
Clean in Place – A Review of
Current Technology and its Use
in the Food and Beverage
Industry
Report for general
circulation

Clean in Place – A Review of Current
Technology and its Use in the Food and
Beverage Industry
October 2005
Report for general circulation

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
1
Deakin Project Team
Dr Laurence Palmowski
A/Prof K. (Bas) Baskaran
Dr Heidi Wilson
Mr Brett Watson
October 2005
Project Contact Details
Dr Laurence Palmowski
School of Engineering and Technology
Deakin
University
Geelong,
VIC,
3217
Tel (03) 5227 2443
Fax (03) 5227 2167
Email:
lpalm@deakin.edu.au
Disclaimer
This publication may be of assistance to you, but Deakin University and their
employees do not guarantee that the publication is without flaw of any kind or is
wholly appropriate for your particular purposes, and therefore disclaims all liability for
any error, loss or other consequence which may arise from you relying on any
information in this publication.

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
2
1
EXECUTIVE SUMMARY
The need to recycle water is becoming increasingly important.
One of the main factors limiting the potential for water recycling
is the high level of Total Dissolved Solids (TDS) found in treated
water. Melbourne Water and City West Water, in their salinity
reduction strategy for the Western Treatment Plant, have set a
target of reducing TDS in treated water by 40% by 2009.
Identified options to reduce TDS level in recycled water include
end-of-pipe desalination technologies, segregation of salty
streams at source, and TDS reduction and substitution at source.
Following the waste hierarchy, TDS reduction and substitution at
the source appear to be the best approaches as they avoid costly
desalination technologies and the difficult handling of the
segregated by-products.
The food and beverage industries are among the main
contributors of TDS loads to the sewer. A large source of TDS,
and particularly sodium, in these factories is the cleaning
chemicals used to maintain high hygienic and quality levels in the
factories. Conventional cleaning agents used in the food and
beverage industry are usually based on sodium hydroxide, and/or
require strong acids or bases for neutralization. This results in
high dissolved solids levels, especially sodium levels, being
discharged in effluent streams from factories. Therefore, to reduce
TDS loads discharged to the sewer it is necessary to review
current industrial cleaning practices.
The aim of this project was two-fold. The first aim was to identify
cleaning chemicals that have the potential to replace traditional
chemicals used in the food and beverage industry and that can
reduce TDS in effluent discharged to the sewer. The second aim
was to identify technologies that can be used to collect, treat and
reuse cleaning solutions for subsequent cleaning cycles. This
could lead to significant reduction in cleaning chemical usage.
The tasks of the project were as follows:
Conduct a critical desk-top review of CIP cleaning agents
containing reduced levels of sodium or no sodium.
Undertake a desk-top review of CIP chemical recovery
technologies via the trade and scientific literature.
There is a wide variety of cleaning agents currently available that
could provide an alternative to sodium hydroxide. The alternative
cleaning agents include built cleaning solutions (contain
additives), low sodium alkaline cleaners, potassium hydroxide
(KOH) based products, NaOH/KOH blends, biotechnology based
Intro-
duction
Project
aims
Alterna-
tive
cleaning
chemicals

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
3
cleaners and further alternatives including plant based products.
Alternatives to conventional acid cleaners were also identified.
From this review, it was found that the use of built cleaning
solutions can reduce cleaning times and/or cleaning chemical
concentrations. The use of alkaline cleaners with medium and
low sodium concentrations can lead to reductions in sodium
discharge from CIP in the range of 78-99%. Even further
reductions in sodium levels can be achieved by using KOH based
products which do not contain sodium at all (almost 100%
reduction in sodium discharge from CIP). However, the cost of
KOH based cleaning agents is higher than that of NaOH, which is
currently limiting its wide spread application in processing plants.
Enzyme based cleaners have been shown to be very effective for
cleaning purposes in the food and beverage industries. However,
the application of enzymes is mainly restricted to cleaning
membranes due to their operating temperature. Further
alternatives to alkaline cleaning agents, including plant-based
products were found to be rarely used in large scale applications.
In addition, there is little information available on these
chemicals.
Alternative acid cleaners, which are mainly based on citric acid,
have been shown to be effective for cleaning purposes but they
have yet to become widely used in the food and beverage
industries.
A number of different CIP systems are currently used in the food
and beverage industries and can be categorised as follows: single
use system, reuse system and multi-use system. A number of
benefits and limitations are associated with each type of system.
Reuse systems collect and reuse used CIP solutions for
subsequent CIP cycles. As a result, reuse systems have lower
running costs due to lower chemical requirements. However, they
require trained operator and a centralised CIP infrastructure. Due
to their simplicity, single use systems may be favoured over reuse
and multi-use systems for certain applications. However, there
will be situations where reuse and multi-use systems will be the
better option. A table summarising the advantages and
disadvantages of each system was produced to provide guidance
for selecting the most appropriate technology for a specific
application.
While reuse systems increase the life of CIP cleaning solutions,
leading to cost and environmental benefits, the use of recovery
technologies can further extend the life of CIP solutions. By
removing organic and inorganic contaminants from cleaning
solutions, recovery technologies such as centrifugation or
membrane separation can reduce chemical usage by up to 97%.
Reuse and
recovery
of cleaning
solutions

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
4
Several optimisation methods can be implemented to help
minimise the consumption of cleaning chemicals, thereby
reducing the TDS load of the effluent. Some of these methods are
the review of cleaning frequency, the use of mechanical action
(pigging systems, high pressure sprayers and floor scrubbers) and
CIP monitoring. Increased intervals between cleaning cycles have
been found to have little or no negative impact on product quality
and hygienic requirements in certain applications. Pigging
systems are effective at removing product from pipes prior to
chemical cleaning while high pressure spray and mechanical floor
scrubbers can enhance the removal of biofilms from equipment.
CIP monitoring systems can be used to fine-tune and optimise the
cleaning operations of factories.
Further work is recommended including laboratory evaluation of
alternative cleaning chemicals, followed by factory trials. Pilot-
scale trials of reuse and recovery systems in factories are
suggested. Of high priority is also the training on CIP practices
and optimisation as well as the transfer of technology and
knowledge to industry.
Re-
commen-
dations
CIP
optimisa
tion

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
5
2 TABLE OF CONTENT
1
EXECUTIVE SUMMARY................................................................................ 2
2
TABLE OF CONTENT ................................................................................... 5
3
ACRONYMS ................................................................................................. 7
4
INTRODUCTION ........................................................................................ 10
4.1
C
LEANING AND
CIP......................................................................................... 10
4.1.1
Key factors for cleaning .....................................................................................................10
4.1.2
The benefits of CIP vs. manual cleaning ............................................................................12
4.1.3
Typical CIP cycle................................................................................................................13
4.2
A
IM AND
O
BJECTIVES
..................................................................................... 13
4.2.1
Background.........................................................................................................................13
4.2.2
Aim and Objectives.............................................................................................................14
4.2.3
Scope of the project ............................................................................................................15
5
IDENTIFICATION OF REDUCED SODIUM AND NON-SODIUM CLEANERS..... 15
5.1
I
NTRODUCTION
............................................................................................... 15
5.2
B
UILT
N
A
OH
OR BUILT
KOH............................................................................ 16
5.3
A
LKALINE CLEANERS WITH MEDIUM OR LOW SODIUM CONCENTRATIONS
................... 16
5.4
P
OTASSIUM HYDROXIDE
(KOH)
BASED PRODUCTS
................................................ 17
5.5
S
ODIUM AND POTASSIUM BLENDS
...................................................................... 18
5.6
E
NHANCED CLEANING CHEMICALS
..................................................................... 19
5.7
B
IOTECHNOLOGY CLEANING AGENTS
.................................................................. 20
5.7.1
Enzyme-based cleaners.......................................................................................................20
5.7.2
Bacteria-based cleaners .....................................................................................................26
5.8
A
LTERNATIVES TO ALKALINE CLEANING AGENTS INCLUDING PLANT
-
BASED CLEANERS
.. 27
5.9
A
LTERNATIVE ACID CLEANERS
........................................................................... 29
5.10
A
LTERNATIVE SANITISERS
............................................................................. 29
5.10.1
Alternative chemical sanitisers...........................................................................................30
5.10.2
Non-chemical sanitisers .....................................................................................................31
5.10.3
Combined acid detergent + sanitiser .................................................................................33
5.11
C
OMPARISON OF CLEANING CHEMICALS
........................................................... 33
5.11.1
Comparison on cleaning performance ...............................................................................33
5.11.2
Comparison of cleaning efficiency for membrane cleaning ...............................................34
5.11.3
Comparison of cleaning efficiency for biofilm removal......................................................36
5.11.4
Comparison of cleaning chemicals through life cycle assessment .....................................36
5.12
D
ESK
-
TOP REVIEW OF THE IMPACT OF IMPLEMENTATION OF ALTERNATIVE CHEMICALS
37
5.12.1
Residue risk, OH&S and corrosion issues..........................................................................37
5.12.2
Sodium discharge reduction ...............................................................................................38
6
REVIEW OF CIP RECOVERY TECHNOLOGIES ............................................ 40
6.1
I
NTRODUCTION
............................................................................................... 40
6.2
S
INGLE USE SYSTEMS
...................................................................................... 41
6.3
M
ULTI
-
USE SYSTEMS
....................................................................................... 42
6.3.1
Benefits of multi-use systems ..............................................................................................42
6.3.2
Case studies ........................................................................................................................43
6.4
CIP
R
EUSE SYSTEMS
...................................................................................... 44
6.4.1
General remarks .................................................................................................................44
6.4.2
Straight reuse vs. treatments...............................................................................................45
6.4.3
Reuse after gravity separation............................................................................................45
6.4.4
Reuse following physicochemical treatments .....................................................................48
6.4.5
Reuse following membrane separation...............................................................................49
6.5
R
EVIEW OF POSSIBLE IMPLEMENTATION OF
CIP
RECOVERY TECHNOLOGIES
.............. 57

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
6
6.5.1
Single use vs. reuse systems................................................................................................57
6.5.2
Selection summary of reuse treatment technologies ...........................................................58
7
OPTIMISATION OF CLEANING TOWARDS REDUCED CHEMICAL USAGE ..... 60
7.1
F
REQUENCY OF CLEANING
................................................................................ 61
7.2
M
ECHANICAL ACTION TO SUPPORT CLEANING
....................................................... 62
7.2.1
High pressure spray and mechanical scrubber ..................................................................62
7.2.2
Pigging systems ..................................................................................................................62
7.3
CIP
M
ONITORING
........................................................................................... 63
7.4
C
ASE STUDIES
............................................................................................... 63
8
RECOMMENDATIONS FOR FUTURE WORK ................................................ 64
9
ACKNOWLEDGMENTS ............................................................................... 66
10
REFERENCES......................................................................................... 67
APPENDICES
Appendix A - Summary and classification of alternative chemicals

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
7
3
ACRONYMS
BSA
Bovine Serum Albumin
CAPEX Capital
Expenditure
CIP Cleaning-In-Place
COD
Chemical Oxygen Demand
CSCCO
Combined Simultaneous Caustic Cleaning and Oxidation
CTAB Cetyle-Trimethyl-Ammonium
Bromide
CWW
City West Water
DEH
Department of the Environment and Heritage
EDTA
Ethylene Diamine Tetra Acetic Acid
EO Electrolysed
Oxidizing
EPA
Environment Protection Authority
ETBPP
Environmental Technology Best Practice Program
H
3
PO
4
Phosphoric
acid
HCl Hydrochloric
acid
HNO
3
Nitric
acid
KMS
Koch Membrane Systems
LCA
Life Cycle Assessment
LPS Lactoperoxidase
System
MF Microfiltration
NaOH Sodium
hydroxide
NF Nanofiltration
NFESC
Naval Facilities Engineering Service Center
OH&S
Occupational Health and Safety
PLC
Programmable Logic Controller
PPM
Parts Per Million
PVC
Poly Vinyl chloride
RO Reverse
Osmosis
RWPC
Reconstituted Whey Protein Concentrate
SDS
Sodium Dodecyl Sulphate
SME
Small and Medium Enterprise
SPC
Standard Plate Count
SS Suspended
Solids

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
8
TAZ Terg-A-Zyme
TOC
Total Organic Carbon
TDS
Total Dissolved Solids
TVC
Total Viable Count
UF Ultrafiltration
UK United
Kingdom
UNEP
United Nations Environment Programme
UV Ultraviolet
Glossary
Caustic or caustic soda
Other name for sodium hydroxide
Diafiltration
Water is added during the filtration
process to reduce the concentration of a
component in the retentate or permeate
(Wagner 2001)
Fouling
Product residues, scale and other
unwanted deposits. Word used inter-
changeably with “Soil”
Flux
Flow rate through a membrane divided by
membrane surface area
Membrane
recovery
Defined as the volume of permeate
obtained per total volume of stream
processed
Permeate
Stream passing through a membrane
Recirculation
In most CIP cycles, there is a step where
cleaning solutions are recirculated, i.e.
pumped in closed loop through the
equipment until an acceptable cleaning
level is reached
Recovery
Collection of cleaning solutions followed
by treatment and subsequent use in
following cleaning cycles
Recycling
In this report, this term is limited to the
recycling of water
Retentate
Stream not passing through a membrane

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
9
Reuse
Collection of cleaning solutions and
subsequent use in following cleaning
cycles
Soil
Product residues, scale and other
unwanted deposits (Romney 1990a)
Specific energy
Energy required in a membrane process
per volume of permeate obtained
Volume
retention
ratio
(VRR) Volume of retentate over volume of
solution treated
Symbols
)
Symbol for a case study
Symbol for a scientific research outcome

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
10
4
INTRODUCTION
4.1 Cleaning and CIP
Trägårdh (1989) defined cleaning as “a process where material is
relieved of a substance which is not an integral part of the material.” In
the food and beverage industry, cleaning is an essential procedure in
the operation of a factory to achieve the following objectives (Garrick
and Schiekowski 1980; Dresch et al. 2001):
Maintain the high hygienic levels required;
Remove soil (or fouling) to restore process performance (heat
transfer, pressure drops). Soil is defined as product residues,
scale and other unwanted deposits (Romney 1990a);
Maintain product quality.
4.1.1 Key factors for cleaning
Cleaning is a combination of physical and chemical action, in which the
following aspects play an important role (Australian Standards 2001):
Contact time. The contact time between the chemical and the soil
is important and needs to cover the following phases:
o
Diffusion of the cleaning chemical into the soil layer
o
Swelling of the soil
o
Mass transfer phase from the soil layer into the liquid
o
Transport away from the surface, flush
Temperature
o
Cold: below 30ºC
o
Warm: 30 - 50ºC
o
Hot: 50 - 80ºC
o
Very hot: above 80ºC
Temperature influences diffusion, mass transfer and fluid
characteristics, the various parameters are thus inter-linked.
Turbulence and resulting shear forces acting on deposits
Type of soil (Romney 1990a; Prasad 2004c)
o
Organic soil: mainly of plant or animal origin, depending on
the industry. Organic soil is usually cleaned by alkaline
detergents, amongst which sodium and potassium
hydroxide are the most common.
o
Inorganic soil: mainly of mineral origin. It is mostly cleaned
by acidic detergents, including inorganic acids (e.g.

