Combustion, Explosion, and Shock Waves, Vol. 38, No. 3, pp. 295–300, 2002
Spontaneous Combustion of Brown-Coal Dust. Experiment,
Determination of Kinetic Parameters, and Numerical Modeling
UDC 536.46+541.124/128
S. P. Amel’chugov,
1
V. I. Bykov,
2
and S. B. Tsybenova
3
Translated from Fizika Goreniya i Vzryva, Vol. 38, No. 3, pp. 48–54, May–June, 2002.
Original article submitted May 7, 2001.
To study spontaneous combustion and explosion of brown-coal dust, an experimental
setup was designed and a method for analysis of these processes was proposed. Kinetic
parameters (activation energy and preexponent) of the brown coal from the Irsha–
Borodino deposit were determined. It was shown in experiments that, for certain
coal/oxidizer ratios, the explosion has a two-stage character. The repeated explosion
of dust is caused by thermal activation of coal at the first stage of the explosion. Math-
ematical models for a qualitative description of spontaneous combustion of brown-coal
dust are considered.
Key words: brown coal, spontaneous combustion of dust, kinetic parameters, nu-
merical modeling.
INTRODUCTION
Fires and explosions are typical of all stages of han-
dling of coal. Spontaneous combustion is the main rea-
son for fires at fuel stores and coal-conveying systems
(50–60%). Every sixth fire at thermal power plants or
boiler houses is also caused by spontaneous combustion.
It was shown in [1–5] that ability of coals to ignite
spontaneously strongly depends on the kinetic param-
eters of this process, i.e., activation energy and preex-
ponent. These parameters were found to change when
coal is heated to 500–600 K; in this case, the coal be-
comes more reactive, i.e., its thermal activation occurs.
