Journal of Chromatography A, 1119 (2006) 51–57
Study of temperature-responsibility on the surfaces of a
thermo-responsive polymer modified stationary phase
Eri Ayano
, Yuji Okada
, Chikako Sakamoto
, Hideko Kanazawa
,
Akihiko Kikuchi
, Teruo Okano
a
Department of Physical Pharmaceutical Chemistry, Kyoritsu University of Pharmacy, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan
b
Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University, 8-1 Kawadacho, Shinjyuku-ku, Tokyo 162-8666, Japan
Available online 17 February 2006
Abstract
We investigated a thermo-sensitive polymer, poly(N-isopropylacrylamide) (PNIPAAm), which is the basis of an HPLC stationary phase. We
prepared a PNIPAAm terminally-modified surface. In this study, we investigated the effect of PNIPAAm on the surface of a stationary phase on
separation based on changes of the retention time with the temperature step gradient. As the temperature changed the surface property of the
stationary phase switched from hydrophilic to hydrophobic. The retention on the polymer-modified stationary phase remarkably changed upon
changing the temperature. Using a column packed with PNIPAAm-modified silica, the separation of steroids was carried out by changing the
temperature. With increasing temperature, an increased interaction between solutes and PNIPAAm-grafted surfaces of the stationary phases was
observed. A temperature-dependent resolution of steroids was achieved using only water as a mobile phase. The PNIPAAm-modified surface of the
stationary phase exhibited temperature-controlled hydrophilic–hydrophobic changes. The drastic and reversible surface hydrophilic–hydrophobic
property alteration for PNIPAAm terminally-grafted surfaces should be due to rapid changes in the polymer hydration state around the polymer’s
transition temperature. A solvent gradient elution-like effect could be achieved with a single mobile phase by programmed temperature changes
during chromatographic runs. This system should be highly useful to control the function and property of the stationary phase for HPLC only by
changing the temperature with an aqueous solvent.
© 2006 Elsevier B.V. All rights reserved.
Keywords: Poly(N-isopropylacrylamide); HPLC; Temperature-responsive chromatography; Temperature step gradient; Oral contraceptive
1. Introduction
Recently, the research of various polymers has been widely
carried out, and investigations on the structure and function play
important roles in various fields
. The structure and function
of these materials, termed ‘intelligent materials’, are controlled
by their response to surrounding conditions, such as the pH
electric field
, light
, chemical species
and tempera-
ture
. Poly(N-isopropylacrylamide) (PNIPAAm) is one of
the intelligent materials that exhibit a thermally reversible phase
transition in response to temperature changes across a lower
critical solution temperature (LCST) of 32
◦
C in aqueous solu-
tion
. In water, the polymer chains of PNIPAAm hydrate and
expand below this LCST, while they dehydrate to form a com-
pact conformation above it. This can possibly be explained by
∗
Corresponding author. Tel.: +81 3 5400 2657; fax: +81 3 5400 1378.
E-mail address: kanazawa-hd@kyoritsu-ph.ac.jp (H. Kanazawa).
hydration/dehydration changes of polymer side-chain isopropyl
groups. Additionally, it has also been confirmed that the effect
of PNIPAAm precipitation by the salt concentrations exists. We
examined the temperature dependence for optical transmittance
of NIPAAm polymer solution of various concentrations in NaCl.
The LCST decreased with increasing concentrations of NaCl,
while the sharp soluble–insoluble changes were maintained. The
LCST remarkably shifted to 20
◦
C in 1 M NaCl solution. The
lowering of the LCST by the addition of salt should, therefore,
be due to acceleration of dehydration, i.e., salting out
Utilizing a change in the property by the response to the
temperature of PNIPAAm, these materials have been widely
utilized in drug-delivery systems
, cell culture dishes
cell sheets
and bioconjugates
. The electrophoresis for
the separation of DNA fragments using PNIPAAm solutions
. Furthermore, the study of the PNIPAAm-modified surface
is also actively carried out, intermolecular force between the
PNIPAAm surface and protein
and surface analysis by the
AFM
are also carried out.
0021-9673/$ – see front matter © 2006 Elsevier B.V. All rights reserved.
doi:10.1016/j.chroma.2006.01.126

52
E. Ayano et al. / J. Chromatogr. A 1119 (2006) 51–57
We previously reported a considerable and reversible change
in the hydrophilic–hydrophobic properties of PNIPAAm-grafted
surfaces in response to a change in temperature
. We have
constructed a novel chromatography system of the temperature-
responsive type, in which we applied PNIPAAm-modified sil-
ica as a column packing material. We previously reported to
have achieved the separation of steroids
and environ-
mental pollutants
using temperature-responsive chro-
matography. Especially, this method was also applicable for the
separation of peptides
and both low-molecular-
weight proteins, such as ribonuclease and chymotrypsinogen,
and high-molecular-weight proteins, such as ovalbumin, cata-
lase, and bovine serum albumin
. The peptides and
proteins retention times increase as the hydrophobicity of the
polymer increases. Many reversed-phase methods for proteins
have employed C18 columns with mobile phases containing
acetonitrile in low-pH buffers. Little attention has been paid to
recovering biological activity. Thus, these conditions should be
avoided in the separation of most proteins. In contrast, because
temperature-responsive chromatography is performed in an
aqueous environment that includes structure-stabilizing salts,
temperature-responsive chromatography allows for the retention
of biological activity. Moreover, the PNIPAAm-modified col-
umn is cleaned by washing with several volumes of cold water,
because the surface property of the stationary phase becomes
hydrophilic at this temperature. This column cleaning is drasti-
cally different from those of reversed-phase columns, where a
high concentration of the organic solvent is used to clean up.
Generally, in conventional chromatography, in order to
achieve the desired resolution in a reasonable time, it may be
necessary to use gradient elution where volumes of an organic
solvent, the composition of the mobile phase, or other proper-
ties of the solvent, such as the pH or ionic strength, are changed
during separation.