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
11
phosphoric, nitric and hydrochloric acids) and to a smaller
extent organic acids (e.g. hydroxyacetic and citric acid)
o
Combined organic/inorganic soil, which is the most
common type
o
Biofilms, which develop on equipment if soils are not
removed frequently enough. Biofilms can lead to hygiene
issues as well as adverse technological effects (Kumar and
Anand 1998)
Concentration and type of cleaning chemical
A wide variety of detergents are used in the food and beverage industry.
They can be classified according to their functions and applications
(Australian Standards 2001). Brief descriptions of the different
detergents commonly used in the food and beverage industry are given
below. The following section has been taken directly from Australian
Standards (2001).
Multi-purpose detergents – Multi-purpose detergents are intended
primarily for use in manual, pressure or foam cleaning of all types of
surfaces, in all areas.
Heavy-duty alkaline detergents – Heavy-duty alkaline detergents are
intended for the removal of proteins, fats and other strongly adherent
organic soils from surfaces.
Enzyme-assisted detergents – Enzyme-assisted detergents are detergent
formulations which contain enzymes, which are intended to break down
and solubilize otherwise difficult-to-remove food soils using relatively
mild detergents and cleaning conditions.
Acidic detergents – Acidic detergents are used to remove mineral soils
and other soils resistant to neutral or alkaline detergents.
Oil-lift detergents – Oil-lift detergents are detergents, typically containing
water soluble solvents and surfactants, intended for the removal of
accumulated grease and oil from walls and floors.
Smokehouse detergents – Smokehouse detergents are designed
primarily for the removal of fats and tar from walls, floors and
equipment in smokehouses.
It is common practice to add additives to pure cleaning chemicals such
as NaOH to improve specific attributes of the chemicals. The attributes
that a detergent should ideally have are described in the following
section, which has been taken directly from Romney (1990a).
Dispersing and suspending power – to bring insoluble soils into
suspension and prevent their redeposition on cleaned surfaces.

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
12
Emulsifying power – to hold oils within the cleaning solution.
Sequestering power – the ability to combine with calcium and
magnesium salts to form water-soluble compounds and to aid
detergency.
Wetting power – to reduce surface tension and thus aid soil penetration.
Rinsing power – the ability to rinse away clearly and completely without
leaving any trace of soil or the detergent chemical on the cleaned
surface.
In membrane cleaning for example, surfactants perform a wide range of
roles: they help to wet surfaces, facilitate soil removal, suspend
materials, stabilize foam, adsorb on surfaces to amend properties of the
surface and act as biocide (D'Souza and Mawson 2005).
4.1.2 The benefits of CIP vs. manual cleaning
Over the last few decades, the use of Cleaning-In-Place (CIP) systems
has brought more reliability in equipment cleaning. CIP is defined as
“the cleaning of complete items of plant or pipeline circuits without
dismantling or opening of the equipment and with little or no manual
involvement on the part of the operator. The process involves jetting or
spraying of surfaces or circulation of cleaning solutions through the
plant under conditions of increased turbulence and flow velocity” (NDA
Chemical Safety Code, 1985).
The use of CIP shows numerous advantages compared to manual
cleaning, including improved cleaning efficiency, shorter cleaning
cycles, improved Occupational Health and Safety (OH&S) and reduced
environmental impact (DEH 2003).
)
As an example, Cascade Brewery applied, extended and automated
the reticulation of cleaning solution throughout their brewery and
beverage plants. As a result, a 60% reduction in cleaning agents was
achieved in the brewery, while the reduction reached up to 80% in the
cider section of the beverage plant (DEH 2003).
)
The introduction of CIP systems in a Small and Medium Enterprise
(SME) can also show economic and environmental benefits. At Food
Spectrum, which produces ingredients for the food manufacturing
industry, it is estimated that 20% of cleaning water can be reused by
introducing a $50,000 CIP system, with a pay-back period of 3 years
(Prasad et al. 2004). It was also reported that the CIP system has the
potential to increase water reuse to 50%, leading to increased water
savings and reduced payback period (EPA 2003).

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
13
4.1.3 Typical CIP cycle
A typical CIP cycle is presented in the sequence below (Romney 1990a;
Australian Standards 2001). It is important to note that this cycle will
differ from one site to another and from one process to another at the
same site.
1. Product flush to remove product residuals. This is often carried
out using water but is not a necessity
2. Pre-rinse to remove any loosely-adherent residuals (and micro-
organisms attached to these residuals). This is usually performed
with water (or slightly alkaline solution) and reduces the amount
of soil, which the main cleaning step has to remove.
3. Main cleaning step to lift the soil from the equipment surface. The
soiling compounds will be suspended or dissolved in the cleaning
solution. This step, which is responsible for removing most of the
soil and micro-organisms attached to surfaces, can be sub-
divided into sub-steps to allow for various cleaning chemicals to
be used. For example:
a. Caustic cleaning, followed by
b. Intermediate rinse, and
c. Acid cleaning step (when required)
4. Final rinse to remove residuals of cleaning solutions
5. Disinfection/sanitising step to reduce the number of micro-
organisms from previously cleaned surfaces
6. Post-rinse might be necessary to remove residuals of sanitisers
Each food and beverage industry type has different CIP requirements.
Furthermore, each area of a food and beverage factory can have
different CIP requirements. For example, the CIP requirements differ in
open systems (e.g. vessels) and in closed systems (e.g. pipes). The CIP
performance in the former is easier to assess visually.
4.2 Aim and Objectives
4.2.1 Background
The need to recycle water in industry is becoming increasingly
important. There is also a growing need to reduce sewer loadings to
achieve a higher quality of trade waste discharges and of treated water.
Total Dissolved Solids (TDS) levels in treated water have been identified
as a key factor limiting water recycling due to their significant impact
on soil productivity (DSE 2004). In the Western Melbourne metropolitan

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
14
region, almost half of the TDS in treated water is produced by industry
and commerce.
The government has shown its commitment to work with industry and
water authorities to improve industrial water management. Urban water
authorities are currently working with industrial and commercial
customers and the Environment Protection Authority (EPA) to develop
cleaner production programs and to reduce TDS discharges. In
particular, Melbourne Water and City West Water in their salinity
reduction strategy for the Western Treatment Plant have set a target of
reducing the TDS content of recycled water by 40% by 2009 (DSE,
2004).
Identified options to reduce TDS content in recycled water include end-
of-pipe desalination technologies, segregation of salty streams at source,
and salt reduction and substitution at source. Following the waste
hierarchy, salt reduction and substitution at the source appear to be
the best approaches as they avoid costly desalination technologies and
the difficult handling of the segregated by-products.
The food and beverage industry, which represents 22% of the total
Victorian manufacturing turnover (ABS 2005), is a significant
contributor to trade waste and TDS discharges. It is estimated that
approximately 50% of the sodium found in trade waste from some of the
food and beverage industries originates from CIP practices. The reason
for this is that conventional cleaning agents used in CIP systems are
usually based on sodium hydroxide, and/or require strong acids or
bases for neutralization. This results in high dissolved solids levels,
especially sodium levels, being discharged from factories in trade waste.
4.2.2 Aim and Objectives
The aim of this project was two-fold. The first objective was to identify
CIP chemicals that have the potential to replace traditional CIP
chemicals used in the food and beverage industry to reduce TDS in
trade waste. The second aim is to identify the technologies that can be
used to collect, treat and reuse cleaning chemicals for subsequent
cleaning cycles.
The tasks of the project were as follows:
Conduct a critical desk-top review of CIP cleaning agents
containing reduced levels of sodium or no sodium. To conduct
this review, published literature, available case studies, and
chemical suppliers have been consulted.
Undertake a desk-top review of CIP chemical recovery
technologies via the trade and scientific literature. Technology
suppliers have also been contacted for additional information.

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
15
4.2.3 Scope of the project
The main focus of this report is on factories in the food and beverage
sector, which are major contributors of TDS within the CWW boundary.
Some information related to the utilisation of alternative chemicals and
technologies has also been found from other industry sectors and has
been included in the report.
All assessments have been made based on published literature,
available case studies and information provided by suppliers of
alternative chemicals and/or technologies. No experimental work was
undertaken at this stage of the project.
5
IDENTIFICATION OF REDUCED SODIUM AND NON-
SODIUM CLEANERS
5.1 Introduction
One of the main purposes of this project was to identify alternative CIP
chemicals and processes to those currently used in the food and
beverage industry with the intention of reducing TDS in effluent
discharged to the sewer. Sodium hydroxide or caustic soda (NaOH) is
the most widely used alkaline detergent in the food and beverage
industry, due to its low price and high cleaning efficiency for fatty-type
and protein soils. The most commonly used acidic detergents are nitric
acid and phosphoric acid. These conventional cleaning chemicals
contribute significantly to the TDS and sodium levels discharged by
food and beverage industries. As a result of high TDS and sodium
concentrations, the recycling of treated water is restricted to avoid any
damage on soils and vegetation. Therefore, there is a clear need to
identify alternative chemicals to reduce the use of traditional chemicals
throughout the food and beverage industry.
The range of alternative cleaning chemicals can be classified as follows:
Built NaOH or built KOH. These chemicals contain additives
which enhance the performance of the sodium and/or potassium
hydroxide. As a result, lower salt/sodium concentrations can be
used.
Low sodium alkaline cleaners
Potassium hydroxide (KOH) based products
NaOH/KOH blends
Biotechnology based cleaners, mainly consisting of enzyme-based
cleaners
Alternatives to alkaline cleaning agents, including plant-based
cleaners