In this work, an explosion of coal dust in a cylindrical
vessel was studied experimentally. It was found that,
for a certain fuel/oxidizer ratio, a second explosion can
follow the first explosion as a result of thermal activa-
tion of coal at the first stage. As mathematical models,
equations governing the material and thermal balance
in a tube reactor were used. Calculations performed for
the kinetic parameters of spontaneous combustion of
1
Institute of Fire Prevention,
Siberian Branch, Krasnoyarsk 660036.
2
Institute of Computational Modeling,
Siberian Division, Russian Academy of Sciences,
Krasnoyarsk 660036.
3
Krasnoyarsk State Technical University,
Krasnoyarsk 660074; bykov@fivt.kgtu.runnet.ru.
brown coal from the Irsha–Borodino deposit show that
the models proposed are in qualitative agreement with
the experiment.
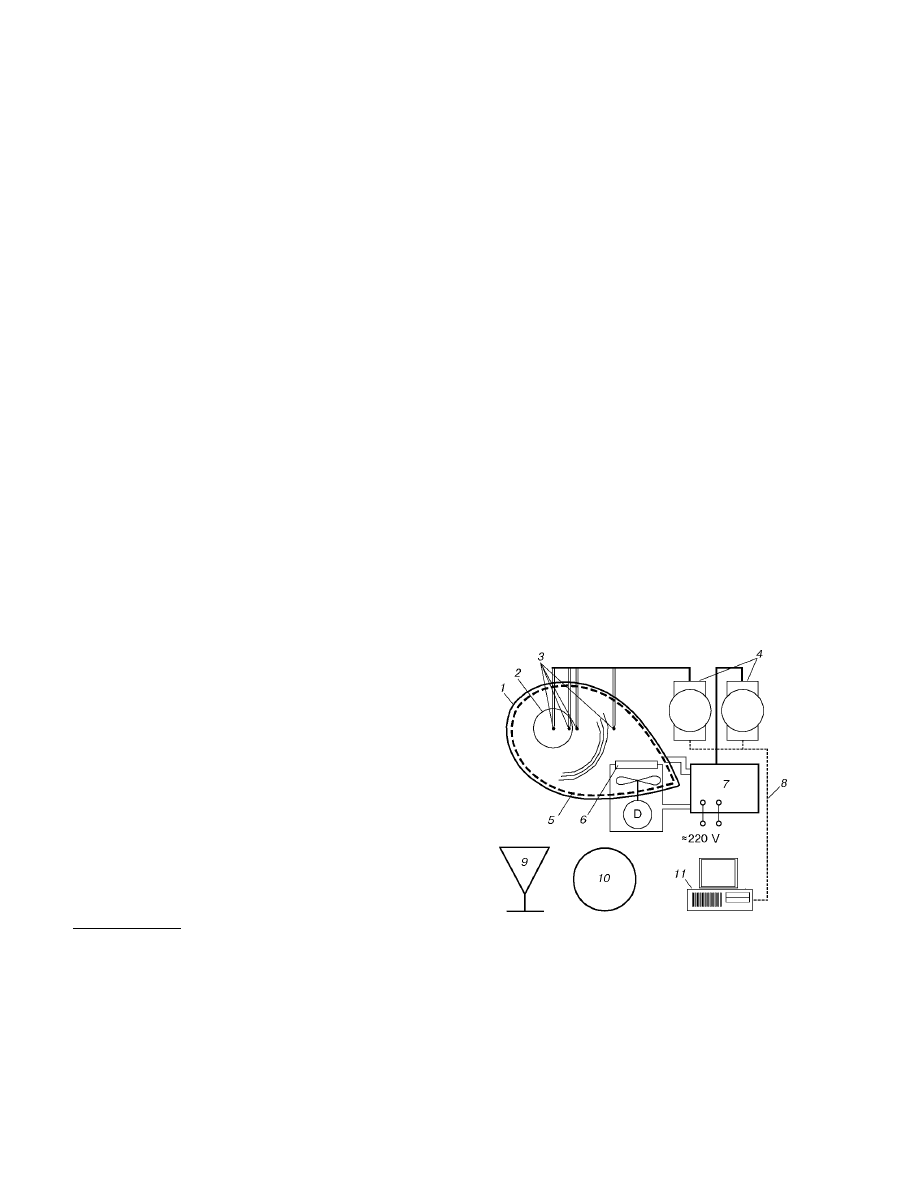
Fig. 1. Diagram of the setup for determining kinetic
parameters of spontaneous combustion of brown
coal: 1) thermostat shell; 2) reaction vessel; 3) ther-
mocouples; 4) modules of data collection and control;
5) main heater; 6) auxiliary heater; 7) module of
power supply and control; 8) RS-485 communication
line; 9) balance; 10) drying cabinet; 11) computer.
0010-5082/02/3803-0295 $27.00 c
2002
Plenum Publishing Corporation
295
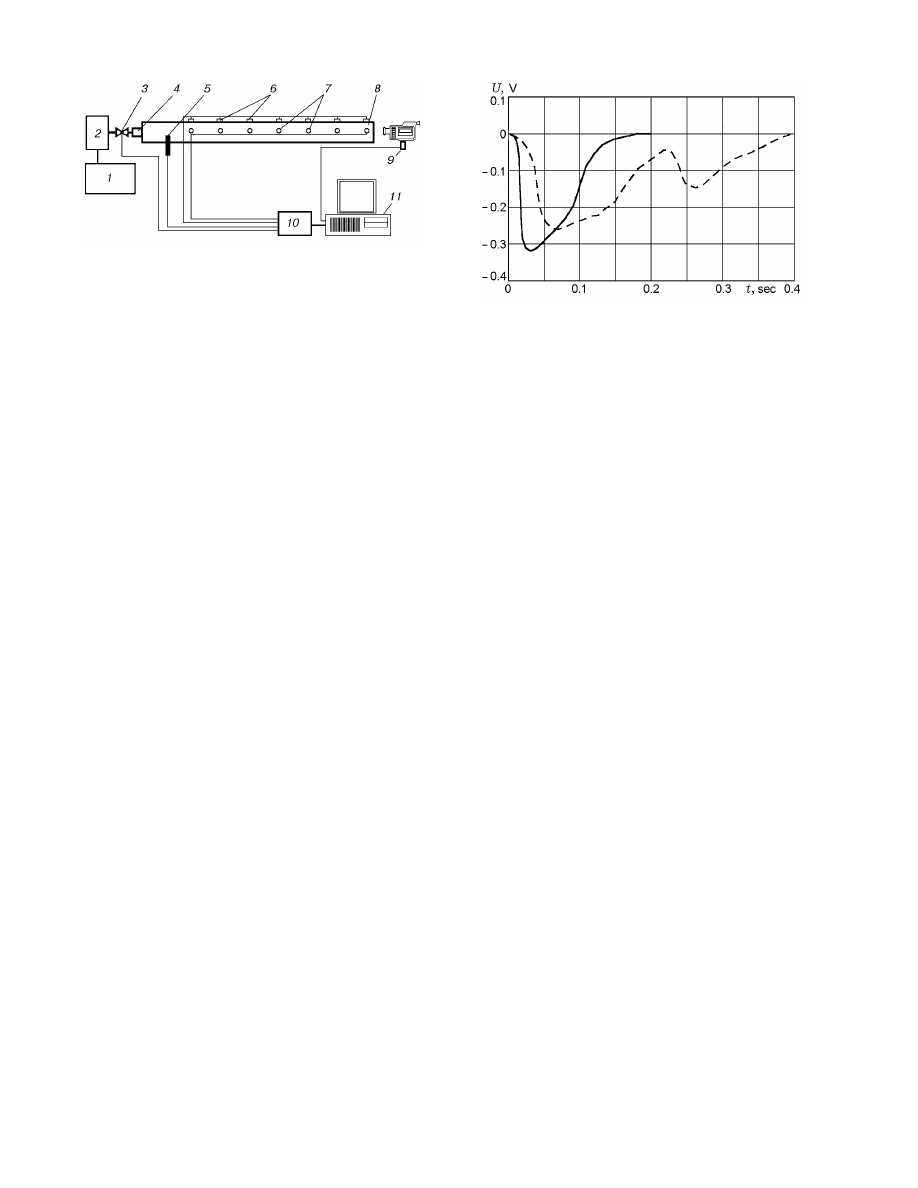
296
Amel’chugov, Bykov, and Tsybenova
Fig. 2. Experimental setup for analysis of explosion
of natural-fuel dust in long channels: 1) compressor;
2) vessel with compressed air; 3) valve; 4) sprayer;
5) igniter; 6) temperature-sensitive elements; 7) pho-
todiodes; 8) explosion channel; 9) video camera;
10) high-speed recorder; 11) computer.