In this study, we prepared a temperature-responsive PNI-
PAAm terminally-modified surface, and investigated the effect
on the retention behavior and the external temperature with a
step temperature gradient using steroids. Furthermore, the speed
in which the surface property of the stationary phase changed
to hydrophilic from hydrophobic was examined. In addition, a
temperature step gradient was also applied to the separation of
an oral contraceptive in human urine.
2. Experimental
2.1. Chemicals
N-Isopropylacrylamide (NIPAAm) was kindly provided by
KOHJIN, Tokyo, Japan, and was purified by recrystallization
from n-hexane. Butylmethacrylate (BMA), ethyl acetate, 1,4-
dioxane, and N-hydroxysuccinimide were purchased from Wako
Pure Chemical Industries. 3-Mercaptopropionic acid (MPA),
2,2
-azobisisobutyronitrile (AIBN), N,N-dimethylformamide
(DMF), and N,N
-dicyclohexylcarbodiimide (DCC) were
obtained from Kanto Chemicals, Tokyo, Japan. Aminopropyl
silica beads (average diameter, 5
m; pore size, 120 ˚A) were
purchased from Nishio Industries, Tokyo, Japan. HPLC-grade
methanol and tetrahydrofran (THF) were purchased from Wako
Pure Chemical Industries.
Analytical-grade standards of steroids were purchased from
Wako Pure Chemical Industries, Osaka, Japan. ANGE
®
28 of
an oral contraceptive was purchased from TeikokuZoki, Co.,
Ltd., Tokyo, Japan. The pure water used for sample preparation
and the LC mobile phase was prepared using a Milli-Q water-
purification system (Millipore, Bedford, MA, USA).
2.2. Preparation of PNIPAAm terminally-modified silica
The synthesis of PNIPAAm and P(NIPAAm-co-BMA), and
a modification of aminopropyl silica with the NIPAAm poly-
mer were carried out by radical polymerization, as previously
reported
2.3. Temperature-responsive LC
A PNIPAAm terminally-modified silica bead support was
packed into a stainless-steel column (150 mm
× 4.6 mm i.d.).
The column was connected to an HPLC system (HITACHI
Model L-7100 intelligent pump; L-7400 UV-detector; D-
7500 integrator). Analysis of the samples was performed on
a temperature-responsive polymer-modified column using a
mobile phase consisting of Milli-Q water. The elution behav-
iors were monitored by 254 nm for steroids and 280 nm for an
oral contraceptive with a flow rate of 1.0 mL min
−1
at various
temperatures with a Coolnics circulator (CTE42W, Yamato-
KOMATSU, Tokyo, Japan) within a derivation of
±0.01 to
±0.03
◦
C.
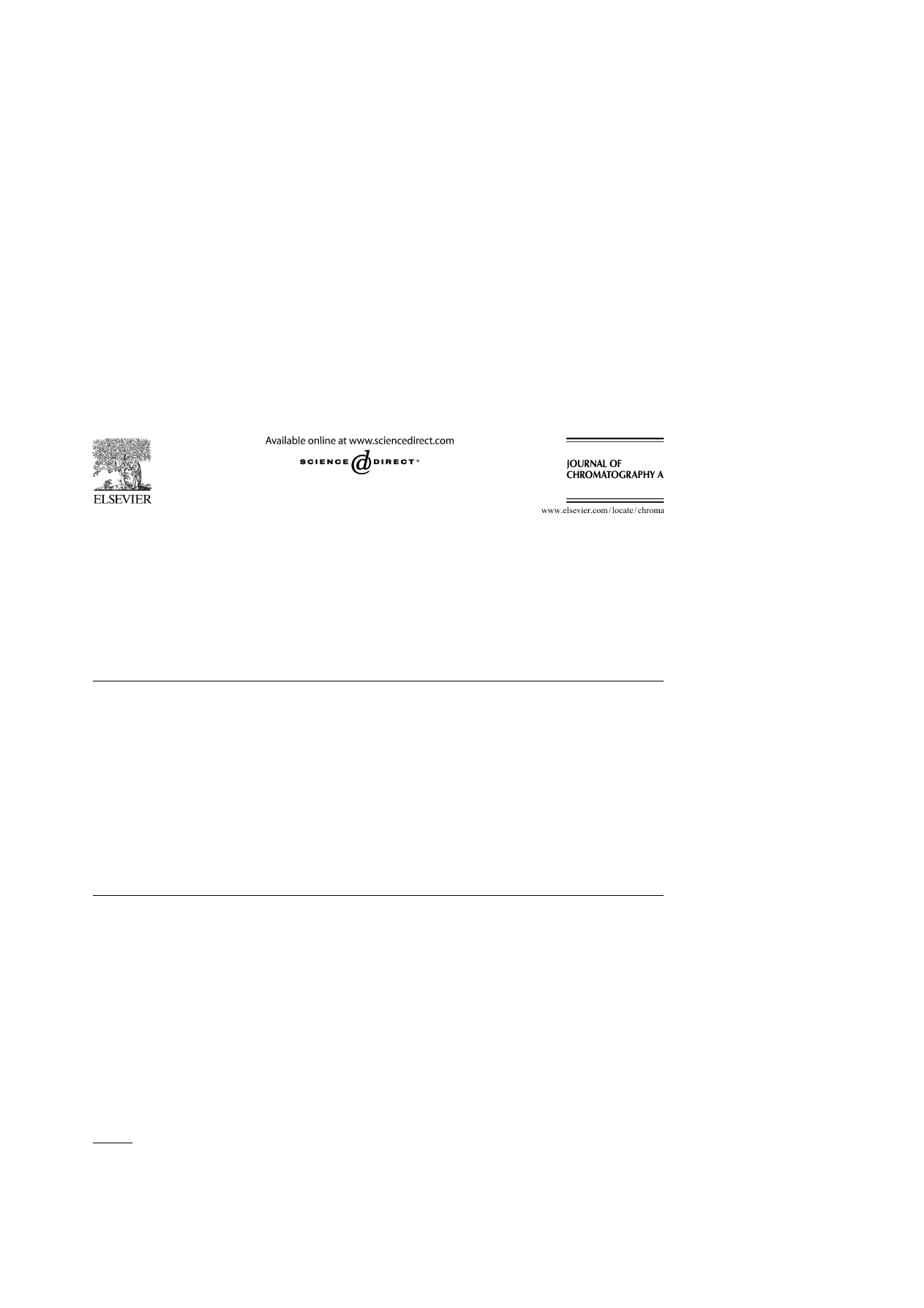
shows a diagram of the apparatus for temperature-
controlled elution. A water jacket was used for changing the
temperature step gradient. The water jacket was connected to
two coolnics with tubing and three-way taps. The coolnics was
first equilibrated at 40
◦
C and, at a given time, the column jacket
temperature was switched to 10
◦
C.