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
16
Alternative acid cleaners
Alternative sanitisers, including non-chemical based sanitisers
All these options, which offer a possible reduction in TDS and/or
sodium in trade waste, are discussed in more detail below. Available
case studies and literature references have been included. A complete
list of all alternative chemicals can be found in Appendix A. It should be
noted that some of the chemicals listed are currently not available in
Australia and would need to be introduced if interest was shown.
Following this presentation of the alternative chemicals, a desk-top
assessment of the possible reduction in sodium discharged to trade
waste, as a result of the change over from traditional cleaning
chemicals, is presented.
5.2 Built NaOH or built KOH
As discussed in the introduction to this report (section 4.1.1), additives
(or builders) are often added to cleaning solutions to improve their
properties and cleaning efficiency. Cleaning solutions containing
additives are called “built” cleaning solutions. The use of built cleaning
solutions can reduce cleaning times, rinse water consumption and/or
cleaning chemical concentrations. This can therefore lead to improved
trade waste discharges.
Typical additives include:
Dispersing and suspending agents
Emulsifiers and surfactants
Sequestrants
Wetting agents
Rinsing agents
As an example, sequestrants are widely used to remove hardness from
water. Prasad (2004c) reported that “hard water can result in scale
build-up, which affects the capacity of detergents and sanitisers to
contact the surface and can lead to excessive scaling in boilers and
cooling towers.” Therefore, hard water may need some treatment such
as ion exchange or the use of detergents and sanitisers containing
specially formulated additives (Prasad 2004c).
5.3 Alkaline cleaners with medium or low sodium
concentrations
While the sodium concentration in chemical cleaners can reach 52%
(pure or bulk caustic – see Appendix A for examples of these
chemicals)), chemical manufacturers have developed products with
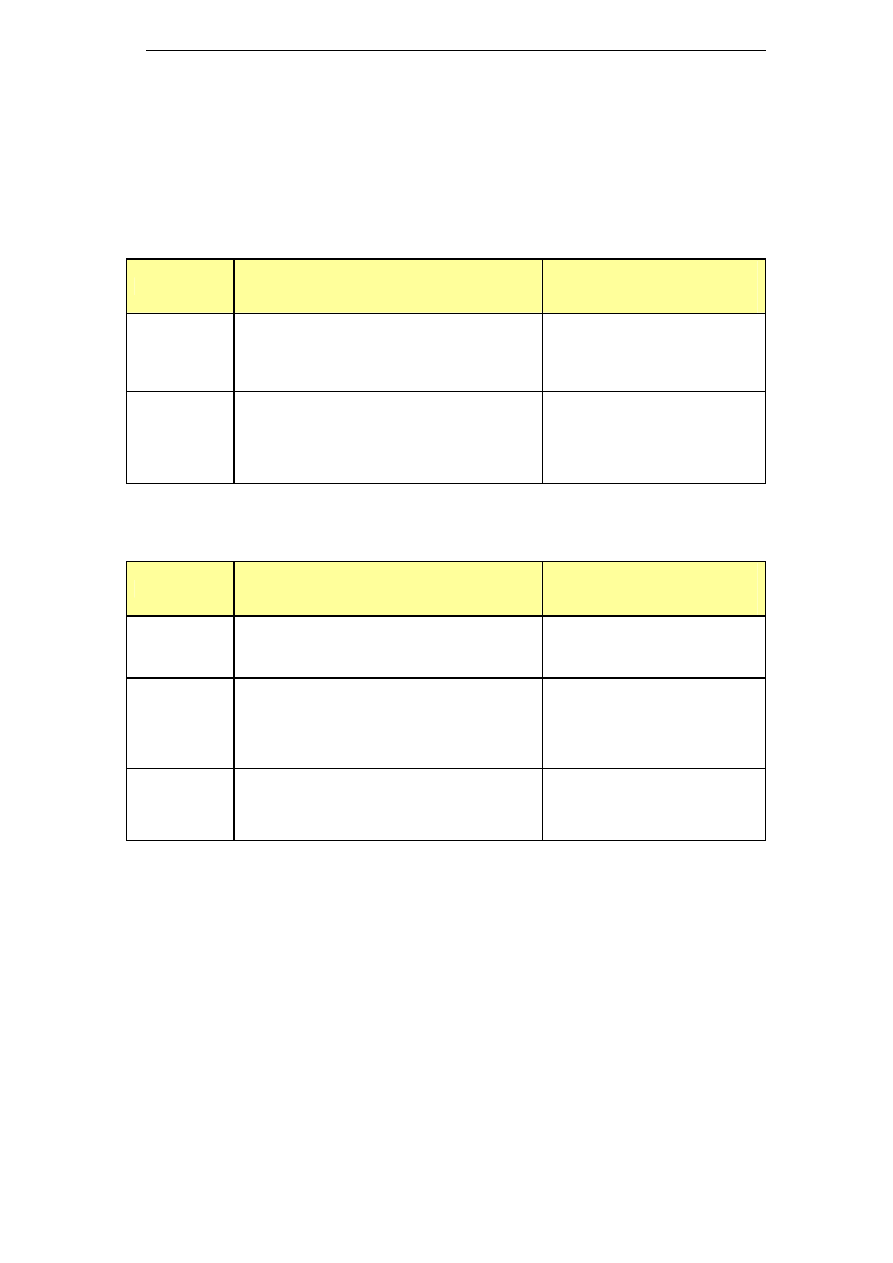
lower sodium concentrations. Table 1 presents alkaline cleaners with
medium sodium concentrations, while Table 2 shows alkaline cleaners
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
17
with low sodium levels. The sodium concentration corresponds to the
sodium concentration in the cleaning solution, after dilution. This has
been calculated using the sodium content of the chemical and its range
of recommended concentration. More details about these chemicals and
their applications can be found in Appendix A.
Table 1: Alkaline cleaners with medium sodium concentration
Name
Composition
Na level in ready-to-use
cleaning solution
[gNa/kg cleaning solution]
Chlorozolv
20% w/v as sodium hydroxide
Active chlorine
Stable chelating and dispersing agents
Na content ≥ 11.5%
1.7 – 2.9
Suma Ilam
L1.8
Sodium Hydroxide < 30%
Sodium Hypochlorite < 4% available
chlorine
Na: 12.6% w/w
Scale inhibitors
0.63 – 1.89
Table 2: Alkaline cleaners with low sodium concentration
Name
Composition
Na level in ready-to-use
cleaning solution
[gNa/kg cleaning solution]
Diverwash
VC24
Na: 1.8%w/w
Wetting agents, buffering agents,
sequestrants & dispersants
0.02 – 0.38
Flowsan
Sodium hydroxide 5-15%
Sodium hypochlorite 5-15%
Chlorine-based bleaching agents 5-15%
Polycarboxylates <5%
Na: 3.1% w/w
0.12 – 0.56
Glide
Alkaline Salts <20%
Sodium Hypochlorite solution <3%
Sodium Hydroxide <2%
Na: 5%w/w
0.4 – 0.8
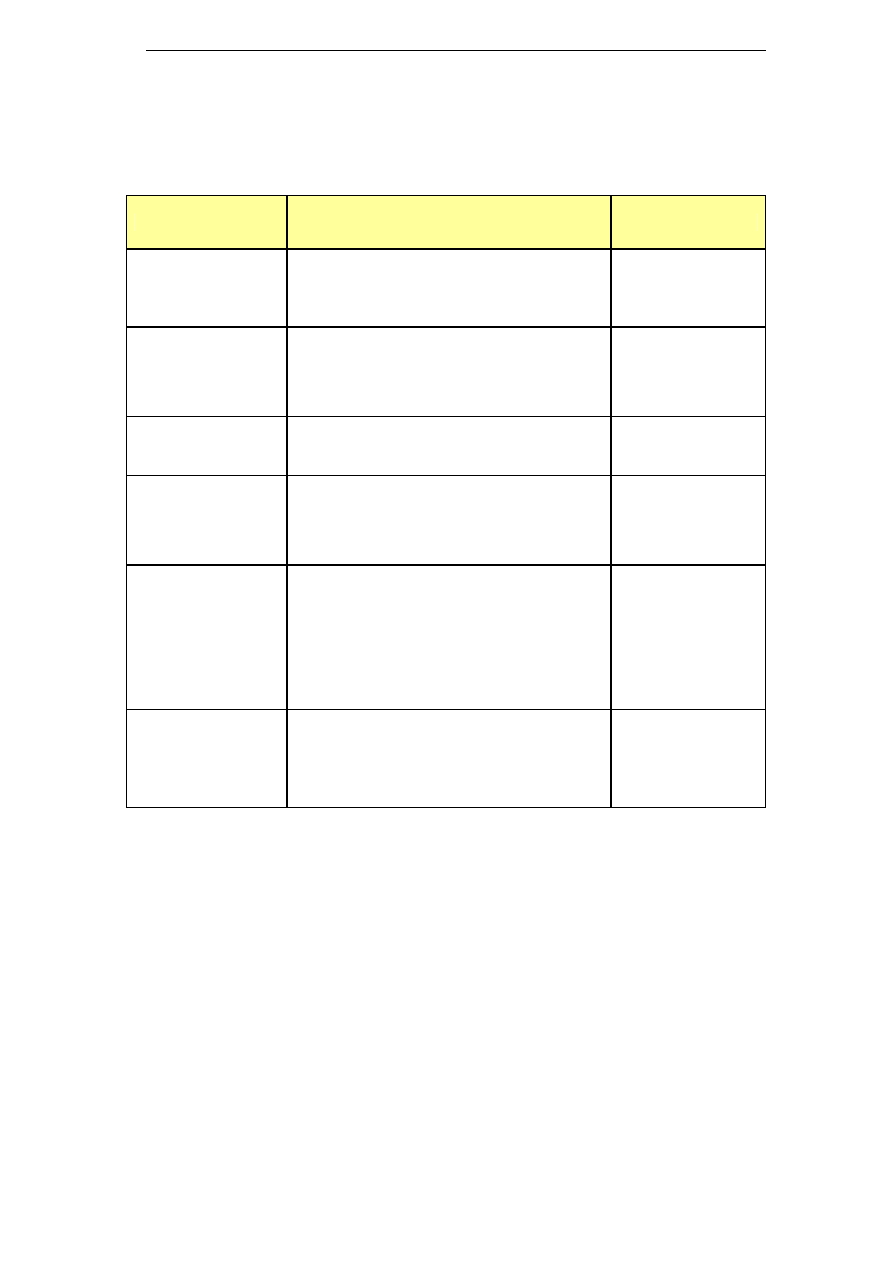
5.4 Potassium hydroxide (KOH) based products
The use of potassium hydroxide based cleaning agents is one of the
approaches to reduce the sodium levels found in trade waste. However,
the main limitation to use potassium hydroxide has been its price.
Similarly to sodium hydroxide, potassium hydroxide is prepared by
electrolysis of a brine solution. In the case of KOH, the brine solution
consists of potassium chloride, which is not as ubiquitous as sodium
chloride and needs to be extracted from mined resources. As a result,
KOH is more expensive than NaOH. Additionally, different market
drivers exist for sodium and potassium hydroxide, leading to different
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
18
price fluctuations (Lech 2005). A list of potassium hydroxide based
chemicals is presented in Table 3.
Table 3: Potassium based cleaning chemicals
Name
Composition
Industry sector
DairyChem (or
Alka-San
Potassium)
Potassium hydroxide >50%
Surfactants <10%
Chlorinated Agent <10%
Sequestrant <20%
DairyChem HT 108
(or Dairy Alkali-
Potassium
Hydroxide
Solution)
Potassium Hydroxide: 50%
Sequestrants <5%
Surfactants <5%
Dairy industry
Divos 100
No sodium
Caustic potash (Potassium hydroxide)
Chelating agents & surfactants
Divos 110
No sodium
Potassium Hydroxide <5%
Dairy industry
Beverage
applications
Pharmaceutical
applications
Solo
Potassium hydroxide 15-30%
Tetrapotassium
ethylenediaminetetraactetate 15-30%
Diethylenetriaminepentaacetic acid
(pentasodium salt) <5%
EDTA 5-15%
Anionic surfactants, phosphonates, non-
ionic surfactants, phosphates <5%
Food industry
Beverage industry
Vegetable
processing
Superquest
Potassium hydroxide ≥ 30%
Tetrasodium ethylenediaminetetraacetate
5-15%
EDTA 5-15%
Phosphonates <5%
Dairy industry
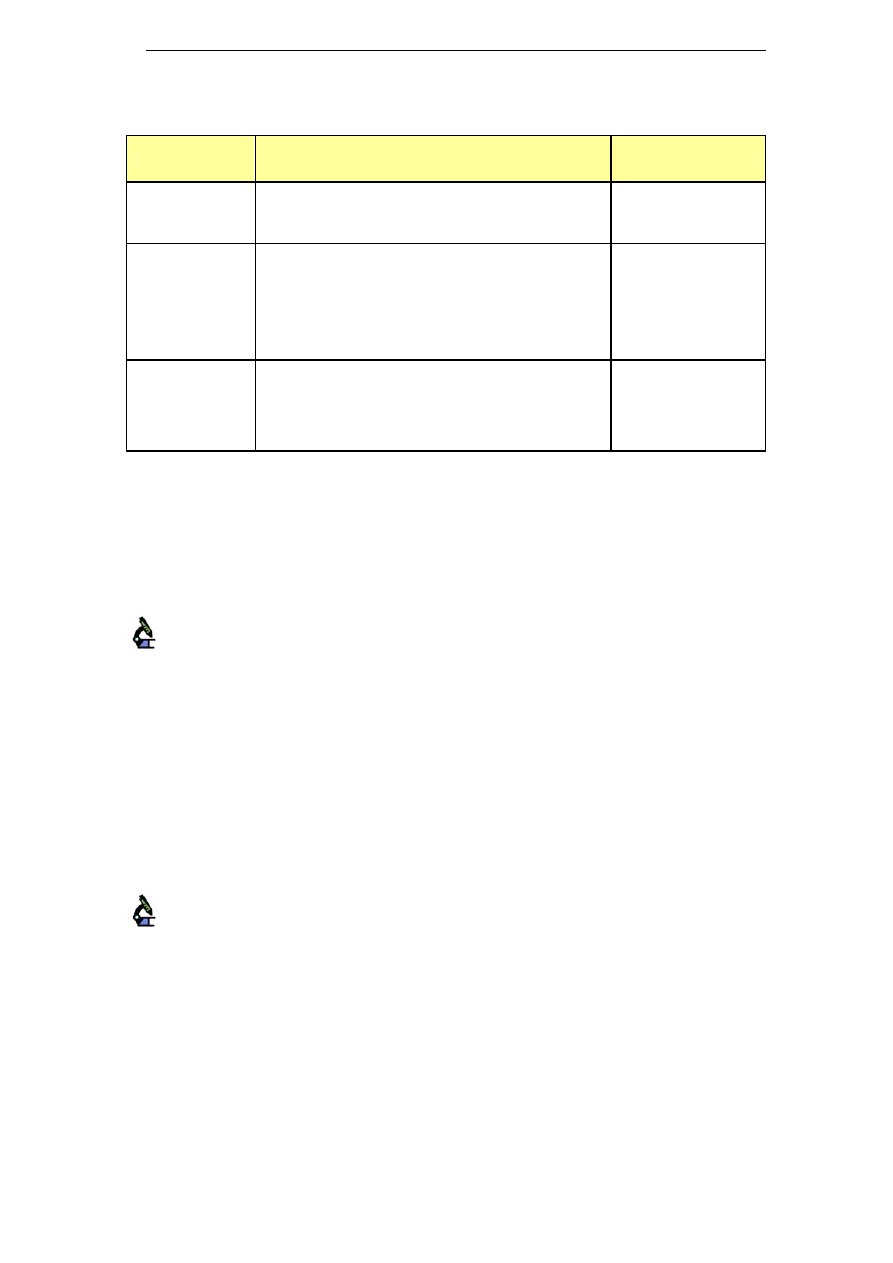
5.5 Sodium and potassium blends
To increase the price competitiveness of potassium hydroxide while
partially maintaining his environmental benefits over pure sodium
hydroxide, blends of potassium and sodium are available on the market
from various suppliers. Table 4 presents some of these products, which
are not a pure blend of NaOH and KOH but also incorporate some
alternative products. As a result, their sodium content is relatively low.
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
19
Table 4: Potassium and sodium blends
Name
Composition
Industry sector
Detojet
Potassium hydroxide (7-13%)
Sodium silicate (5-10%)
Sodium hypochlorite (1.5%)
Food industry
Profile
Potassium hydroxide <5%
Sodium hypochlorite <5%
Sodium hydroxide <5%
Phosphates, chlorine-based bleaching agents
<5%
Na content: 5.2% w/w
Meat processing
industry
Redes
Disodium/dipotassium metasilicate <5%
Sodium hypochlorite <5%
Phosphates 15-30%
Chlorine-based bleaching agents <5%
Na content: 4.4% w/w
Food and beverage
industries
5.6 Enhanced cleaning chemicals
While additives can be added to cleaning solutions to improve their
performance, the combined used of oxidation agents and cleaning
chemicals has also been investigated.
The effectiveness of alkali cleaning combined with ozone pre-
treatment was investigated for removing protein from equipment
surfaces (Takehara et al. 2000). The authors used bovine serum
albumin (BSA) as the model protein and particles of alumina (Al
2
O
3
),
which is widely used as a ceramic membrane material. The Al
2
O
3
particles were fouled with the BSA and then pre-treated using 0.3%
(v/v) gaseous ozone. Takehara et al. (2000) found that the pre-treatment
of the BSA-fouled Al
2
O
3
particles markedly accelerated the removal of
the BSA during alkali cleaning through partial decomposition of the
BSA molecule. The authors concluded that ozone pre-treatment has the
potential to reduce NaOH concentrations required for adequate alkali
cleaning by at least one order of magnitude.
Gan et al. (1999) also developed and tested a combined
simultaneous caustic cleaning and oxidation (CSCCO) process in a
single stage cleaning operation. The cleaning solution used in the
CSCCO process was comprised of NaOH and H
2
O
2
at optimised levels of
concentration. It was demonstrated that the CSCCO process had a
greater cleaning power than the single-step caustic cleaning and the
successive two-step process. In relation to this result, the authors
stated that “the synergy achieved between caustic cleaning and
oxidation has suggested that the combined chemicals provide a fast and
effective cleaning process” (Gan et al. 1999).