METHOD OF ANALYSIS
OF SPONTANEOUS COMBUSTION
The kinetic parameters of spontaneous combustion
were determined by the calorimetric method. The dry-
air thermostat was the main part of the setup (Fig. 1).
A cylinder made of thin brass mesh served as a reac-
tion vessel. In the tests, Chromel–Alumel thermocou-
ples were used to record the temperature at four or more
points (in the thermostat, near the wall, and at the cen-
ter and boundary of the reaction vessel).
The experiments were performed on coal in the fol-
lowing states: just after mining, after oxidation in air
for 36 h (which corresponds to transportation time),
and after thermal activation. The thermal activation
was performed by heating a sample of brown coal in air
until indications of combustion were observed (intense
smoking and smouldering grains) and subsequent cool-
ing in an inert medium. Experimental results are listed
in Table 1.
It was found that the reactivity and, hence, fire
and explosion hazard of brown-coal dust increase in the
series: oxidized, recently mined, and thermoactivated
coals.
METHOD OF ANALYSIS
OF COAL-DUST EXPLOSION
IN A LONG CHANNEL
The propagation velocity of the combustion front of
suspended dust was studied using an experimental setup
shown schematically in Fig. 2. The explosion channel
was a steel tube 100 mm in diameter and 2000 mm
long. To produce a uniform dust cloud inside the ex-
plosion chamber, a conical spayer with an injector was
used.
The dust was ignited when it passed through
Fig. 3. Dynamics of signals from photodiodes for
explosion of coal dust: the solid curve refers to active
dust and the dashed curve to oxidized dust.
a tube heater. The temperature variation during for-
mation and propagation of the combustion front along
the channel was recorded by Chromel–Alumel thermo-
couples distributed along the channel.
Signals from
the thermocouples and photodiodes were recorded by
a high-speed recorder (the measurement interval was
1–10 msec), displayed on the screen of a digital os-
cilloscope, and recorded on a magnetic medium. The
concentration of suspended dust was determined by the
mass of the dust sample. Moreover, high-speed record-
ing of the dynamics of the brown-coal dust ignition was
performed with variation in different parameters of the
process.
Figure 3 shows the dynamics of coal-dust explosion
according to optical measurements performed for sam-
ples of coal dust (fraction to 80 µm) of different degree
of oxidation. The corresponding experimental data for
dust of different fractions and degree of oxidation are
given in Table 2. An analysis of these data shows that,
during explosion, the brown-coal dust is activated upon
thermal heating and becomes more reactive, whereas
the effective activation energy of spontaneous combus-
tion decreases.
MATHEMATICAL MODELING
We use three simple models that describe qualita-
tively spontaneous combustion of coal dust in a cylindri-