Standard solutions of steroids were prepared with hydro-
cortisone (0.101 mg mL
−1
), predonisolone (0.166 mg mL
−1
),
dexamethasone
(0.061 mg mL
−1
),
hydrocortisone
acetate
(0.007 mg mL
−1
), and testosterone (0.027 mg mL
−1
) in Milli-Q
water.
2.4. Preparation of a standard sample for an oral
contraceptive
A ground oral contraceptive including levonorgestrel
0.075 mg and ethinylestradiol 0.040 mg in one tablet was dis-
solved in 1 mL of THF. After ultrasonication for 2 min, it was
centrifuged at 3000 rpm for 10 min. The supernatant was fil-
trated, and was made to be a standard sample.
2.5. Urinary sample preparation
The ground oral contraceptive was spiked in 1 mL of urine
and dissolved. After ultrasonication for 2 min, it was centrifuged
at 3000 rpm for 10 min. The supernatant was filtrated. The spiked
urinary sample was pretreated with a solid-phase extraction as
E. Ayano et al. / J. Chromatogr. A 1119 (2006) 51–57
53
Fig. 1. Diagram of a system for temperature step gradient elution.
follows: after a supernatant was loaded into a Sep-pak C
18
cartridge (Waters, Milford, MA, USA), which has been pre-
conditioned with methanol and Milli-Q water, a 5 mL volume
of Milli-Q water as a washing solution was passed through the
cartridge. The sample fraction was then obtained by elution with
5 mL of methanol. After evaporation under reduced pressure, the
residue was dissolved in 1.0 mL of THF.
3. Results and discussion
3.1. Effect of PNIPAAm modified surface morphology on
separation
The effects of the graft polymer chain conformation were
examined on a PNIPAAm terminally-modified surface using
steroids. We grafted PNIPAAm onto aminopropyl silica beads,
and used them as a packing material for an HPLC column.
shows typical chromatograms of steroids on a PNI-
PAAm teminally-modified column at 10 and 50
◦
C. In reversed-
phase HPLC using an ODS column, the retention times should
decrease with increasing temperature. However, in the PNI-
PAAm terminally-modified column, the opposite behavior of
the retarded retention times was observed with increasing tem-
Fig. 2. Chromatograms of steroids on a PNIPAAm terminally-modified column
at 10 and 50
◦
C using pure water as a mobile phase. Peaks: (1) hydrocortisone;
(2) prednisolone; (3) dexamethasone; (4) hydrocortisone acetate and (5) testos-
terone. HPLC conditions: flow-rate, 1.0 mL min
−1
; monitoring, UV at 254 nm.
perature. It was considered that a hydrophobic interaction exists
between steroids and the PNIPAAm terminally-modified col-
umn.
We previously demonstrated that effect of salt addition in the
mobile phase was examined. The effects of column temperature
and NaCl in the mobile phase on the retention profile of steroids
are observed. The retention times of steroids were much longer
with 1 M NaCl than with pure water as a mobile phase. Some
extent of resolution was obtained even at 5
◦
C. This should result
from a lowering of the LCST by changing the mobile phase. The
fact suggests that we can control LCST and the hydrophobicity
of the surface of the stationary phases, and thus elution time, by
salt concentration of mobile phase
shows the variation of the ln k values with the tem-
perature changes of a PNIPAAm terminally-modified column
with water as the mobile phase. The stationary phase showed
a greater affinity for steroids at a higher temperature (50
◦
C)
compared with those at a lower temperature (10
◦
C). These
Fig. 3. Comparison of ln k on a PNIPAAm terminally-modified column at 10
and 50
◦
C using pure water as a mobile phase. Samples: (1) hydrocortisone;
(2) prednisolone; (3) dexamethasone; (4) hydrocortisone acetate and (5) testos-
terone. HPLC conditions: flow-rate, 1.0 mL min
−1
; monitoring, UV at 254 nm.
54
E. Ayano et al. / J. Chromatogr. A 1119 (2006) 51–57
observations would be due to a temperature-responsive confor-
mational change of the NIPAAm polymer.
We previously reported that a hydrophobic interaction
between steroids and PNIPAAm modified surfaces was readily
modulated by the temperature
. As described above, the
PNIPAAm terminally-modified column showed drastic changes
in the retention of solutes by changes in the column temper-
ature. There should be interactions between the solutes and
polymer chains of the surface on the stationary phase in our
system. Okano et al.
and Yakushiji et al.
that the molecular mobility and density of a PNIPAAm chain
are greatly influenced by the difference of the construction
structure of the PNIPAAm modified surfaces by the tempera-
ture. It is considered that it maintains high mobility by fixing
the PNIPAAm molecule at the end on the modified terminal,
and quickly responds to any temperature change. The degree
of freedom of a PNIPAAm terminally-modified polymer chain
is bigger, and temperature-controlled hydrophilic–hydrophobic
changes seem to be big. The temperature-responsive interac-
tion between PNIPAAm terminally-modified silica and steroids
should be due to changes in the surface properties of the
PNIPAAm-grafted stationary phase by a reversible transition of
hydrophilic–hydrophobic PNIPAAm-grafted surface properties.
By utilizing the features of such modified columns, application
possibilities to various fields were indicated.
3.2. Effect of a temperature step gradient
In the isocratic elution of samples containing solutes with a
wide range of polarity, it is sometimes difficult to achieve the
desired resolution in a reasonable time. It may be necessary to
use gradient elution where volumes of an organic solvent, the
composition of the mobile phase, or other properties of the sol-
vent (e.g., pH or ionic strength) are changed during separation.