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
20
5.7 Biotechnology cleaning agents
Biotechnology based cleaning agents include bacteria-based agents and
enzyme-based agents, the latter being far more widely used in
industries. The main advantages and disadvantages of biotechnology
based cleaning agents include (ETBPP 1998):
Advantages
Usually less harmful to the environment
Very specific cleaning action
Can be used at lower temperatures than conventional chemical
May be cheaper
Reduce effluent disposal costs as they produce an effluent with a
lower COD
Non-corrosive
Disadvantages
May take longer to act than traditional chemical cleaners
)
ETBPP (1998) reported that a poultry processing company had
extreme difficulty cleaning an area that was soiled with faeces, blood,
urine, grease, fat and feathers, even with sodium hydroxide. They
applied a biotechnology cleaning agent and found that all traces of
organic mater were removed efficiently. There was a reduction in
cleaning time as well as energy consumption because hot water was not
required.
5.7.1 Enzyme-based cleaners
Enzyme-based cleaners in the food industry are becoming increasingly
popular. There has been a resurgence of interest in enzymes because
they offer a number of advantages over traditional caustic or acid
cleaning regimes (D'Souza and Mawson 2005). One of the main factors
responsible for the growing popularity of enzyme-based cleaners is new
developments in enzymology (Kumar et al. 1998). Enzymes used for
detergent production comprised 28% of the global market for industrial
enzymes in 1994 (Kumar et al. 1998). A non-exhaustive list of enzyme
based cleaning agents available on the market is presented in Table 5,
while more details on these products can be found in Appendix A.
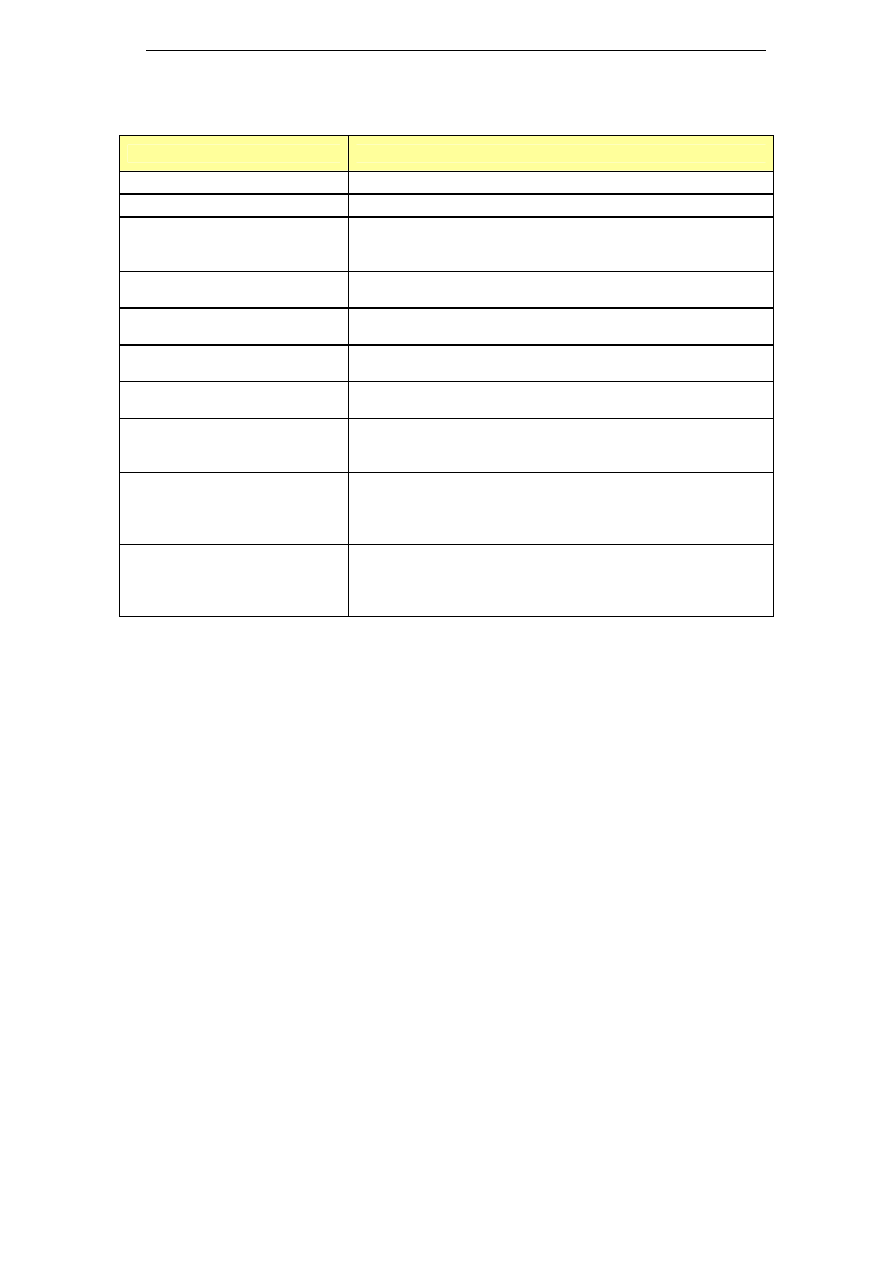
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
21
Table 5: Enzyme based cleaning agents
Name
Composition
Cipzyme P
Contains proteolytic enzymes
Divos 80-4
Enzyme cleaner
Paradigm
Protease cleaner
0.8% P2010
0.9% P2030
Properase 1600L
Protease enzyme (liquid)
Subtilisin (1-5%)
PURADAX EG 7000L
Fungal cellulase enzyme (liquid)
Cellulase (1-5%)
Purafect 4000E
Protease enzyme (granulated)
Subtilisin (1-5%)
Purastar ST 15000L
Bacterial alpha-amylase enzyme (liquid)
Amylase (1-5%)
Reflux E 2001
Enzyme cleaner
NH compounds >60%
Subtilsin (CAS 9014-01-1) <10%
Terg-a-zyme
Protease enzyme
Sodium dodecylbenzenesulfonate (10-30%)
Sodium carbonate (7-13%)
Sodium phosphate (30-40%)
Zymex Enzymatic Cleaner
Enzymatic cleaning solution concentrate
Aqueous mixture of enzymes and surfactants
Isopropyl Alcohol (<10%)
Triethanolamine (<10%)
A number of studies have been carried out in laboratories around the
world comparing the cleaning abilities of enzyme-based cleaners against
the cleaning abilities of conventional cleaning agents. However, most
applications of enzyme-based cleaners in industry have mainly been
reserved for the cleaning of membranes. This is due to the expense of
purchasing large quantities of enzymes and formulating them into
effective detergents (Trägårdh 1989). Therefore, a significant proportion
of the following section is dedicated to the utilisation of enzyme-based
cleaners for cleaning membranes.
5.7.1.1 Introduction to membrane cleaning
Trägårdh (1989) provided a comprehensive review of the state-of-the-art
of membrane cleaning up till 1989. A number of important factors
related to membrane fouling reduction and membrane cleaning were
reviewed and discussed including flow conditions, pre-treatment,
membrane properties, water quality, cleaning agents, and cleaning
performance. D’Souza and Mawson (2005) presented a further
comprehensive review of membrane cleaning in the dairy industry. They
reviewed the key mechanisms governing cleaning performance.

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
22
The characteristics of effective membrane cleaners can be summarised
as follows (Trägårdh 1989; D'Souza and Mawson 2005):
ability to loosen and dissolve the fouling material, and keep the
dislodged foulants in dispersion or solution to prevent the
refouling of already cleaned surfaces
optimal active compound concentration, keeping the soil in
dispersion and/or solution to avoid new fouling
good solubility and rinsing characteristics
low or moderate foam level
good compatibility with the membrane
good buffering capacity and stability with time
ability to promote disinfection of the wet surfaces
Trägårdh (1989) listed and briefly discussed the main cleaning agents
and additives used to clean membrane plants. They are:
alkalis - hydroxides, carbonates and phosphates
acids - nitric and phosphoric
enzymes
surface-active agents - anionic, cationic and non-ionic
sequestering agents - ethylene diamine tetra acetic acid (EDTA)
formulated cleaning agents
combined cleaning and disinfecting agents
disinfectants - H
2
O
2
, metabisulphite, hypochlorite and heat
treatment
Trägårdh (1989) also reported that “the choice of cleaning agents and
cleaning conditions depends not only on the type of components
deposited, but also on the chemical and thermal resistance of the
membrane, the module and the rest of the equipment.”
Enzymatic cleaners are usually employed if the pH limitation of the
membrane is at or below 10, or if a high level of soil is present. Enzymes
offer a number of advantages over traditional caustic or acid cleaning
regimes (D'Souza and Mawson 2005):
enzymes are biodegradable and cause fewer pollution problems
enzymes are less aggressive to the membranes and can therefore
lengthen the lifespan of the membrane
rinsing volumes are reduced which in turn lower wastewater
volumes
enzymatic agents can improve cleaning efficiency

