed oil. utili/ing mcthyl pentadccanoateas an inlcmal standard.
3. Kesult and discussion
3.1. Effcct of ratio of rapeseed oil and methanol on FAMĘ production
The ery stal lattice of ABE is a threc-laycred struclurc. namcly. silica-alumina-silica. which confers a large specific surfacc arca and largo adsorplion capacily bccausc of its porous structure. Rapeseed oil and methanol in liquid phase were immediately adsorbed onto the ABE generating a rapeseed oil— mcthanol-ABE complcx. Thereforc. the balance of iiKthanol and rapeseed oil is important in solvent-free methanolysis. To optimize the molar ratio of methanol to rapeseed oil. the addilion of methanol to the reaclion mixlurc was invcsligalcd using molar ratios of methanol to rapeseed oil from 1:1 to 1:8 (Fig. I). The theoretical molar ratio of methanol to rapeseed oil is 1:3. Whcn the ralio was 1:1 the FAMĘ comcrsion was lower than 50%. and whcn thc ratio was 1:3 it was 67%. This might he due lo a diflusion limitation of methanol in the lipasc-catalyzed transesterification on thc surface of the ABE. Whcn the molar ratio was 1:4, lite FAMĘ conversion reached to 81% in a reaclion tirne of 4 h. Howcvcr. in the casc of a methanol molar ratio higher than 1:4. thc com crsion of rapeseed oil to FAMĘ decreased and was lower than 60%. The Iow comersion efficiency using a high molar ratio of methanol is caused by methanol inhibition of thc catalytic rcaction. which was shown elsewhere 1111.
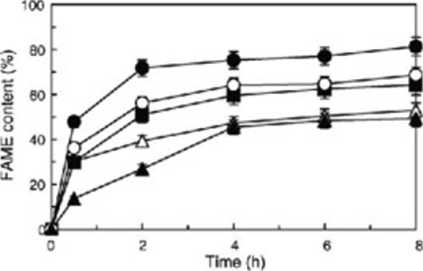
Fig. I. Effcct uf mcihanol to rapeseed od molar ratio on lipa-c-catuly/ed methanolysis of rapeseed oil in solvent-free system. Symbols: 1:1. closed trianglcs: 1:3. open circtcs: 1:4. closed cirelcs: 1:6. closed squares: 1:8. open trianglcv
Wyszukiwarka
Podobne podstrony:
14 H. Pridalova et al.INTRODUCTION Goats are bred worldwide and therefore there is a great variance
155 B. Rihn et aL / Toxicology 109 (1996) 147-156 toe, 1983) using relatively high dust concentra-io
134 T. Wilgał et al. drying, a change in hydraulic gradients and a shift of ground-watersheds. The z
2 M. Sajewicz et al.Introduction Identification of plant species and varieties can be performed by a
190 M. Sajewicz et al.References [1] M.E. Guynot, S. Martin, L. Seto, V. Sanchis,
39 BLATTER et WIER, 1994; HIRAMATSU et al.t 1994). L inhibition de la Liberation de calcium du retic
Cerdan M. [et al.], Reussir le Delf: niveauA2: du Cadre europeen commun de reference, Paris, 2005. C
228 M. Yacoubi-Khebiza et al. - Un nouveau Microparasellidae du Haut-Atlas Derivatio nominis. - Le n
M Yacoubi-Khebiza et al. - Un nouveau Microparasellidae du Haut-Atlas 230 Fig. 2. Microcharon ourike
232 M. Yacoubi-Khebiza et al. - Un nouveau Microparasellidae du Haut-Atlas relation avec 1’allongeme
234M Yacoubi-Khebiza et al. - Un nouveau Microparasellidae du Haut-Atlas Coineau, N., 1986. Isopoda:
Porównanie NOSów Alderton et al. Biochem J. 2001
Murray Robert K. [et al. ; tł. Zenon Aleksandrowicz et al.]. Biochemia Harpera. Wyd. 5. (red. nauk.
42 E. Cadier et al.LE NORDESTE BRESILIEN Le Nordeste du Bresil couvre un peu moins de 1 600 000 km2
138 dćveloppement du squelette (Hankenson et al., 2010). Par contrę, Pexpression de Rspo2 n’a pas et
144 1’angiogenese et la mediation du signal antiangiogenique (Jimenez et al., 2000), il serait inter
Rev. Sci. Technol., Synthese 26: 65 - 74 (2013) T.Hamel et al. 2.2 Mćthodologie La realisation du pr
więcej podobnych podstron