On HPLC columns packed with temperature-responsive
polymer-modified silica, temperature programming is used
in lieu of a gradient solvent
. In this study, the
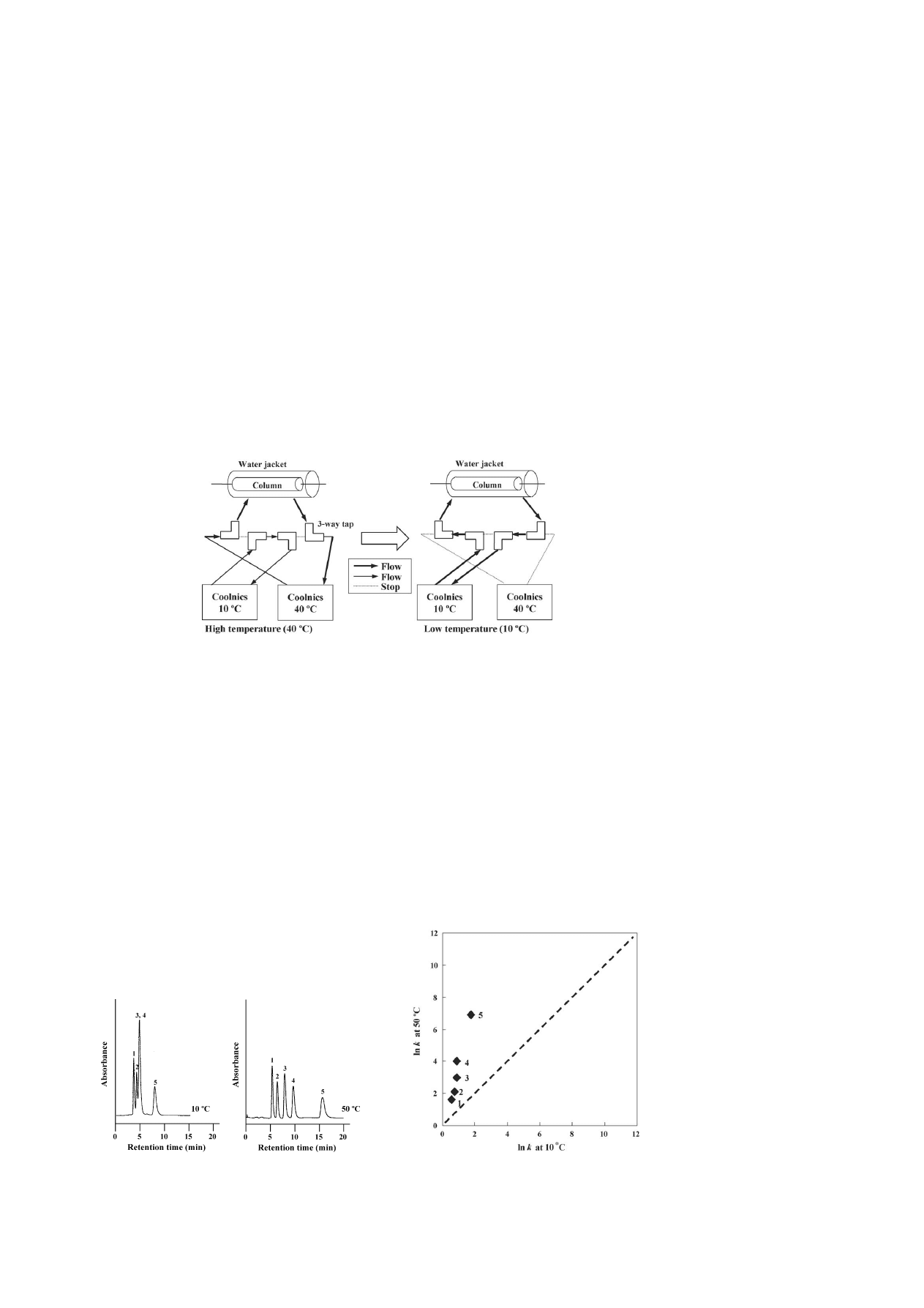
Table 1
Regression equations and R
2
for PNIPAAm terminally-modified surface when
the temperature was changed from 40 to 10
◦
C
Compounds
Equations
R
2
Hydrocortisone
y = 0.281x + 3.262
0.997
Hydrocortisone acetate
y = 0.341x + 3.592
0.998
Testosterone
y = 0.326x + 5.060
0.997
a
x, temperature changing time and y, retention time.
temperature-dependent elution behavior of steroids was exam-
ined on a PNIPAAm terminally-modified surface. These consid-
erations were supported by the elution profiles of steroids after
applying a temperature step gradient. A temperature step gradi-
ent from 40 to 10
◦
C was applied during the elution of three
steroids (hydrocortisone, hydrocortisone acetate, and testos-
terone).
shows the change in the retention time with
the temperature step gradient on the elution of three steroids.
The horizontal axis represents the time at which the column
temperature was switched from 40 to 10
◦
C; the sample injec-
tion time is indicated at 0 min time. The retention time equaled
the equilibrium retention time at 10
◦
C with isocratic elution,
even though the column temperature was changed before sam-
ple injection. Because the change in the surface property of
the stationary phase from hydrophobic to hydrophilic was very
rapid, it was suggested that the surface property of the stationary
phase became hydrophilic, until the analytes reached the col-
umn. Moreover, the retention times changed linearly when the
column temperature was changed while the analytes were dis-
tributed in the column. The regression equations were required,
because the retention times changed linearly. The equations are
shown in
. For each sample, a good correlation (R
2
) was
obtained during the time for switching the column temperature
and the retention time. It is indicated that the reproducibility of
the analytes for the column temperature is good, even if the tem-
perature is changed at same point of time. These features seem
to be due to rapid changes in the polymer conformation, which
are attributed to the mobility of terminally-grafted polymers.
Fig. 4. (a) Effect of the temperature step gradient on steroid elution from 40 to 10
◦
C change. (b) Regression line on a PNIPAAm terminally-modified column. (
)
Hydrocortisone; (
), hydrocortisone acetate and () testosterone.
