8589356371
L Kozhitov. A. Kostikova. V. Kozlov. Zh. Myrkhalykov, A. Saipov.
FEA TURES OF FeNi3 NANOPARTICLES IN POL YACRYLONITRILE FORMA TION UNDERINFRARED HEA TING
The XP A spectra of composites FeCl3*6H20/NiCl2*6H20/PAN with CFc and Cn, concentrations from 5 to 20 mass % prepared by the IR-heating at 500 and 700 °C are obtained (Fig. 3). In the XPA spectra of samples the halo at 20 - 4-r30° confirming the formation of amorphous C from PAN under the destruction
as a result of the IR-heating is observed. Peaks at 26 ~ 45, 52 and 76° corresponding to the FeNij crystalline inter-metallide formation are defined. As far as Cmc increases from 5 to 20 mass %, the average size of FeNi3 nanoparticles in nanocomposites increases from 20 to 60 nm (Table 2).
O.f
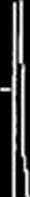
“I-'-I---1-1-1-
8
1* 2C 22 *0 *8 i* 72 80
:e.'
Fig. 3 - XPA spectrum of FeNi3/C nanocomposite with initial CFc and CNl equal to 20 mass % after IR-
heating at 500°C
The phase diagram of a Fe-Ni system confirms the possibility of FcNi3 obtaining at Iow temperaturę [14]. Crystalline FeNi3 formation is determined by SEM (Fig. 4). FeNi3
nanoparticles are distributed in the carbon materiał obtained from the polymer. In this case, the carbon materiał has the porous surface obtained by the polymer destruction and volatile Products emission under IR-heating (Fig. 4).
Table 2 - X-ray data of FeNij/C composites obtained under the IR-heating
|
X2 |
cFe, mass % |
CN„ mass % |
T °C |
Particie composition |
Average particie size, nm |
|
1 |
20 |
20 |
500 700 |
FeNi3 FeNi3 |
53 |
|
57 | |||||
|
FeNi, |
20 | ||||
|
500 |
43 | ||||
|
2 |
10 |
10 |
Fe | ||
|
700 |
FeNi3 |
40 | |||
|
500 |
FeNi3 |
47 | |||
|
3 |
5 |
5 |
700 |
FeNi3 |
10 |
8
Journal of Industrial Technology and Engineering, 2012, 2(3): 5-10
Wyszukiwarka
Podobne podstrony:
L. Kozhitov, A. Kostikova. V. Kozlov. 7Ji. Myrkhalykov. A. Saipov. FE A TURES OF FeNi3 NANOPA R TICL
L Kozhitov. A. Kostikova. V. Kozlov. Zh. Myrkhalykov. A. Saipow FEATURES OFFeNi3 NANOPARTICLES IN PO
L. Kozhitov, A. Kostikova. V. Kozlov, Zh. Myrkhalykow A. Saipow FEATURES OF FeNi3 NANOPARTICLES IN
L. Kozhitow A. Kostikoia. V. Kozlov. Zh. fyrkhalvkov. A. Saipow FEATURES OFFeNi3 NANOPARTICLES IN
Vlasenko, N. A., and Zh. A. Pukhliy (6). Solid 6tate laser Iwith reduced threshola level of current
skanuj0031 (133) AM. jl oju 48/K(Jckl0tMAŁci zoc&t <o wunebAiockhcmk- piłuzcniM (fea^mj/ozi^a
foto (22) b) kombinacja wynikowa:Yju +W-ZH-^t*K idzie: /, - obciążenie wyjątkowe. W stanach graniczn
Zdj?cie2548 Efekt przyjęcia p*trc h Sem i wari ogram zh=20m Semiwariogram z h-200m 101
Ćwiczenia dla 5 6 latków 4 (2) Wypisz nazwy zwierząt i przedmiotów na ilustracji, które zaczynają s
img380 ERRATA STRONA JEST POWINNO BYĆ 35 ŻH 2 / ■ 1 J n- 1 S n - 1 42 - ~al(/«-i) s - a1
IMGW34 43 43 jąco lego iwa- Wu- ości 1-Fe ulku feą jes. od ru- -3d -
I I Demon v.c. C hien Ch aiurdelrtun, ?5 tri tures dc Malik 164 T n iin V % cira
PolandP131b 100Zlotych 1947 donatedELH f 0000000 > NARODOW BANK POL-SKI 0000000ZŁOTYCHZ ŁOT Y€H W
więcej podobnych podstron