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
23
enzymes can reduce energy costs and the amount of chemical
needed by working at a lower temperature
Leaver et al. (1995) conducted a study to test the effect that cleaning
agents had on four coupling and four seal types. The cleaning solutions
that the test pieces (coupling and seal) came into contact with were
NaOH (1M) and Terg-A-Zyme (TAZ). Each test piece was filled with the
cleaning agents and left for 24 hours at room temperature before being
rinsed with tap water left to dry (Leaver et al. 1995). Pressure hold tests
were then conducted to determine leak diameters. It was found that the
couplings did not release liquid at the test conditions and that changes
in leak diameters were relatively small. Leaver et al. (1995) reported that
the largest increase in leak diameter was 9 µm when exposed to NaOH.
It was acknowledged that the testing offered only a limited challenge to
the seals and that further work was required to supplement these initial
results (Leaver et al. 1995).
5.7.1.2 Case studies
)
Several milk processing plants have adopted alternative cleaning
chemicals for CIP systems. Murray Goulburn used cold surface cleaners
(enzymes in conjunction with mild detergents) to reduce caustic-based
cleaners (Prasad, 2004c).
)
Dairy Farmers replaced phosphoric acid with nitric acid after it was
found that equipment was not being cleaned properly. This initiative
resulted in a superior clean and reduced phosphate load in the water
used for irrigation.
)
Kumar et al. (1998) reported that the use of alkaline proteases from
Bacillus sp. strain MK5-6 have proved successful in laboratory scale
tests. They also conducted a pilot scale evaluation of the same enzyme
preparation for UF membrane cleaning. It was found that the enzyme
preparation resulted in 100% of the flux being restored whereas TAZ
only achieved an 80% restoration of the flux.
Allie et al (2003) used lipases and proteases to clean flat-sheet
polysulphone membranes fouled in abattoir effluent. The motivation for
this study was to demonstrate that enzymatic cleaning regimes are
effective at removing foulants adsorbed onto these membranes and also
increasing flux recovery. The lipases used were Candida cylindracea,
Pseudomonas mendocina and Aspergillus oryzea. The proteases used
were Bacillus licheniformis, Protease A (protein engineered protease) and
Aspergillus oryzea. When the Candida, Aspergillus and Pseudomonas
lipases were used alone in the cleaning mixtures, the lipid content on
the membranes were reduced by 33, 46 and 55% respectively. The
highest lipid removal was obtained with the Pseudomonas lipase, while
the lowest percentage lipid removal was obtained with the Candida
lipase. A significantly greater lipid removal was observed after the
membranes were cleaned with the lipases in conjunction with the
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
24
proteases than when the lipases were used alone. The Pseudomonas
lipase was found to reduce lipids by 70% when used in conjunction
with proteases. Allie et al. (2003) stated that these results indicate
enzyme-based cleaning regimes are useful for membranes operating on
abattoir effluents (Allie et al. 2003).
Maartens et al. (1996) tested the ability of a number of enzymes for
cleaning polysulphone membranes fouled with abattoir effluent. The
purpose of the study was to determine whether the different enzyme
and enzyme/detergent mixtures could restore pure-water flux when
used to treat the fouled membranes. The enzymes evaluated were
protease A, lipase A, Alkazyme, Zymex, sodium dodecyl sulphate (SDS)
and Triton X100. Maartens et al. (1996) compared the ability of each
cleaning agent to remove adsorbed protein and lipid material from the
membranes. Increasing the concentration of protease A, lipase A and a
mixture of lipase A and Triton X100 beyond 3 mg/mL did not lead to
further decreases in protein removal. In fact, no significant increase in
protein removal was observed for concentrations beyond 1mg/mL.
However, for the removal of lipid material, the optimal concentration
was found to be 3 mg/mL for each enzyme.
In terms of incubation time, maximum protein removal was achieved
after 60 min for lipase A while protease A and the lipase A:Triton X100
mixture required an incubation time of 90 min to achieve maximum
protein removal. Lipase A required an incubation time of 90 min to
effectively remove lipids, whereas protease A and the lipase A:Triton
X100 mixture only required an incubation time of 60 min to achieve
maximum lipid removal. Maartens et al. (1996) concluded that enzymes
can be used effectively as cleaning agents in biological effluent streams.
However, they stipulated that the effluent and fouling agents must be
well characterised and identified to ensure that the correct enzymes or
enzyme/detergent mixtures are selected.
In another study, the performance of two proteolytic enzymes was
evaluated for cleaning inorganic membranes fouled by whey protein
solutions (Argüello et al. 2003). The two cleaning agents, Maxatase
®
XL
and P3-Ultrasil
®
62, adopted for this study were enzymatic
formulations. Tami
®
150 + 4T membranes were employed. Argüello et
al. (2003) reported that very high efficiencies (~100%) were achieved in
short operating times (20 min). It was also found that higher amounts of
enzyme resulted in a slight decrease in cleaning efficiency. The authors
also stated “the optimum values of the operating conditions tested were
related to the best conditions to hydrolyze whey proteins in a
discontinuous reactor using the same enzyme preparations.”
Also investigated in the study was the potential to reuse the enzyme
solutions for consecutive cleaning steps. It was shown that the
enzymatic solutions could be reused used for consecutive steps.
However, it was observed that there was a 30% loss in enzymatic

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
25
activity during each cleaning cycle, regardless of the initial activity of
the solution (Argüello et al., 2003).
In a later study, the ability of P3-Ultrasil
®
62 was tested for cleaning
a Carboseo
®
M1 membrane (Argüello et al. 2005). As with the earlier
study (Argüello et al., 2003), very high cleaning efficiencies (~100%)
were reached in short operating times (20 min). It was reported that the
cleaning efficiency depended on the operating conditions. A decrease in
the pH of the cleaning solution during the cleaning process was
attributed to protein hydrolysis. However, Argüello et al. (2005) reported
that chemical cleanliness was not achieved because residual matter was
detected on the membrane surface after cleaning. This phenomenon
was observed even when the hydraulic cleaning efficiency was 100%.
Muñoz-Aguado et al. (1996) investigated the effects of enzyme and
surfactants on a totally retentive polysulfone membrane fouled with
bovine serum albumin (BSA) and a reconstituted whey protein
concentrate (RWPC). The cleaning agents employed were CTAB (cetyl-
trimethyl-ammonium bromide), TAZ and
α
-CT (
α
-chymotryspin). It was
found that the cationic surfactant, CTAB, was more effective when the
pH of the fouled membranes was 7 than when the pH of the fouled
membranes was only 5. The authors reported that the cleaning
efficiency of CTAB increased with temperature and surfactant
concentration. They also investigated the impact that the cleaning time
had on cleaning efficiency. The optimum cleaning time for CTAB was
found to be 1 hour. A concentration of approximately 0.01 wt%
achieved the maximum flux recovery and resistance removal for
α
-CT.
Increasing the concentration actually led to a decrease in cleaning
efficiency. They also showed that cleaning the fouled membranes with
α
-CT before CTAB resulted in an improvement of the cleaning efficiency.
The use of a water rinse was shown to be an effective method of
removing loose foulant pieces at little additional cost. However, this can
only be carried out at the same temperature as the chemical cleaning,
otherwise the fouling layer will be compacted. Muñoz-Aguado et al.
(1996) conclude that the main disadvantage of the multi-step cleaning
process is the time taken to carry out the cleaning, while a major
advantage is that the milder cleaning conditions result in lower cleaning
costs and a longer membrane lifespan (Muñoz-Aguado et al. 1996).
Sakiyama et al. (1998) compared the performance of various
proteases for the removal of proteinaceous deposits from stainless steel
surfaces. The protease solutions were fed into a packed column of
stainless steel particles fouled with
β
-lactoglobulin and gelatin. The
proteases used in the study were crystalline trypsin, crystalline
thermolysin, several crude powder protease preparations (Protin AC10,
Protin PC10, Thermoase PC10 and Tunicase FN), and several
thermostable alkaline proteases (B21-2, B18’ and KuAP). Sakiyama et
al. (1998) found that the cleaning kinetics depended greatly on the kind

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
26
of protease used as well as on the type of protein to be removed. They
also found that regardless of the protease used, the cleaning kinetics
increased with protease concentration and became constant above a
certain protease concentration. It was also shown that a small amount
of
β
-lactoglobulin was left on the stainless steel surface after 120
minutes of enzymatic cleaning, irrespective of the protease used. The
results of this study indicate that the choice of an enzyme to remove
fouling deposits is critical for establishing an efficient enzymatic
cleaning procedure (Sakiyama et al. 1998).
Flint et al. (1999) conducted a pilot-scale trial to evaluate the
effectiveness of Paradigm in removing biofilms of thermo-resistant S.
thermophilus from a pasteuriser. Following cleaning with acid and
caustic cleaners the reduction in the total number of bacteria was less
than 10-fold. However, when Paradigm was used the total number of
cells was reduced by approximately 100-fold. Flint et al. (1999)
concluded that the use of a proteolytic enzyme-based cleaning system
may be more effective than acid or alkali cleaning in removing biofilms
of thermo-resistant streptococci from the surface of commercial
manufacturing plants.
)
An ice-cream manufacturing plant in Asia uses enzymes to remove
milk protein from cold milk surfaces (UNEP, 2004). “A secondary
component of the cleaning product removes fats and minerals, resulting
in a single-phase clean and allowing the acid phase of the cleaning to be
eliminated” (UNEP, 2004). An acid sanitiser is used after the enzymatic
clean.
It is evident from the literature that a considerable amount of research
has been carried out to evaluate the effectiveness of enzyme-based
cleaners for cleaning membranes. However, little work has been done to
date on determining the applicability of enzyme-based cleaners for
cleaning larger pieces of equipment in factories, particularly pipes and
tanks. This is primarily due to the expense of the enzymes. Therefore,
most of the research has been confined to laboratory scale experiments
which are of little value at the plant scale. In terms of being used for the
cleaning of membranes, enzymes have been shown to perform as
effectively as traditional cleaning agents. Given the results reported in
the literature, it would be expected that the utilisation of enzyme-based
cleaning agents for cleaning membranes will increase significantly in
the future.
5.7.2 Bacteria-based cleaners
Several case studies of companies adopting biotechnology cleaning
systems to replace more conventional cleaning methods have been
reported in the literature. It is important to point out that the
companies are not within the food industry.

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
27
)
Wyko EMS, an electrical engineering company that specialise in
refurbishing electrical motors and components, utilise a biological
cleaning agent that contains bacteria that digest the oil and grease left
on electrical components. The alternative product was found to be just
as effective as the solvent-based cleaner that was previously used but
has environmental and financial benefits as well (BIO-WISE 2002).
)
BIO-WISE (2002) also reported that a company specialising in heat
treatment and electron beam welding has managed to save almost
£3,000 per year since it began using a biological system to remove
manufacturing oils from parts instead of an alkaline solution.
)
An electroplating company also installed a biological cleaning system
to replace the utilisation of an alkaline solution needed to clean metal
parts prior to electroplating (BIO-WISE 2001a). This measure helped the
company reduce the cost of purchasing chemicals, saved time and
labour and eliminated production downtime.
)
Glacier Vandervell, a company that manufactures bearings and
bushes for the motor industry, used a biological cleaning system to
clean and remove oils from bushes instead of a highly caustic detergent
solution. It was reported that the biological cleaning system had
considerably lower annual running costs than the original cleaning
process (BIO-WISE 2001b). Two important aspects are also mentioned.
Firstly, it was found that a sludge containing a mixture of particulates
and dead bacterial cells settled to the bottom of the control unit.
However, only a small volume (3 litres) needed to be removed from the
system every three to four weeks. Secondly, the bacteria in the cleaning
solution were found to attack natural rubber seals. This problem was
solved simply by using PVC or silicone rubber seals instead (BIO-WISE
2001b).
5.8 Alternatives to alkaline cleaning agents including
plant-based cleaners
Alternative cleaning agents, such as plant-based cleaners, are used in
some circumstances as replacements for traditional alkaline cleaners.
Although some information was obtained from suppliers or through a
comprehensive internet search, it was often incomplete. In addition,
there were very few references in the scientific literature on alternatives
to alkaline cleaning agents and only a handful of cases studies could be
found. Table 6 presents examples of these alternative cleaners, while
further details about these products can be found in Appendix A. The
categories of products include:
plant-based products, which can be of various origin:
o
tall oil fatty acids, which are derived from pine pulp
production
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
28
o
citrus based products, containing concentrated d-limonene
chemical origin, including ethylene and glycol derivatives
products of unknown composition or origin
Table 6: Examples of alternative cleaning chemicals as alkali replacement
Name
Composition
Industry
sector
Colloidal
Concentrate
Non-ionic surfactant, as alcohols C12-
16 ethoxylated 5%
Tall oil fatty acids 0.5%
Organic butter, as sodium
iminodisuccinate 0-1%
Supersolve
Tall oil fatty acids
Heavy Duty
Surfactants < 5%
Tall oil fatty acids <5%
Succinimide <5%
Dairy farms
Food
preparation
Plant-based
products
Citra-Solv
Concentrated d-limonen based product
80-95 wt% limonene fraction terpenes
1-10 wt% ethoxylated alcohols C9-C11
1-10 wt% coconut diethanolamide
Manufacturing
Food Process
Cleaner
Ethylene Glycol Monobutyl Ether (%wt)
< 15%
Canneries
Dairies
Bakeries,
Seafood
processing
Bottling plants
Red meat
processing
Poultry
processing
Breweries
EASY-CLEAN
Rig Wash
Alkyl aryl sulfonates & builders
Nonhazardous blend (100% wt)
Meat and
poultry
Chemical
compounds
4171 TRITON
X-100
Diethylene ether,1,4-dioxane
Ethylene oxide
Polyethylene glycol
Triton X-100
Actisolve
Not available
Unknown
origin
Concept C20
Not available
Dairy plants
The SGS U.S. Testing Company performed a 28-day
biodegradability test on Citra-Solv
®
Cleaner and Degreaser to determine
the biodegradability of this cleaning agent in a closed aqueous system.
Citra-Solv
®
is a concentrated d-limonene based product derived from
the extract of orange peels. The results of the study showed that Citra-
Solv
®
degraded 75.6% as determined by Total Organic Carbon (TOC)
reduction and 209% by CO
2
evolution within 28 days (NFESC 1999).
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
29
5.9 Alternative acid cleaners
Acids are used principally to dissolve precipitates of inorganic salts or
oxide films. Conventional acid cleaners contain nitric and/or
phosphoric acids, which can lead to nutrient problems in effluent
discharges.
Alternative acid cleaners are mainly based on citric acid, as presented
in Table 7. In membrane applications, citric acid is favoured over nitric
acid because of its mildness. It also rinses easily and does not corrode
surfaces (D'Souza and Mawson 2005).
)
In an interesting case study, Bonlac Foods replaced the nitric and
phosphoric acid normally used in their CIP process with Stabilon®
(DEH, 2005a,b). Prior to the changeover, 200 litres of nitric and
phosphoric acid were used every day for CIP processes in the cheese
manufacturing plant. DEH (2005b) reported that the use of Stabilon®
decreased the CIP wash time by 1.5 hours per day. Consequently, this
enabled the plant to increase production time by 9 hours per week. The
net savings to the factory was $312 per day. Although phosphorus and
nitric acid levels were reduced by using Stabilon®, the total wastewater
volume actually increased. This was because more production took
place each day. When the volume of wastewater was related to the
amount of cheese produced, it was found that utilising Stabilon®
resulted in the roughly the same volume of effluent discharged per
tonne of cheese produced as from using nitric and phosphoric acid.
Table 7: Examples of alternative cleaning chemicals – acid replacement
Name
Composition
Industry sector
Citrajet
Citric acid (10-30%)
Phosphorus compounds < 1%
Organic Carbon (%w/w) – 14%
Blend of organic acids and
surfactants Dairy
industry
Citranox
Citric acid (10-30%)
Blend of organic acids, anionic and
non-ionic surfactants and
alkanolamines.
Organic Carbon (%w/w) – 17%
Phosphate free.
Food industry
Enviroscale
Citric acid, anhydrous <2%
Lactic acid <2%
Surfactant <1%
5.10
Alternative sanitisers
Many detergents have been found to have a disinfecting ability.
However, the stand-alone application of a sanitizer (see sections 5.10.1
and 5.10.2) or the application of combined acid + sanitisers (see section