E. Ayano et al. / J. Chromatogr. A 1119 (2006) 51–57
55
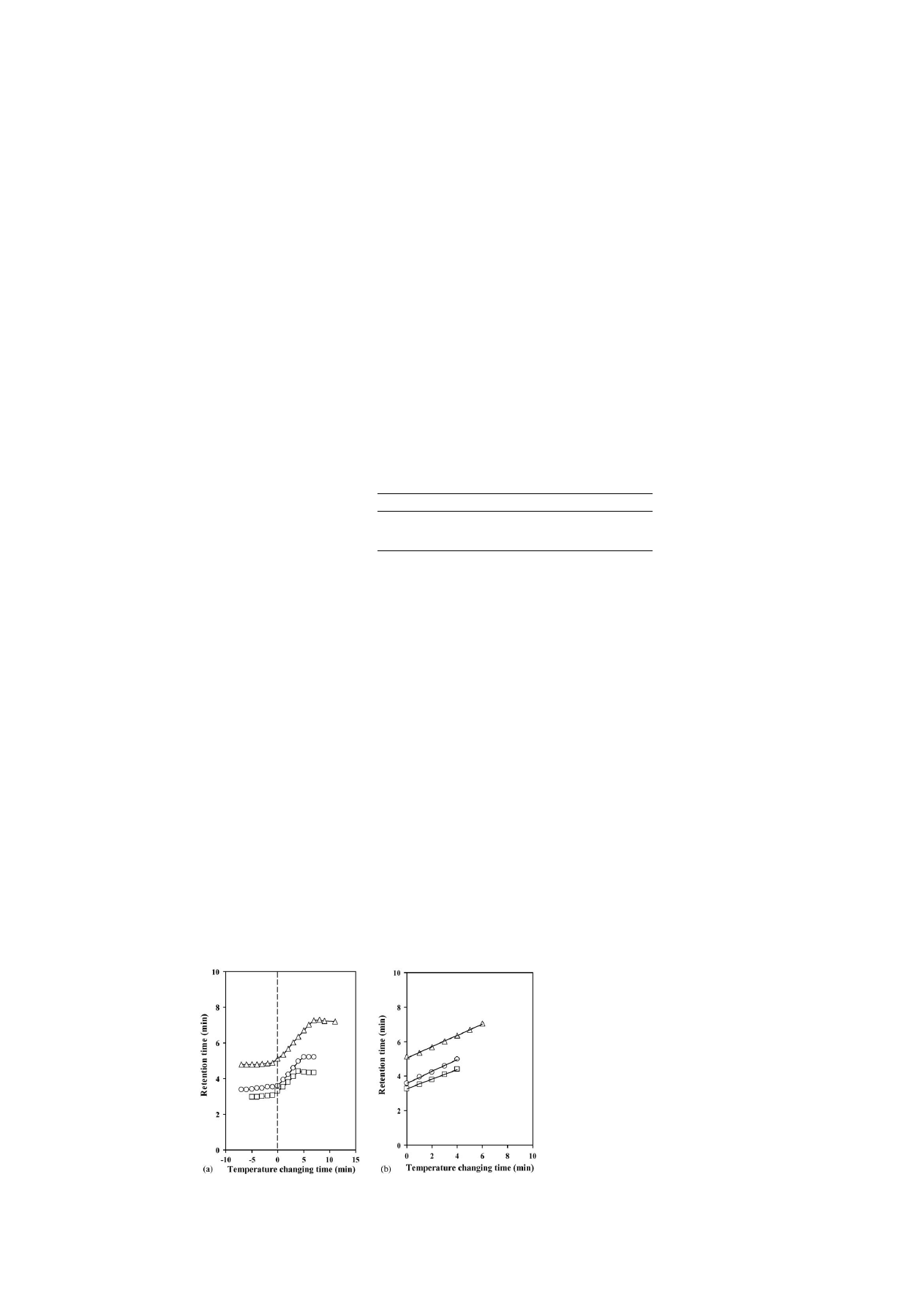
Fig. 5. Changes in the column back pressure for a PNIPAAm terminally-
modified column after temperature step changes from 40 to 10
◦
C.
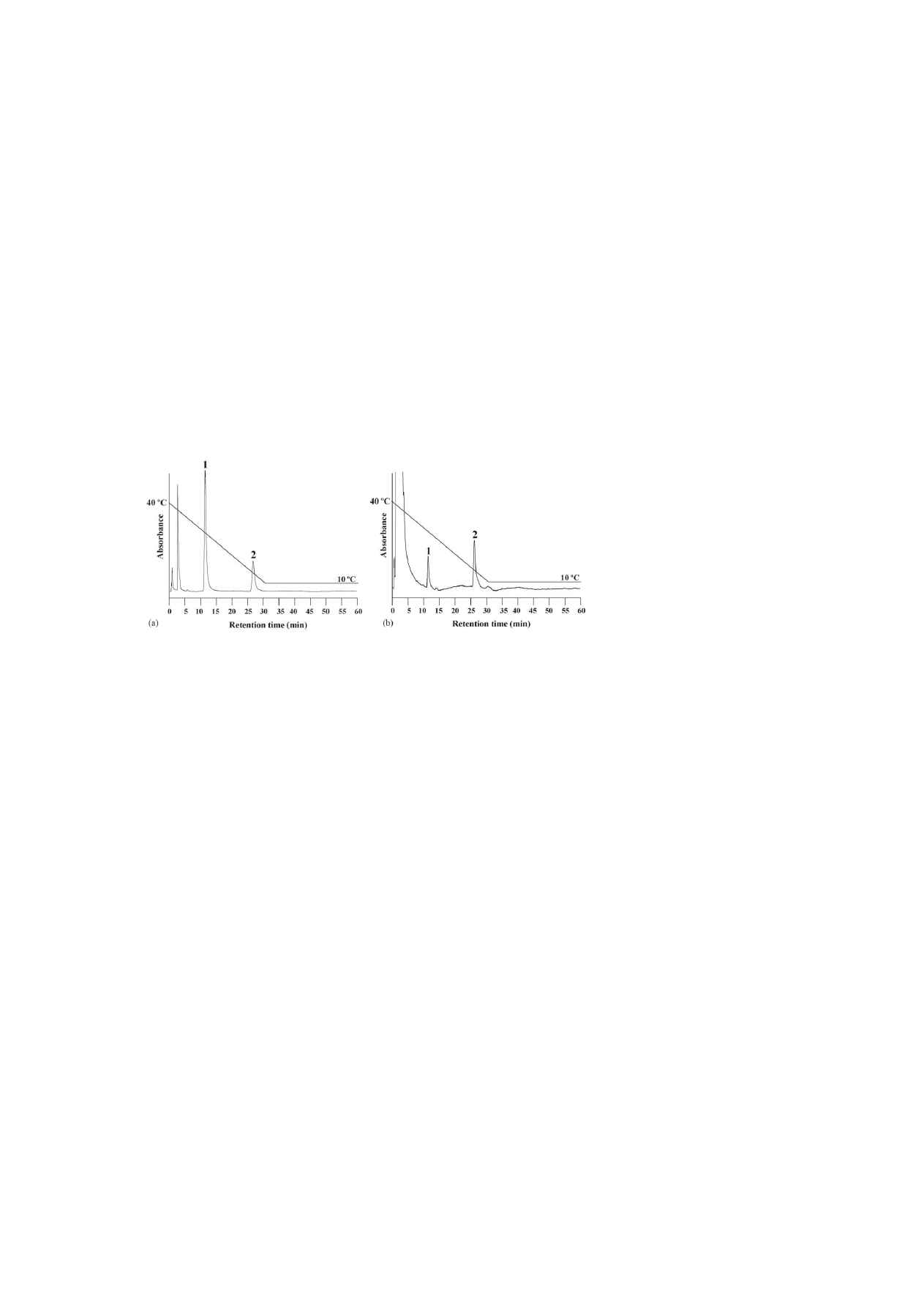
Fig. 6. Effect of a temperature change (stepwise) on the retention times of
levonorgestrel and ethinylestradiol of an oral contraceptive on a PNIPAAm
terminally-modified column at 10 and 40
◦
C, and from 40 to 10
◦
C using pure
water as a mobile phase. Peaks: (1) levonorgestrel and (2) ethinylestradiol. HPLC
conditions: flow-rate, 1.0 mL min
−1
; monitoring, UV at 280 nm.
This shows that an examination of the surface mobility is pos-
sible from the size of the slope. These facts can be discussed as
follows: (1) the expectation of the retention time from the tem-