cal reactor under various thermal and physicochemical
assumptions. First of all, this is an ideal-displacement
regime in a tube. In this case, one-dimensional steady
profiles of the temperature and concentration distri-
bution along the reactor were considered. Moreover,
regimes of spontaneous combustion for excess oxygen
were studied with allowance for a considerable change
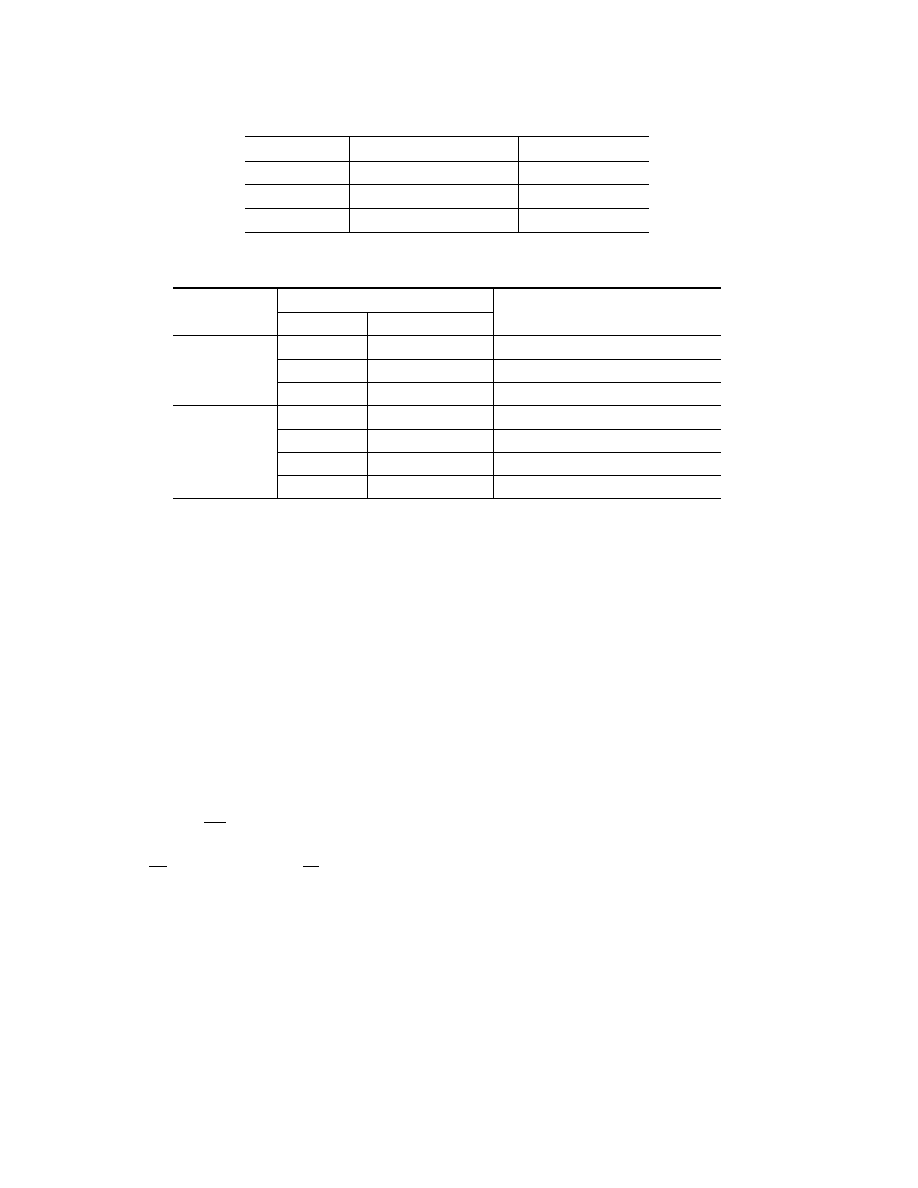
Spontaneous Combustion of Brown-Coal Dust
297
TABLE 1
Kinetic Parameters of Spontaneous Combustion of Brown Coal
of the Irsha–Borodino Deposit
Coal
Activation energy, kJ/mole
Preexponent, sec
−1
Recently mined
40.4
0.61
· 10
8
Oxidized
53.2
0.84
· 10
9
Activated
35.0
0.62
· 10
7
TABLE 2
Experimental Data on Explosion of Brown-Coal Dust
Type of sensor
Characteristics of coal dust
Mean time
Fraction, µm
Degree of oxidation
required to reach the maximum, msec
<80
A
27
Photoelectric
<80
O
60
80–160
A
45
<80
A
390
Thermoelectric
80–160
A
430
80–160
O
460
160–180
A
350
Note. A and O stand for activated and oxidized coal dust, respectively.
in the latter. Particular attention was given to sponta-
neous combustion with allowance for the so-called ther-
mal activation of coal. In the first two cases, a one-
stage process occurred, whereas a three-stage process
was observed in the third case. The aim of modeling
was to describe qualitatively the main specific features
of spontaneous combustion of coal dust and to deter-
mine physicochemical and thermophysical parameters
responsible for spontaneous combustion.
Reaction X
→ P (Coal → Product). In the
steady case, the mathematical model of a tube reactor
with a single exothermic reaction comprises the equa-
tions of material and thermal balance [6, 7]:
u
dX
dl
=
−k(T )X,
(1)
c
p
ρu
dT
dl
= (
−∆H)k(T )X +
4h
d
(T
w
− T ).
Here
k(T ) = k
0
exp(
−E/RT )
(2)
is the reaction-rate constant, k
0
is the preexponent, E is
the activation energy, R is the universal gas constant,
T is the current temperature, X is the current concen-
tration of coal dust, T
w
is the temperature of the reactor
wall, l is the variable length of the reactor, h is the co-
efficient of heat transfer from the wall of the reactor
to its volume, d is the diameter of the reactor, ρ, c
p
,
and u are the density, heat capacity, and feeding rate
of the reactive mixture, respectively, and (
−∆H) is the
thermal effect of the reaction. The length of the reactor
varies within the limits
0 6 l 6 l
f
,
(3)
where l
f
is the total length of the cylindrical reactor.
The conditions at the input of the reactor are written
as
X(0) = X
0
,
T (0) = T
0
,
(4)
where X
0
and T
0
are the concentration and temperature
of the reactive mixture at the input of the reactor. In
model (1), the first-order reaction is considered, and it is
assumed that the process occurs under oxygen excess,
which can be accepted as a first approximation if the
concentration of coal dust is small.