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
30
5.10.3) is common practice to ensure adequate reduction in microbial
numbers.
Typical sanitisers are based on chlorine, sodium hypochlorite, hydrogen
peroxide and quaternary ammonium compounds. Details about several
different alternative sanitisers are presented below.
5.10.1 Alternative chemical sanitisers
A wide range of chemical sanitisers are used within the food industry.
(ADHS 2005) listed a number of criteria that the ideal chemical sanitiser
should meet for application in the food industry. The criteria that the
ideal chemical sanitiser should meet are as follows (taken directly from
ADHS, 2005):
be approved for food contact surface application
have a wide range or scope of activity
destroy micro-organisms rapidly
be stable under all types of conditions
be tolerant of a broad range of environment conditions
be readily solubilised and possess some detergency
be low in toxicity and corrosivity
be inexpensive
It is impossible for any single sanitiser to meet all of these criteria.
Therefore, it is important that the properties, advantages and
disadvantages of a sanitiser are evaluated being used for a specific
application (ADHS, 2005).
Dufour et al (2004) developed a laboratory scale system to quantify
the effectiveness of chlorine and alternative sanitizers in reducing the
number of viable bacteria attached to stainless steel surfaces. The
experimental system, which consisted of a continuous flow reactor and
recirculating test loop, was used to model the development of dairy
biofilms on stainless steel surfaces under conditions of growth and
cleaning regimes typically encountered in dairy processing plants.
Stainless steel tubes were placed in the recirculating loop and exposed
to a standard CIP regime. The tubes were then exposed to chlorine (200
ppm) and combinations of nisin (a natural antimicrobial agent, 500
ppm), lauricidin (a natural microbial product, 100 ppm), and the
lactoperoxidase system (LPS) (enzyme-based, 200 ppm) for different
lengths of time (10 min or 2, 4, 8, 18 or 24 h) (Dufour et al. 2004).
It was found that increasing the concentration of the chemicals did not
always lead to a greater reduction in the number of attached cells. Log
reductions varied between 0 and 2.1. Dufour et al. (2004) also
investigated the effectiveness of chlorine, nisin + LPS, and lauricidin +
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
31
LPS against biofilms following the standard CIP regime. They reported
that none of the sanitizers significantly reduced the number of attached
cells after a 10-min treatment. However, after 2h of exposure, all three
treatments significantly reduced bacterial counts on the stainless steel
tubes. Exposure times greater than 2h did not achieve further
significant microbial reductions.
Langsrud et al. (2000) carried out a study to determine the effects
that peroxygen have on Bacillus cereus spores. They also investigated
whether alkali treatment sensitised spores to the effect of peroxygen.
The cleaning agents employed in this study were sodium hydroxide
(NaOH), nitric acid (HNO
3
), Paradigm enzyme 10/30. The two peroxygen
based sanitisers used were Parades and Oxonia aktiv (Langsrud et al.
2000).
The sporicidal effect of 1% Oxonia aktiv was generally poor at 20 and
30°C, even when exposed for 30 min. However, when the temperature
was increased to 40°C the reduction in viable spores was significantly
larger. Pre-treatment of spores with 1% NaOH at 60°C made the spores
susceptible to even low concentrations of Oxonia aktiv. It was shown
that pre-exposure of the spores to 0.25 and 0.5% NaOH was not as
effective as 1% NaOH. Langsrud et al. (2000) investigated the influence
of cleaning temperature on the potentiating effect of alkali. They found
that alkali treatment alone only reduced spores significantly at 80°C,
whereas alkali treatment followed by exposure to Oxonia resulted in
significant spore reductions at 40°C. It was also shown that pre-
exposure to Paradigm potentiated the effect of Parades. The results of
the study indicated that peroxygen-based disinfectants have a good
effect at lower concentrations and temperatures if the pores are exposed
first to alkali or an enzyme based cleaner (Langsrud et al. 2000).
The use of ozone in CIP processes has been tested in the form of
ozonated water. Lagrange et al. (2004) carried out a study to determine
the antimicrobial efficiency of ozonated water applied in a CIP system
on the surfaces of food processing plants. Under optimal conditions
ozonated water showed excellent microbicidal and fungicidal
characteristics within seconds. However, these characteristics were
extinguished in the presence of protein soil. It was concluded that a
suitable use of ozonated water for sanitation was only possible after
efficient cleaning (Lagrange et al. 2004).
5.10.2 Non-chemical sanitisers
A number of non-chemical sanitisers have been reported in the
literature including thermal sanitising, steam and hot water (ADHS
2005). UNEP (2004) report that two alternatives to using sanitation
chemicals are ionisation and ultraviolet light. Ionisation involves the
use of an electrode cell to release silver and copper ions into a stream of
water. The positively charged silver and copper ions are attracted to the
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
32
negatively charged surface of the micro-organisms, distorting the cell
structure and preventing the absorption of nutrients (UNEP, 2004).
Ultraviolet (UV) disinfection systems destroy micro-organisms through
interaction with microbial DNA (UNEP, 2004).
)
A carrot processing plant in Australia is trialling a new ionisation
system to sanitise 80 000 tonnes of fresh carrots using 200 000 L of
sanitised water per day (UNEP, 2004). Although the trials are only in
the preliminary stages, it is expected that ionisation will be just as
effective as chlorination. This is based on overseas experience.
)
A cheese processing plant in South Africa required a non-chemical
brine disinfection system that would not alter the quality of the cheese
and would also be simple and easy to maintain (UNEP, 2004). The
company installed an UV disinfection system. The operating costs for
the UV system were reported to be far lower than the operating costs of
pasteurisation.
)
A food processing plant in the UK has installed a medium-pressure
UV disinfection system to treat water originating from a private borehole
(UNEP, 2004). The water is treated using an iron and manganese filter
before being passed through a membrane filter. The final stage of the
treatment process is to pass the water through the UV system.
Approximately 95% of the UV-treated water is used for washing and
treating equipment while the remaining 5% is used in product make-up.
The products from the plant are not affected in any way by using this
source of water.
A number of physical methods have also been tested for the control of
biofilms including (Kumar and Anand 1998):
super-high magnetic fields
ultrasound treatment
high pulsed electrical fields on their own and in combination with
organic acids
low electrical fields both on their own and as enhancers of
biocides
low electrical currents in combination with antibiotics
The utilisation of the last two methods for controlling biofilms appears
to be very promising. Several studies reported in the literature have
successfully employed low electrical currents to control biofilms (Davies
et al. 1991; Costerton et al. 1994; Jass et al. 1995; Jass and Lappin-
Scott 1996; Kumar and Anand 1998).
1
Walker et al (2005) conducted a study to determine whether
electrolysed oxidizing (EO) water could be used as an acceptable
1
Cited in Kumar and Anand (1998).
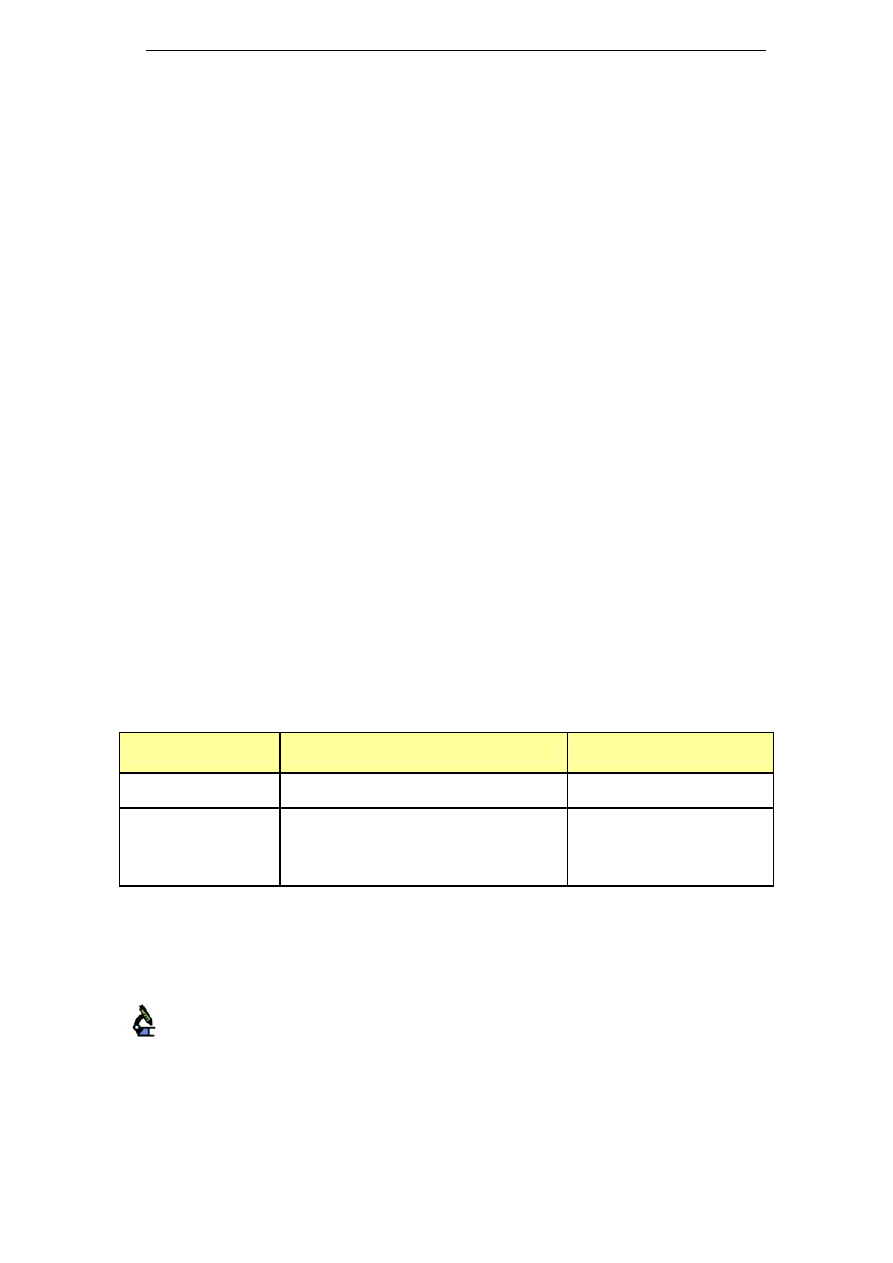
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
33
cleaning and disinfecting agent for pipeline systems. EO water is
produced by electrolysis of a weak salt solution into sodium and
chlorine, with a membrane between the electrodes to separate the ions
from each other, yielding alkaline and acidic EO water (Walker et al.
2005). Small pieces of materials commonly used in the milk processing
industry, including stainless steel sanitary pipe, PVC milk hose, rubber
liners, rubber gasket material and polysulfone plastic, were soiled using
raw milk inoculated with a cocktail of four bacterial cultures similar to
those commonly found in raw milk. The materials were then soaked in
the alkaline EO water before being transferred to the acidic EO water.
The materials were soaked for a series of time and temperature
combinations.
It was found that most of the treatments at 60°C and several treatments
at lower temperatures successfully removed all detectable bacteria.
Based on these results, Walker et al. (2005) stated that EO water has
the potential to be used as a cleaning and disinfecting agent for a range
of materials commonly found in the milk processing industry.
5.10.3 Combined acid detergent + sanitiser
In many food processing plants it has become common practice to
combine detergency and sanitisation to form one stage in the cleaning
process instead of two separate stages. The main benefit of this
approach is that it saves considerable time. However, it is important to
realise that there can be a loss of disinfection action so it is important
to consider the final effect of combing detergency and sanitising
(Loghney and Brougham 2005).
Table 8: Examples of alternative combined acid cleaner + sanitisers
Name
Composition
Industry sector
Envirowash
No phosphates or nitrates
Dairy plants
Iodosan (Triple 7)
Iodine
Iodophor
Abattoirs
Dairies
Livestock/Poultry Farms
Wineries
5.11
Comparison of cleaning chemicals
5.11.1 Comparison on cleaning performance
Parkar et al. (2004) carried out a comprehensive study to determine
the cleaning and sanitisation mechanisms that caused the removal
(cleaning) and inactivation (sanitisation) of 18-h biofilms of a
thermophilic Bacillus species growing on stainless steel. They tested a
number of different cleaning strategies. The success of the cleaning
regimes was determined by the removal of cells and organic debris and

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
34
the elimination of viable cells. A number of different cleaning agents
were selected for the study including:
alkaline cleaners
enzyme based cleaners
oxidizing chemicals
a quaternary ammonium chloride
detergents
Parkar et al. (2004) found that caustic and acid cleaning with 2% NaOH
at 75°C for 30 min was the most effective of all the caustic and acid
treatments used to remove and kill biofilms. They also found that when
the temperature of the full strength alkali and acid was reduced to 60
and 50°C the cleaning efficacy was reduced. A reduction in the
temperature of the full strength caustic acid
2
also led to a decrease in
cleaning efficacy.
Parkar et al. (2004) reported that when Paradigm, an enzyme based
cleaner, was used according to the manufacturers’ instructions at 60°C,
no viable cells or cell debris were left behind on the stainless steel. The
other enzyme preparations analysed in the study, namely Purafect
®
,
Purastar™ and Cellulase
L
, were not as effective as Paradigm. Parkar et
al. (2004) suggested that low wetability
and the fact that the enzyme
cleaners target only one part of the biofilm were possible causes for this.
The oxygen based agent Perform
®
was found to remove 100% of cells
and attached polysaccharides. The other oxygen based agents, namely
Oxine
®
, Halamid and sodium hypochlorite, did not perform as well as
Perform
®
in terms of total cell reduction. However, in terms of loss of
viability of the biofilms, Oxine
®
and Perform
®
were found to be the
better performing agents (Parkar et al. 2004).
Parkar et al. (2004) stipulate that it is very important to use the right
concentrations of agents and the recommended temperatures to achieve
the best results. A decrease in the strength of the agents can kill the
cells but can fail to remove all the cells from the surface. Parkar et al.
(2004) concluded that several procedures, including caustic/acid and
enzyme based cleaners, produce satisfactory results in terms of the
removal and inactivation of biofilms from stainless steel, provided that
the correct process parameters are observed.
5.11.2 Comparison of cleaning efficiency for membrane
cleaning
Gan et al. (1999) carried out a series of experiments to formulate
and optimise chemical cleaning methods for a chemical microfiltration
2
Parkar et al. (2004) consider caustic acid to be a combination of 2% NaOH and 1.8%
HNO
3
.