perature changing time became possible, and (2) an estimate of
the temperature switching time from the retention time became
possible. With a single mobile phase of water and by controlling
the external temperature, it was possible to obtain an effect sim-
ilar to the solvent gradient. Therefore, it was considered that it
might become possible to separate those compounds for which
separation has been difficult until now only in the temperature
program. Also, it seems to be possible to use a chromatographic
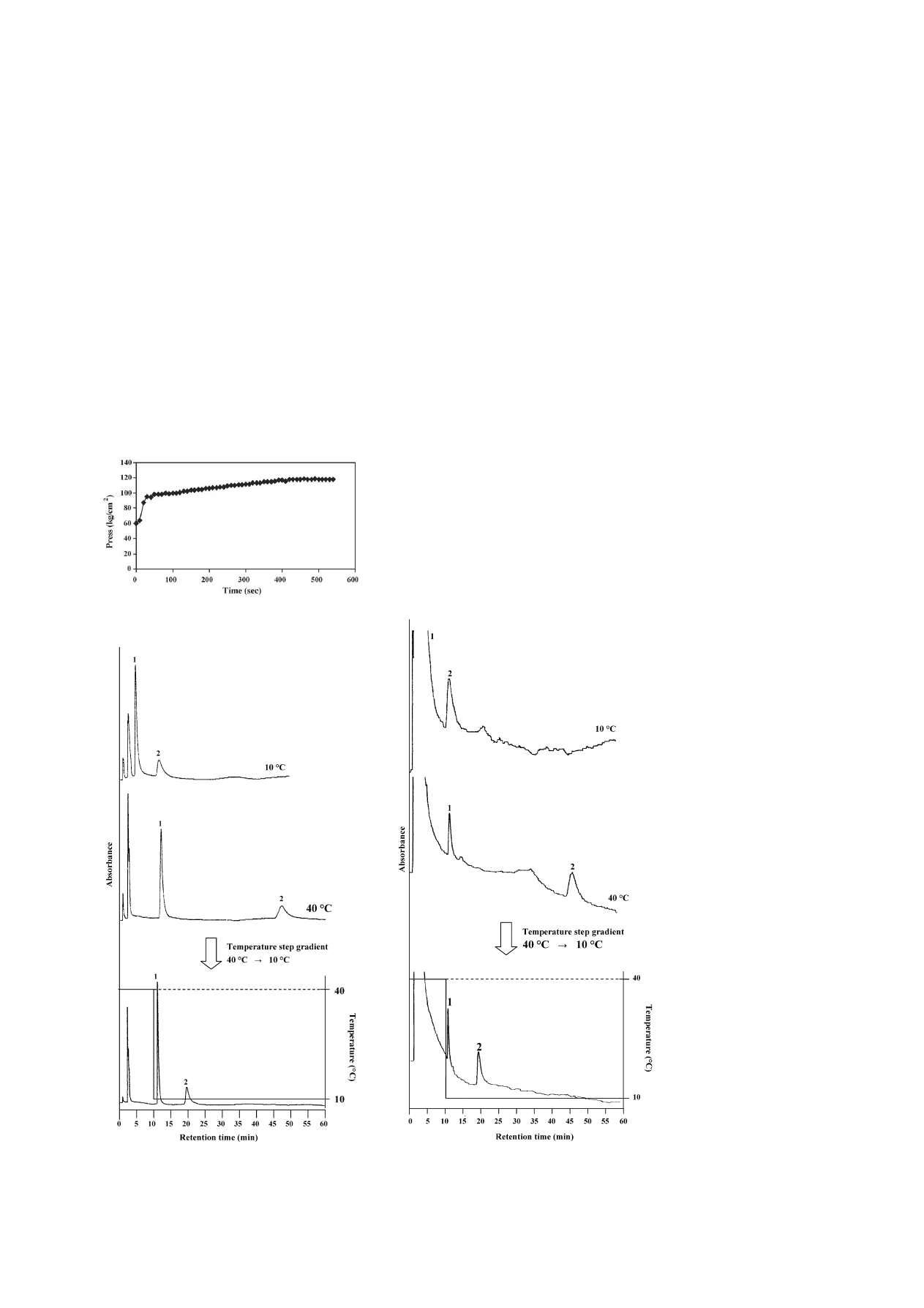
Fig. 7. Effect of a temperature change (stepwise) on the retention times of lev-
onorgestrel and ethinylestradiol in urine on a PNIPAAm terminally-modified
column at 10 and 40
◦
C, and from 40 to 10
◦
C using pure water as a mobile phase.