Calculations according to model (1) were per-
formed for the following parameters:
k
0
= 8.4
×
10
7
sec
−1
, E = 53,200 J/mole, R = 8.31 J/(mole
· K),
(
−∆H) = 900 J/mole, T
w
= 283 K, T
0
= 325 K,
X
0
= 5
· 10
−4
g/cm
3
, l
f
= 200 cm, d = 10 cm,
ρ = 5
· 10
−4
g/cm
3
, c
p
= 1.13 J/(g
· K), u = 100 cm/sec,
and h = 0.006 J/(cm
2
· sec· K). Conditions under which
coal dust is ignited spontaneously were determined for
298
Amel’chugov, Bykov, and Tsybenova
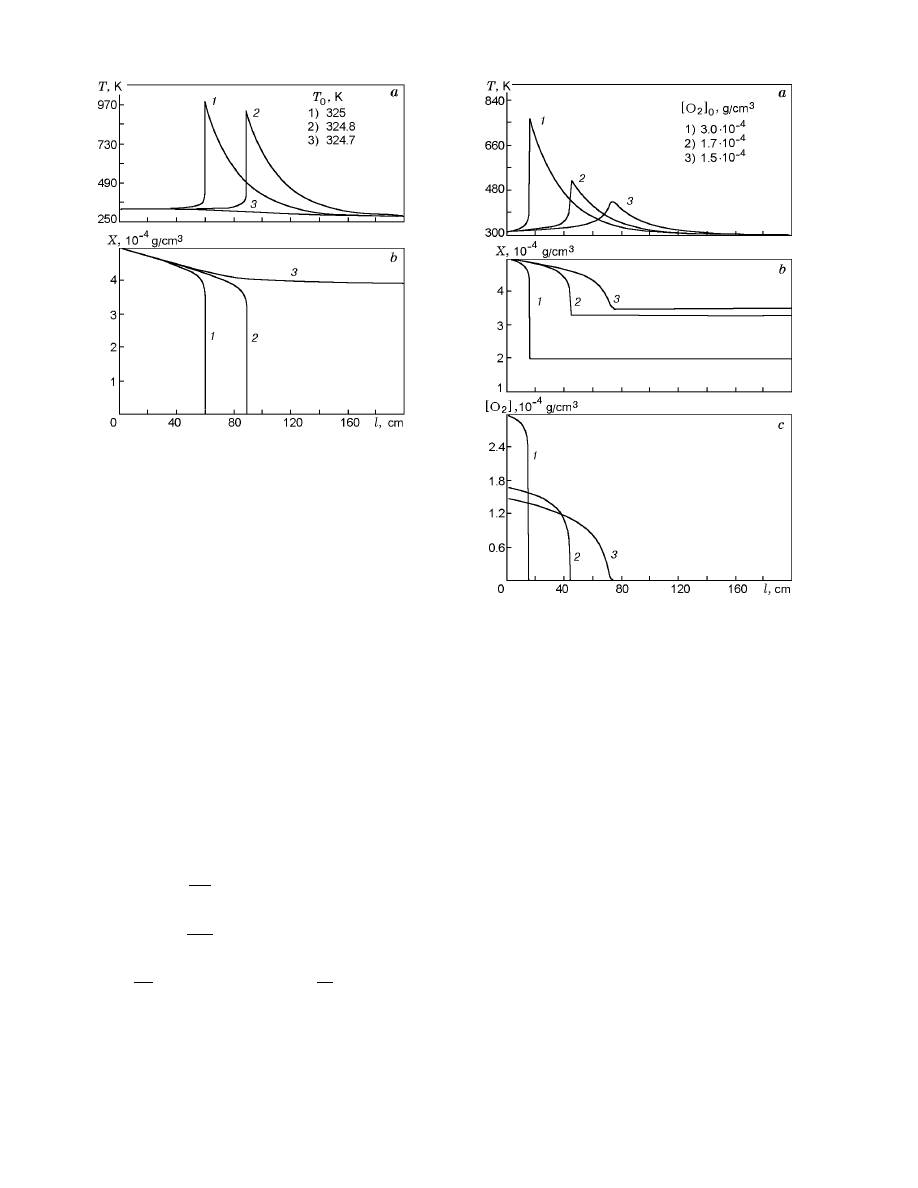
Fig. 4.
Profiles of temperature (a) and concentra-
tion of coal dust (b) in a cylindrical reactor for varied
temperature at the input of the reactor.
varied parameters T
0
, X
0
, h, T
w
, and u. Some calcula-
tion results are shown in Fig. 4.
It was found that the conditions of spontaneous
combustion and location and temperature of the “hot
spot” are highly sensitive to the conditions at the in-
put to the reactor and heat-exchange conditions: for a
certain critical value of the parameter varied, the tem-
perature increases abruptly, which can be characterized
as a thermal explosion of coal dust in the tube. An
example of this steady temperature distribution along
the reactor is shown in Fig. 4, where the input tem-
perature is the varied parameter. A similar effect is
observed when the temperature of the reactor wall is
varied. Thus, model (1)–(4) describes the phenomenon
qualitatively and can be used as the simplest model of
spontaneous combustion of coal dust in the tube.