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
35
membrane, which had been severely fouled by beer microfiltration. The
cleaning agents considered in this study were NaOH, HNO
3
, H
2
O
2
and
Ultrasil 11 (consists of sodium hydroxide and unspecified anionic
surfactants). The membranes used in the beer microfiltration rig were
fouled during a typical 10h run. Sodium hydroxide was found to have
the highest cleaning power, followed by Ultrasil 11 and last HNO
3
.
Gan et al. (1999) also investigated the effect of altering the
concentration of the chemicals. Sodium hydroxide produced a similar
result with a concentration of 0.3 wt% as it did for a concentration of
0.5 wt%. The optimal concentration for Ultrasil 11 was 0.3 wt%.
Concentrations higher than 0.3 wt% were shown to have an adverse
effect on cleaning. Chemical oxidation using hydrogen peroxide at
ambient temperatures was found to be a very slow and ineffective
cleaning process on its own. However, when oxidation was employed as
a second cleaning step, it was found that the water flux recovery
increased by between 8 and 18% for the three chemical agents (Gan et
al. 1999).
A further study was carried out on the cleaning of reverse osmosis
membranes fouled by whey (Madaeni and Mansourpanah 2004). A wide
variety of agents were used to clean the fouled membranes, including
acids, bases, enzymes and complexing agents. The authors employed
two parameters, resistance removal and flux recovery, to evaluate
cleaning efficiency. They found that hydrochloric acid (0.05 wt%,
pH = 3) resulted in the maximum flux recovery and complete resistance
removal. In contrast, the resistance removal for one of the other acids
analysed, H
2
SO
4
, was considerably lower than that achieved by HCl,
regardless of the concentration.
Furthermore, the study provided some interesting insights into how the
concentration of different chemicals affected the cleaning effectiveness.
Increasing the concentration of H
2
SO
4
led to lower resistance removal
and flux recovery. In the case of HCl, the cleaning efficiency increased
with the cleaner concentration, reached an optimal value and then
continually decreased. It was found that the cleaning efficiency of NaOH
gradually increased up to a concentration of 0.1 wt%, but there was
evidence to suggest that this cleaning agent caused damage to the
membrane at high concentrations. Of the acids considered by Madaeni
and Mansourpanah (2004), HNO
3
was found to be the best for
resistance removal, followed by H
3
PO
4
, NH
4
Cl, and oxalic acid. Of the
surfactants, CTAB resulted in the greatest resistance removal followed
closely by SDS. The other surfactant, Triton-x100, had a very poor
resistance removal. NH
3
exhibited a reasonably high resistance removal,
whereas urea and EDTA had only moderate effects (Madaeni and
Mansourpanah 2004).
A field study investigated the fouling of a reverse osmosis
desalination system installed at a refinery thermo-power plant in China
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
36
(Luo and Wang 2001). Citric acid based cleaning solutions were used in
the CIP process: Cleaner A (citric acid, special cleaner aids and buffer
corrosives) and Cleaner B (citric acid and Na
2
EDTA). Cleaner A was
found to restore 90.8% of the membrane performance. Although
Cleaner A was originally designed for the cleaning of silica colloids only,
it was shown that other foulants were removed simultaneously thus
improving the overall cleaning performance.
5.11.3 Comparison of cleaning efficiency for biofilm removal
Bremer et al. (2005) used a laboratory scale bench top flow system
to quantify the effectiveness of caustic and acid wash steps in reducing
the number of viable bacteria attached to stainless steel surfaces. The
system was designed to reproduce dairy plant conditions under which
biofilms form. They found that a standard CIP regime (water rinse, 1%
sodium hydroxide at 65°C for 10 min, water rinse, 1.0% nitric acid at
65°C for 10 min, water rinse) did not remove all the bacteria. The
addition of a caustic additive (Eliminator) was found to enhance biofilm
removal while the substitution of nitric acid with Nitroplus increased
the cleaning efficiency. It was also reported that the incorporation of a
sanitiser step into the CIP did not appear to enhance removal. The
results of this study indicate that the effectiveness of a standard CIP
can potentially be enhanced through the testing and use of caustic and
acid blends (Bremer et al. 2005).
Kumar and Anand (1998) cover a number of studies reported in the
literature detailing various chemical methods used to remove biofilm. It
has been shown that enzymes can be effective in cleaning the
extracellular polymers which form the biofilm, thereby helping in the
removal of biofilms. The microflora making up the biofilm will largely
determine the enzymes that should be used for cleaning (Kumar and
Anand 1998).
5.11.4 Comparison of cleaning chemicals through life cycle
assessment
A life cycle assessment (LCA) approach has been used to compare
four scenarios of CIP methods for dairy plants (Eide et al. 2003). The
CIP methods investigated were:
conventional alkaline/acidic cleaning by nitric acid and sodium
hydroxide followed by hot-water disinfection
one-phase alkaline cleaning with acid chemical cleaning
enzyme-based cleaning with acid chemical disinfection
conventional alkaline/acidic cleaning with disinfection by cold
nitric acid at pH 2
The main objective of the study was to compare the environmental
impact of new and commonly used CIP methods, simulated in a model

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
37
dairy. The LCA covered the production of detergents, transport to the
plant, the user phase and waste management of the packaging. Eide et
al. (2003) found that the CIP methods with small volumes and low
temperatures, such as enzyme-based cleaning and one-phase alkaline
cleaning, were the best alternatives for the impact categories energy
use, global warming, acidification, eutrophication and photo-oxidant
formation.
However, Eide et al. (2003) also stated that the LCA did not give a clear-
cut conclusion regarding the choice of CIP method because of the
difficulty associated with assessing the toxicity impact of the cleaning
agents. They reported that the one-phase alkaline method is likely to be
the best alternative from an environmental point of view, but
acknowledged that further research on the assessment of toxic
substances is needed to reduce the uncertainty of this conclusion.
Finally, Eide et al. (2003) pointed out that regardless of the choice of the
CIP method, hygienic design and optimisation of the cleaning process
are the most important effective steps to reduce the environmental
impact (see section 7 for further information on CIP optimisation).
5.12
Desk-top review of the impact of
implementation of alternative chemicals
The following section presents a desk-top assessment on the possible
implementation of alternative chemicals. Where information was
available from the chemical suppliers, literature references, internet
sites or factories, this assessment has been done in terms of:
Sodium discharge reduction
residue risk (including product and environmental impacts)
OH&S of factory and sewer workers
Corrosion issues: in-factory, sewer infrastructure and treatment
plants
No information could be obtained in relation to anticipated
operational/capital cost-benefit to be gained from using alternative
chemicals.
5.12.1 Residue risk, OH&S and corrosion issues
While some cleaning chemicals are food compatible, most of them will
require a water rinse at the end of the CIP cycle before the food
production can resume. Where information was provided by chemical
suppliers on food compatibility or need for rinsing, this has been
included in Appendix A.