Peaks: (1) levonorgestrel and (2) ethinylestradiol. HPLC conditions: flow-rate,
1.0 mL min
−1
; monitoring, UV at 280 nm.
56
E. Ayano et al. / J. Chromatogr. A 1119 (2006) 51–57
Fig. 8. Effect of a temperature change (linear) on the retention times of levonorgestrel and ethinylestradiol of (a) an oral contraceptive (b) in urine on a PNIPAAm
terminally-modified column from 40 to 10
◦
C using pure water as a mobile phase. Peaks: (1) levonorgestrel and (2) ethinylestradiol. HPLC conditions: flow-rate,
1.0 mL min
−1
; monitoring, UV at 280 nm.
methodology for a kinetic analysis of a stimulus-response poly-
mer in the surface by the new technique of the temperature step
gradient in the LC method.
The column back pressure in switching the column tempera-
ture was determined in order to observe the changing rate on the
polymer surface (
). Coolnics was first equilibrated at 40
◦
C
and, at a given time, the column jacket temperature was switched
to 10
◦
C. In fact, the column back pressure was abruptly elevated
within 30 s, and re-equilibrated at 10
◦
C within 7 min after tem-
perature changes. It can be examined whether the changing rate
on the polymer surface is rapid by observing the column back
pressure.
3.3. Application of an oral contraceptive
Optimization of the analysis was attempted regarding two
components, levonorgestrel (log P = 2.871) and ethinylestradiol
(log P = 4.017) of an oral contraceptive using the temperature
step gradient. Those components that are not metabolized in
the urine of a woman remain. As a result, it can efflux to
rivers, causing water pollution that effects fishes and animals
that inhabit the rivers
. It was determined using a PNI-
PAAm terminally-modified column in which the surface prop-
erty changes were rapid.
shows chromatograms of lev-
onorgestrel and ethinylestradiol of an oral contraceptive that
was obtained at 10 and 40
◦
C, and with a step gradient by
changing the column temperature. For levonorgestrel with lower
hydrophobicity, the retention time hardly changed at 10 and
40
◦
C. However, for ethinylestradiol with a higher hydropho-
bicity, the retention time was increased too much, and influ-
enced the hydrophobic interaction at 40
◦
C. To move strongly
retained components of the oral contraceptive and to optimize
the analysis, we used a temperature-programming technique.
With a single mobile phase of water and by controlling the
external temperature from 40 to 10
◦
C, the analytical time was
reduced. An excellent resolution of the oral contraceptive was
achieved using a temperature step gradient. This was caused
because the PNIPAAm-modified surface property of the sta-
tionary phase changed to hydrophilic at decreased tempera-
ture, and the hydrophobic interaction between the component
and the stationary phase was decreased. Additionally, this fact
indicated the effectiveness of ‘thermo-responsive’ and ‘thermo-
reversible’ property alterations of a PNIPAAm-modified
surface.
3.4. Application of a biological sample: human urine
A sample in which an oral contraceptive was spiked in urine
was examined in order to investigate whether this analytical
system can be applied to biological samples.
shows chro-
matograms of levonorgestrel and ethinylestradiol of an oral
contraceptive spiked in urine that was obtained at 10 and 40
◦
C,
and with a step gradient by changing the column temperature.
At 10
◦
C, the peak of levonorgestrel was disturbed at a con-
taminant peak, which was derived from urine; they were not
properly resolved. At 40
◦
C, the peaks were well-resolved with-
out affecting from the contaminants; however, the analytical
time was increased too much. Especially, the retention time of
ethinylestradiol was greatly retarded. The levonorgestrel well-
resolved without affecting of the contaminants by delaying the
retention time when the temperature was changed from 40 to
10
◦
C. Moreover, the retention time of the ethinylestradiol was
also reduced to about half, and optimization of the analysis was
possible. It was also possible to confirm that the temperature
gradient was effective in those samples when a commercially
produced column oven was used (
). The oral contra-
ceptive spiked in human urine could be well-resolved using
a temperature linear-gradient with a single mobile phase of
water.
4. Conclusions
In a temperature-responsive chromatographic system using a
PNIPAAm-modified column, temperature step-gradient exper-
iments revealed that stationary phase alterations are very rapid
after the application of aqueous mobile-phase temperature
changes. These facts were concluded by investigating the reten-
tion behavior of steroids with temperature changes in aque-

E. Ayano et al. / J. Chromatogr. A 1119 (2006) 51–57
57
ous mobile phases. From these results, the degree of freedom
of a PNIPAAm terminally-modified surface was large, and
it was indicated to be excellent in temperature response. In
this study, we have succeeded in separating levonorgestrel and
ethinylestradiol in an oral contraceptive using only water as the
mobile phase. Optimizing the analysis of these components was
achieved using a temperature step gradient. Moreover, in this
system, it was confirmed that it could be applied to not only
standard compounds, but also biological samples, such as drugs
in urine.
In the future, a temperature-gradient method based on
temperature-responsive chromatography should allow an effec-
tive separation and purification of bioactive compounds, such as
peptides and proteins, and even cells. This system, without the
use of an organic solvent as a mobile phase, is advantageous for
green technology and, as a result, applications to materials that
previously could not be analyzed are expected.