Reaction O
2
+ X
→ P. To take into account
the variation in the oxidizer concentration, we modify
system (1)–(4):
u
dX
dl
=
−k(T )X · O
2
,
u
dO
2
dl
=
−k(T )X · O
2
,
(5)
c
p
ρu
dT
dl
= (
−∆H)k(T )X · O
2
+
4h
d
(T
w
− T ).
Here the kinetics corresponds to the second-order reac-
tion and O
2
is the concentration of oxygen. The no-
Fig. 5. Profiles of the coal-dust temperature (a) and
concentrations of coal dust (b) and oxygen (c) with
varied concentration of the oxidizer at the input to
the reactor.
tation used in system (5) coincides with that consid-
ered above, and conditions (2)–(4) are the same. Fig-
ure 5 shows the characteristic profiles of coal and oxy-
gen concentrations and temperature for different con-
centrations O
2
at the input of the reactor.
These
profiles were obtained for the following parameters:
k
0
= 0.84
· 10
9
sec
−1
, E = 53,200 J/mole, R =
8.31 J/(mole
· K), (−∆H) = 900 J/mole, T
w
= 300 K,
T
0
= 310 K, X
0
= 5
· 10
−4
g/cm
3
, u = 100 cm/sec, and
h = 0.006 J/(cm
2
· sec · K). Here the preexponent k
0
dif-
fers from that in the above-considered scheme. The rea-
son is that the kinetic parameters correspond to differ-
ent conditions: above, we considered the reaction under
the assumption of constant amount of oxygen, whereas
its variation is taken into account in system (5).
Calculations show that the degree of the coal burn-
out strongly depends on the oxidizer content. For a
sufficient amount of oxygen, the temperature increases
Spontaneous Combustion of Brown-Coal Dust
299
abruptly near the input to the reactor, which can be
interpreted as intense spontaneous combustion of coal
dust. As the concentration O
2
at the input to the re-
actor decreases, the temperature profiles become more
smooth, and the characteristic time of variation in the
concentration of reagents increases. Thus, the concen-
tration of the oxidizer is one of the factors determining
spontaneous combustion of brown-coal dust.
Three-Stage Scheme with Thermal Activa-
tion. A physicochemical analysis shows that coal can
be activated upon heating, i.e., its ability to ignite
spontaneously increases since the activation energy in
(2) decreases. Therefore, in addition to the one-stage
reactions considered above, we study the three-stage
scheme:
Stage 1.
O
2
+ X
1
→ P;
Stage 2.
X
1
→ X
2
;
Stage 3.
O
2
+ X
2
→ P
(6)
(X
1
and X
2
are the initial and activated coals, respec-
tively). Stage 1 corresponds to the above-considered
reaction of coal oxidation, stage 2 to coal activation
(transition from the initial coal X
1
to the activated coal
X
2
), and stage 3 to oxidation of the activated coal. The
activation energy of this reaction is much smaller than
the activation energy in the first stage. Scheme (6) cor-
responds to the following thermokinetic model:
u
dX
1
dl
=
−k
1
(T )O
2
X
1
− k
2
(T )X
1
,
u
dX
2
dl
=
−k
2
(T )X
2
− k
3
(T )O
2
X
2
,
u
dO
2
dl
=
−k
1
(T )O
2
X
1
− k
3
(T )O
2
X
2
,
(7)
c
p
ρu
dT
dl
= (
−∆H
1
)k
1
(T )O
2
X
1
+ (
−∆H
2
)k
2
(T )X
1
+ (
−∆H
3
)k
3
(T )O
2
X
2
+
4h
d
(T
w
− T ).
Here k
i
(T ) (i = 1, 2, and 3) are the constants of
the rates at stages 1, 2, and 3 [see (6)] and (
−∆H
i
)
(i = 1, 2, and 3) are the thermal effects of these re-
actions. Figure 6 shows some profiles of the reagent
concentrations and temperature. In the calculations,
the following kinetic and thermophysical parameters
were used:
k
0
1
= 0.84
· 10
9
sec
−1
, k
0
2
= 1200 sec
−1
,
k
0
3
= 0.62
· 10
9
sec
−1
, E
1
= 53, 200 J/mole, E
2
=
20,000 J/mole, E
3
= 40,000 J/mole, (
−∆H
1
) =
900 J/mole, (
−∆H
2
) = 400 J/mole, (
−∆H
3
) =
1100 J/mole, T
w
= 300 K, and T
0
= 310 K.
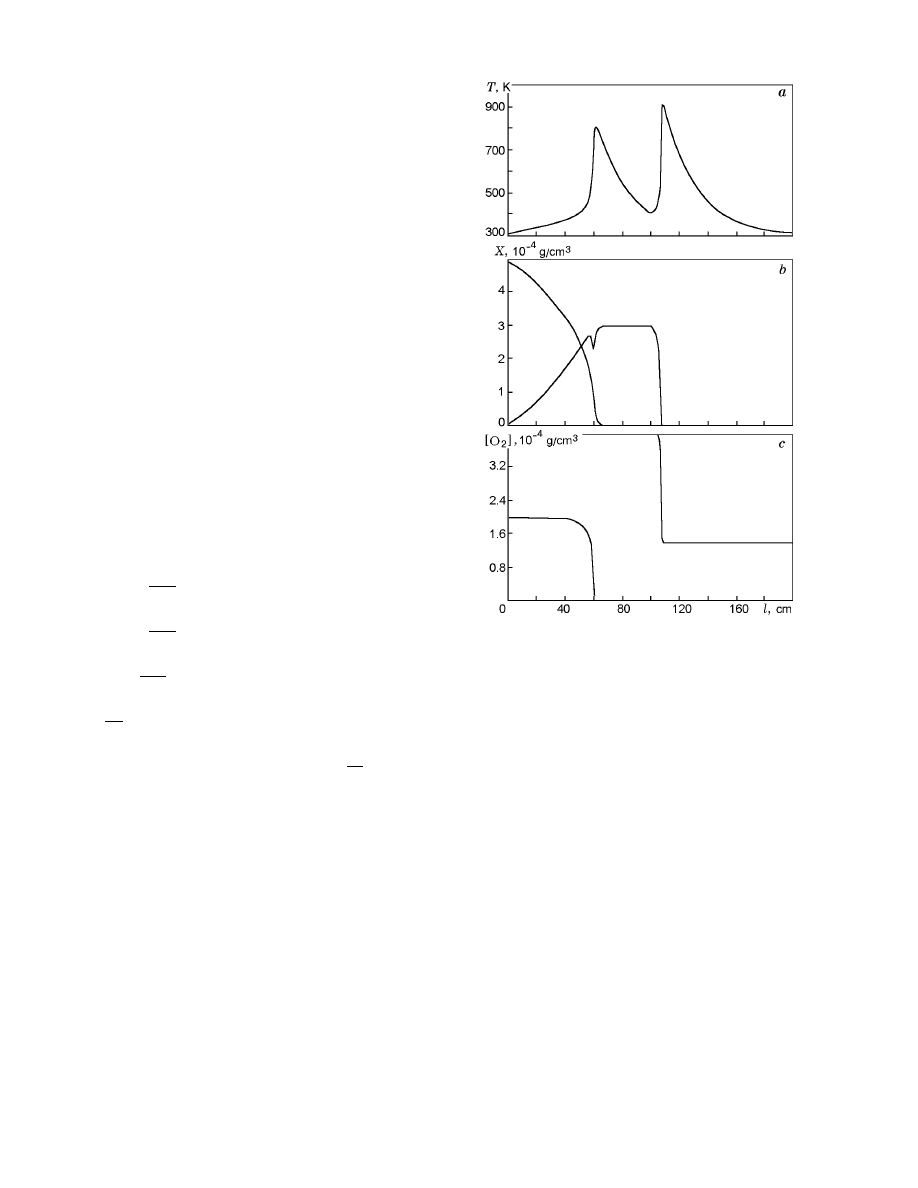
The first peak of temperature in Fig. 6 corresponds
to spontaneous combustion of the initial coal. In this
case, oxygen burns out completely, and the combustion
Fig. 6. Profiles of coal-dust temperature (a) and con-
centrations of coal dust (b) and oxygen (c) for the
three-stage scheme with thermal activation.
of dust is terminated. The concentration of the acti-
vated coal increases in the first interval of combustion
and stabilizes at the middle of the cylindrical reactor.
For l
≈ 60 cm, a small-amplitude jump in the con-
centration of X
2
is observed. This is an indication of
combustion of the activated coal, which is rapidly ter-
minated due to the lack of the oxidizer. A new portion
of the oxidizer is supplied at the middle of the reactor
(l = 100 cm), which leads to a secondary burst of coal
dust. In this case, the activated coal burns.
As in the case of one-stage reactions, the combus-
tion regimes and ignition conditions in model (7) are
highly sensitive to the input conditions and conditions
of heat exchange with the ambient medium. The three-
stage scheme (6) taking into account thermal activation
of coal allows one to describe qualitatively the process of
secondary spontaneous combustion of coal dust within
the framework of the simple model (7).

300
Amel’chugov, Bykov, and Tsybenova
CONCLUSIONS
The calculation results are in qualitative agreement
with the experimental data. It was not the aim of this
work to obtain a quantitative description of the pro-
cesses using the models considered. Therefore, detailed
statistic characteristics of the experiment are not given
in this paper. For calculations, the standard thermo-
physical parameters were taken from [8–10] and the ki-
netic parameters E and k
0
for gas suspension of coal
were borrowed from [2, 5].
Experiments and mathematical modeling of spon-
taneous combustion of brown-coal dust allowed us to
reveal the main factors that determine the critical con-
ditions of combustion. The fire and explosion safety of
coal dust is determined by the kinetic processes of its
spontaneous combustion, ignition, and explosion prop-
agation.