References
[1] M. Heskins, J.E. Guillet, E. James, J. Macromol. Sci. Chem. A 2 (1968)
1441.
[2] Y.H. Bae, T. Okano, S.W. Kim, J. Polym. Sci. Polym. Phys. 28 (1990)
923.
[3] Y.-H. Kim, Y.H. Bae, S.W. Kim, J. Control. Rel. 28 (1994) 143.
[4] T. Tanaka, I. Nishio, S.-T. Sun, S. Ueno-Nishio, Science 218 (1981)
467.
[5] I.C. Kwon, Y.H. Bae, S.W. Kim, Nature 354 (1991) 291.
[6] A. Suzuki, T. Tanaka, Nature 346 (1990) 345.
[7] K. Ishihara, N. Muramoto, I. Shinohara, J. Appl. Polym. Sci. 29 (1984)
211.
[8] G. Chen, A.S. Hoffman, Bioconjugate Chem. 4 (1993) 509.
[9] A. Gutowska, Y.H. Bae, H.A. Jacobs, J. Feijen, S.W. Kim, Macro-
molecules 27 (1994) 4167.
[10] H. Kanazawa, K. Yamamoto, Y. Matsushima, N. Takai, A. Kikuchi, Y.
Sakurai, T. Okano, Anal. Chem. 68 (1996) 100.
[11] H. Kanazawa, Y. Matsushima, T. Okano, in: P.R. Brown, E. Grushka
(Eds.), Advances in Chromatography, vol. 41, Marcel Dekker, 2002, p.
311, Chapter 8.
[12] T. Okano, N. Yamada, H. Sakai, Y. Sakurai, J. Biomed. Mater. Res. 27
(1993) 1243.
[13] T. Shimizu, M. Yamato, A. Kikuchi, T. Okano, Tissue Eng. 7 (2001)
141.
[14] M. Matsukata, T. Aoki, K. Sanui, N. Ogata, A. Kikuchi, Y. Sakurai, T.
Okano, Bioconjugate Chem. 7 (1996) 96.
[15] F. Xu, Y. Baba, Electrophoresis 25 (2004) 2332.
[16] E.C. Cho, Y.D. Kim, K. Cho, J. Colloid Interface Sci. 286 (2005) 479.
[17] X. Cheng, H.E. Canavan, M.J. Stein, J.R. Hull, S.J. Kweskin, M.S.
Wagner, G.A. Somorjai, D.G. Castner, B.D. Ratner, Langmuir 21 (2005)
7833.
[18] H. Kanazawa, Y. Kashiwase, K. Yamamoto, Y. Matsushima, A. Kikuchi,
Y. Sakurai, T. Okano, Anal. Chem. 69 (1997) 823.
[19] H. Kanazawa, T. Sunamoto, E. Ayano, Y. Matsushima, A. Kikuchi, T.
Okano, Anal. Sci. 18 (2002) 45.
[20] K. Yamamoto, H. Kanazawa, Y. Matsushima, K. Oikawa, A. Kikuchi,
T. Okano, Environ. Sci. 7 (2000) 47.
[21] E. Ayano, Y. Okada, C. Sakamoto, H. Kanazawa, T. Okano, M. Ando,
T. Nishimura, J. Chromatogr. A 1069 (2005) 281.
[22] H. Kanazawa, Y. Kashiwase, K. Yamamoto, Y. Matsushima, N. Takai,
A. Kikuchi, Y. Sakurai, T. Okano, J. Pharm. Biomed. Anal. 15 (1997)
1545.
[23] H. Kanazawa, T. Sunamoto, Y. Matsushima, A. Kikuchi, T. Okano, Anal.
Chem. 72 (2000) 5961.
[24] K. Yamamoto, H. Kanazawa, Y. Matsushima, N. Takai, A. Kikuchi, T.
Okano, Chromatography 209 (2000) 21.
[25] R. Yoshida, K. Uchida, Y. Kaneko, K. Sakai, A. Kikuchi, Y. Sakurai,
T. Okano, Nature 374 (1995) 240.
[26] T. Yakushiji, K. Sakai, A. Kikuchi, T. Aoyagi, Y. Sakurai, T. Okano,
Langmuir 14 (1998) 4657.
[27] H. Kanazawa, E. Ayano, K. Chiba, A. Kikuchi, T. Okano, Anal. Sci. 17
(2001) 875.
[28] H. Kanazawa, Anal. Bioanal. Chem. 378 (2004) 46.
[29] T.A. Ternes, M. Stumpf, J. Mueller, K. Haberer, R.D. Wilken, M. Servos,
Sci. Total. Environ. 225 (1999) 81.
[30] G.S. Stokes, Drugs 12 (1976) 222.
Document Outline
- Study of temperature-responsibility on the surfaces of a thermo-responsive polymer modified stationary phase
Wyszukiwarka
Podobne podstrony:
Formation and growth of calcium phosphate on the surface of
Interruption of the blood supply of femoral head an experimental study on the pathogenesis of Legg C
Pancharatnam A Study on the Computer Aided Acoustic Analysis of an Auditorium (CATT)
An experimental study on the development of a b type Stirling engine
Study of the temperature?pendence of the?initic transformation rate in a multiphase TRIP assi
70 1003 1019 Influence of Surface Engineering on the Performance of Tool Steels for Die Casting
Interruption of the blood supply of femoral head an experimental study on the pathogenesis of Legg C
Pancharatnam A Study on the Computer Aided Acoustic Analysis of an Auditorium (CATT)
The effect of temperature on the nucleation of corrosion pit
The incorporation of carboxylate groups into temperature responsive poly(N isopropylacrylamide) base
An experimental study on the drying kinetics of quince
Adsorption of active ingredients of surface disinfectants depends on the type
1955 Some critisism of On the Origin of Lunar Surface Features Urey
Vergauwen David Toward a “Masonic musicology” Some theoretical issues on the study of Music in rela
Notes on the Study of Merkabah Mysticism
więcej podobnych podstron