This is of significant practical importance.
Explosions of coal dust mixtures at heat power engi-
neering plants are characterized by secondary explo-
sions (puffs) in the suspended mixture. These explo-
sions also occur when fire extinguishers are used or when
the direction and velocity of air flows change abruptly,
etc.
If, at a certain moment of time, ignition con-
ditions are formed in the presence of a combustible
medium and oxidizer (oxygen contained in air), an ig-
nition source appears. Ignition of this mixture occurs
in the deflagration-combustion regime. Some dangerous
factors of combustion are observed: combustion prod-
ucts are formed, volume temperature increases, and coal
dust is activated.
This implies the following important conclusion.
To prevent the action of dangerous factors of an ex-
plosion on objects, it is necessary to control these pro-
cesses. At present, however, explosion and fire protec-
tion of heat power engineering plants is based on passive
systems (easily removable structures, explosion valves,
filling of window openings, etc.), which are not effec-
tive means of prevention of accidents in coal-feed paths.
This problem can be solved with the help of mathemat-
ical modeling of spontaneous combustion of coal dust.
Detailed calculations of the macrokinetics of these pro-
cesses for parameters varied within a wide range allow
one to develop scientifically justified methods and spec-
ifications of estimating the fire and explosion safety of
coal-feed paths at modern power plants.
REFERENCES
1. Ya. S. Kiselev, V. Ya. Kiselev, and S. P. Amel’chugov,
“Conditions for spontaneous ignition of the Eastern
coals,” Pozharovzryvobezopasnost’, No. 3, 7–21 (1992).
2. S. P. Amel’chugov, “Effect of carbon dioxide on spon-
taneous combustion of brown coals,” Pozharovzryvobe-
zopasnost’, No. 2, 25–27 (1992).
3. D. M. Zakharenko and S. P. Amel’chugov, “Automated
system of explosion suppression and fire protection of
fuel-feed paths at the Abakan thermoelectric plant,” Sib.
Vestn. Pozhar. Bezopasnosti, No. 2, 42–47 (1999).
4. S. P. Amel’chugov, V. I. Bykov, and S. B. Tsybenova,
“Simulation of coal-dust ignition in a tube reactor,”
in: Problems of Usage of the Kansk–Achinsk Coals at
Electric Power Plants (Abstracts of All-Russian Conf.),
Krasnoyarsk (2000), pp. 320–321.
5. S. P. Amel’chugov, “Thermophysical characteristics of
spontaneous combustion of brown coals and methods of
its suppression,” Candidate Dissertation in Tech. Sci.,
Krasnoyarsk (1996).
6. S. B. Tsybenova, “Parametric analysis of the basic mod-
els of the theory of chemical reactors and combustion
theory,” Candidate Dissertation in Tech. Sci., Krasno-
yarsk (1999).
7. V. I. Bykov and S. B. Tsybenova, “Parametric anal-
ysis of some basic models of the combustion theory,”
in: Chemical Physics of Combustion (collected scien-
tific papers dedicated to the 70th Anniversary of Acad.
G. I. Ksandopulo) [in Russian], Almaty (1999), pp. 133–
135.
8. T. V. Vilenskii and D. M. Khzmalyan, Dynamics of
Combustion of Dust-Like Fuel [in Russian], ´
Energiya,
Moscow (1977).
9. V. I. Babii and Yu. F. Kuvaev, Combustion of Coal
Dust and Calculation of a Coal-Dust Plume [in Russian],
´
Energoatomizdat, Moscow (1986).
10. A. A. Shatil’, Furnace Processes and Devices (Analy-
sis and Calculations) [in Russian], Joint-Stock Company
“NPO CKTI,” St. Petersburg (1997).
Wyszukiwarka
Podobne podstrony:
Influence of extraction parameters and medium on efficiency
Computer Modelling with CATT Acoustic Theory and Practice of Diffusion Reflection and Array Modelin
Prowstawanie pzebieg pożaru Process of spontaneous combustion
Historical Dictionary of Medieval Philosophy and Theology (Brown & Flores) (2)
56 793 814 Thermal Fatique of a Tool Steel Experiment and Numerical Simulation
Martin Predicted and experimental results of acoustic parameters in the new Symphony Hall in Pamplo
Insensitive Semantics~ A Defense of Semantic Minimalism and Speech Act Pluralism
Estimation of Dietary Pb and Cd Intake from Pb and Cd in blood and urine
Development of Carbon Nanotubes and Polymer Composites Therefrom
Analysis of soil fertility and its anomalies using an objective model
Modeling of Polymer Processing and Properties
DICTIONARY OF AUSTRALIAN WORDS AND TERMS
A Chymicall treatise of the Ancient and highly illuminated Philosopher
Song of Myself Individuality and Free Verse
Extensive Analysis of Government Spending and?lancing the
Comparison of Human Language and Animal Communication
The?onomic Emergence of China, Japan and Vietnam
Hoban The Lion of Boaz Jachin and Jachin Boaz
więcej podobnych podstron