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
38
In relation to OH&S issues, the most relevant information has been
extracted from chemical supplier information and Material Safety Data
Sheets. The three major OH&S issues found with chemical cleaners are:
Corrosive substances due to alkaline or acid nature of the
products (Class 8). This applies particularly to NaOH, KOH,
NaOH/KOH blends, built NaOH and KOH
Toxicity of chlorinated alkali cleaners, which in contact with acid
can produce toxic substances.
Oxidising substances (Class 5) such as hydrogen peroxide
Similarly, the corrosiveness of the cleaning agents is reported (where
available) in the specification sheet for each product.
5.12.2 Sodium discharge reduction
To assess the potential reduction in sodium discharge that could be
achieved through the change over to alternative cleaning chemicals, the
sodium concentration in the cleaning solution itself (after dilution of the
bulk chemicals) has been used. This was calculated using the sodium
concentration of the bulk chemical as well as the chemical in-use
concentration (as recommended by the chemical suppliers). The
reference for comparison is pure sodium hydroxide, with a
recommended in-use concentration of 0.5 – 4%, depending on the
process and equipment to be cleaned. Sodium hydroxide was chosen as
a reference because of its large use across many food and beverage
industries.
The cleaning chemicals have been grouped according to their categories
as identified in the sections above (where enough information about the
product was available). The lower recommended concentration of each
chemical was compared with the 0.5% NaOH solution, while the higher
recommended concentration was compared with 4% NaOH solution.
The results are presented in Table 9.
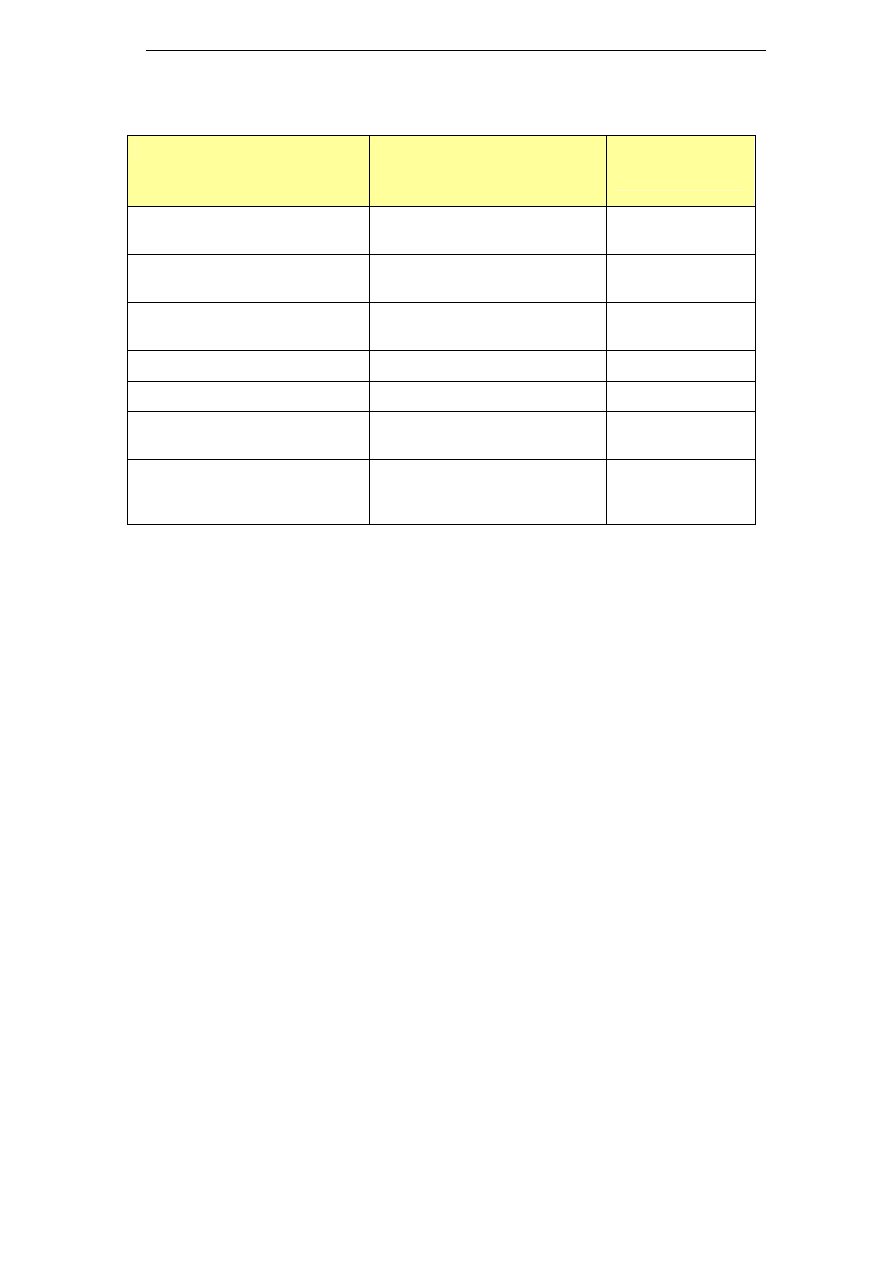
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
39
Table 9: Sodium concentration in various cleaning solutions
Sodium concentration in
cleaning solution
[gNa/kg cleaning solution]
Reduction
%
Pure NaOH (at 0.5 to 4%
NaOH)
2.88 – 23
Reference
a
Alkaline cleaners with
medium sodium levels
0.63 – 2.9
78 – 87%
Alkaline cleaners with low
sodium levels
0.02 – 0.8
99.3 – 96.5%
KOH based products
0 or negligible
Almost 100%
NaOH/KOH blends
0.13 – 0.78
95.5 – 96.6%
Enzymes and other
biotechnology based cleaners
0 or negligible
Almost 100%
Alternatives to alkaline
cleaning agents, including
plant-based cleaners
0 or negligible
Almost 100%
a
Pure NaOH has been used as the reference for comparison with all other chemicals
Table 9 shows that the use of alkaline cleaners with medium and low
sodium concentrations can lead to significant reductions in sodium
discharge from the alkaline step of a CIP cycle. Large savings can also
be obtained from NaOH/KOH blends, which can be attributed to the
use of KOH instead of NaOH and also to the use of alternative chemicals
in the blends. Furthermore, the use of potassium based cleaners,
enzyme products or other alternative cleaning agents can reduce the
sodium concentration to almost zero.
However, while these preliminary results are encouraging, it is
necessary to test the efficiency of these alternative cleaning chemicals in
factory environments and for specific processes and equipment. In some
cases, the limitations of a cleaning agent are known, e.g. it is known
that enzymes will not be able to operate at temperatures above 60ºC. In
many cases however, the performance of a cleaning chemical for specific
fouling and under various process conditions is unknown. Some
information from case studies and literature references has been
presented in this section. Further studies are required to measure
cleaning performance of selected cleaning chemicals in factories.
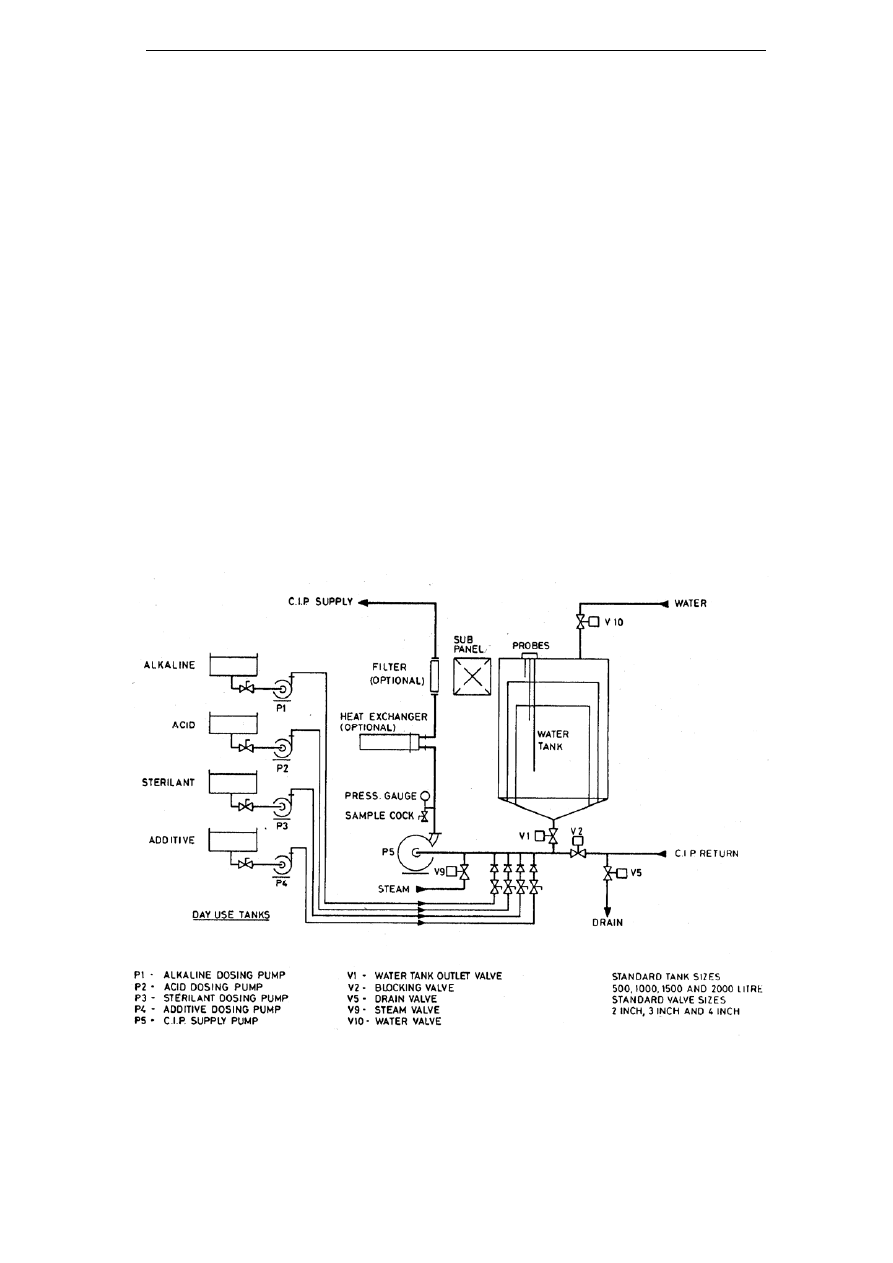
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
40
6
REVIEW OF CIP RECOVERY TECHNOLOGIES
6.1 Introduction
While following the same aim as the previous section to reduce the
impact of cleaning chemicals on trade waste discharges, this section is
taking a different approach. The approach in this section is to reuse the
used cleaning solutions in subsequent cleaning cycles, extending its
lifespan.
As a result of cleaning, the cleaning solution contains soil and an
increased COD (mainly in soluble form), and has lost some active
detergent compounds. This used cleaning solution can either be
discharged (single-use CIP) or reused (multi-use or reuse systems). The
different types of CIP systems are defined below (Davis 1980; Hamblin
1990):
Single use CIP system: The required amount of CIP solution is made
up at the lowest possible concentration. The solution is used,
recirculated during cleaning where appropriate, and then discharged
to drain (see Figure 1).
Figure 1: Single-use CIP system (Hamblin 1990)
Reuse system: The same cleaning solution is used for a large number
of cleaning operations. After use, the cleaning solution is returned to
the multi-tank, where it can be treated to remove some
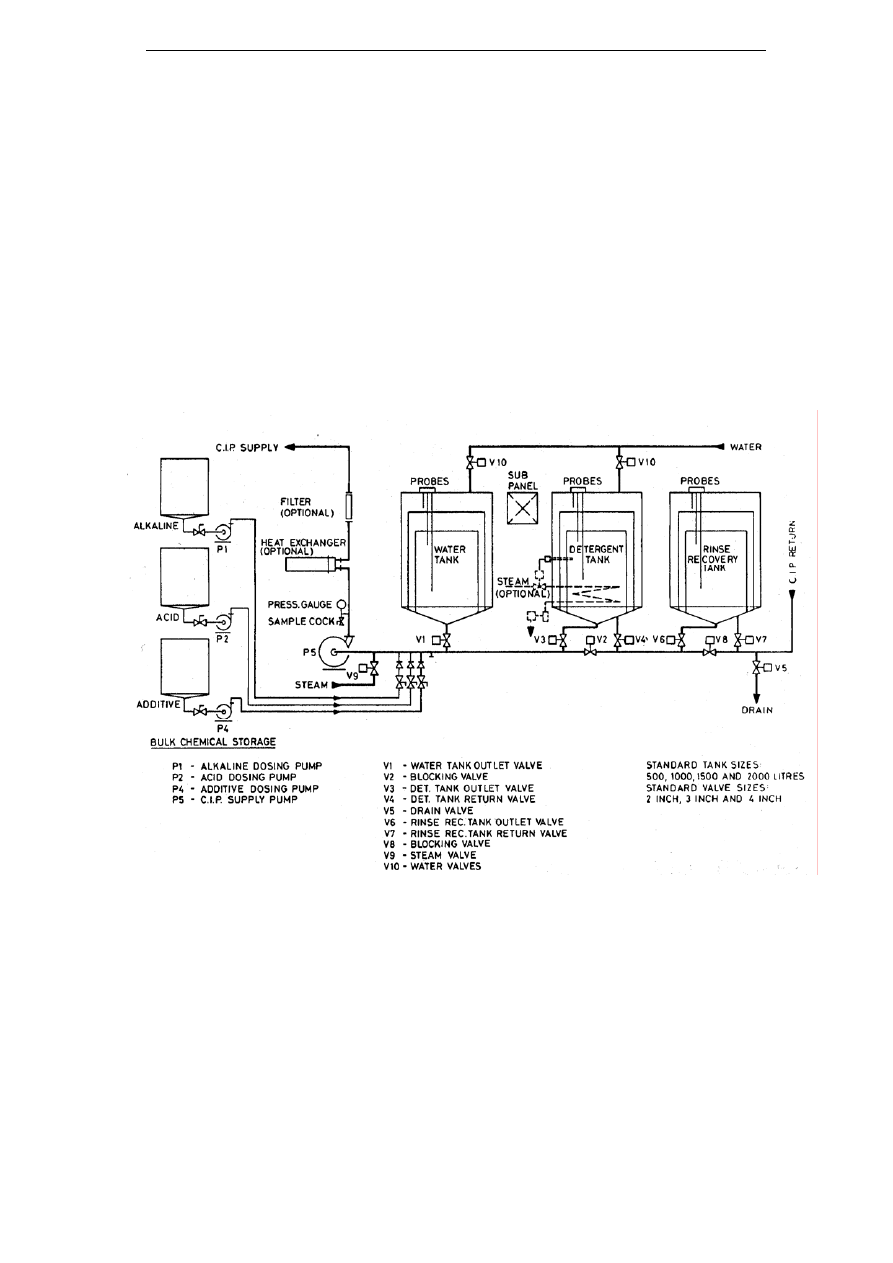
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
41
contamination, before being reused for further cleaning applications
(see Figure 2)
Multi-use system: After use, the cleaning solution is returned to a
collection tank. It is subsequently reused for pre-wash and rinses
before being discharged. This system presents the advantage of
increasing the cleaning efficiency of the pre-rinses due to the
presence of cleaning chemicals and also of reducing overall water
consumption.
Note that all configurations can exist in one plant. Reuse and multi-use
systems are often combined in one CIP system.
Figure 2: Reuse CIP system (Hamblin 1990)
6.2 Single use systems
In a single use system, the soiled cleaning solution is discharged to the
drain after use. Historically, the first CIP systems installed were single
use. Davis (1980) compared both single use and reuse systems and
strongly recommended single use for almost all applications. The trend
has now changed. However, some of the arguments in favour of single
use system are:

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
42
Reduced risk of cross-contamination
Lower initial capital costs
More applicable for decentralised CIP system, in which various
CIP loops are specifically operated for one piece of equipment or
process. In decentralised CIP systems, pipes are not connected
centrally, making the use of reuse tanks and treatment
technologies difficult to impossible. The costs associated with re-
piping all CIP loops to transform a decentralised system into a
centralised system are significant, leading to unattractive pay-
back periods.
More appropriate for cleaning solutions with high contamination
after the first use (e.g. evaporators)
More appropriate for applications requiring special cleaning
regimes: different chemicals, concentrations, temperatures, etc.
(e.g. membrane processes)
According to Davis (1980), single use systems are recommended for
tank cleaning because lower chemical concentrations and lower
temperatures are required for tanking than most other processes. The
dilution effect in reuse system amounts to the quantity of fresh caustic
for single use. There is therefore no incentive in reusing CIP solutions in
the application (Davis 1980).
Single use systems are also recommended in the biotechnology area and
for the cleaning of bioreactors (Chisti and Moo-Young 1994; Forday
2005). Single use systems avoid contamination with soil and microbial
spores, which have long survival periods. Such systems also enable a
higher quality control as the characteristics of the starting cleaning
solution for each clean are well known. In contradiction to the previous
authors, the company Koch Membrane Systems (KMS) suggests the use
of a nanofiltration membrane (AlkaSave®) for the recovery of used
cleaning solutions from fermentation equipment, which can enable
reuse in subsequent cleaning cycles (KMS 2005). More details about the
AlkaSave® process are provided in section 6.4.5.
6.3 Multi-use systems
6.3.1 Benefits of multi-use systems
In multi-use systems, used CIP solutions are collected in tanks and
reused for pre-rinses in subsequent cleaning cycles. The introduction of
multi-use systems leads primarily to savings in water consumption.
Additionally, the presence of residual cleaning agents in the pre-rinse
solution increases the efficiency of the pre-rinse, thus reducing the load
on the main cleaning step.

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
43
With the multi-use approach, where a used CIP solution is reused in
the next cleaning cycle for pre-wash and rinses, the following
achievements are reported (Davis 1980):
30% reduction in water consumption
15% reduction in energy consumption (due to heat recovery)
10-13% reduction in chemical usage
6.3.2 Case studies
)
For Pauls Limited, the disadvantages of single use CIP were
summarised by (DEH 2001):
Cost inefficiency
Excessive use of cleaning chemicals
High time out of production schedule to clean on a continuous
basis
Pauls Limited conducted a major upgrade to install multi-use CIP to
clean and sanitise all milk lines and pasteurised milk vats. All used
cleaning chemicals (acid, sanitiser and sodium hydroxide) are returned
to respective holding vats. In the holding vats, conductivity and
temperature are measured and used to control the length of the
following CIP cycles to ensure that specifications are met. After many
cleaning cycles, when the organic build-up exceeds a set value, the
spent CIP solution is discarded (DEH 2001). The new CIP system saves
the dairy company $40,000 per year, with a payback period of 1 year
(Prasad 2004b).
)
Golden Circle, QLD, installed a collection system to hold final rinses
from CIP. The collected solutions are then used in pre-rinses for the
next cleaning cycle. This has resulted in a saving of 4.35 ML of water
per year, which is equivalent to $10,300 (UNEP 2004).
)
Schweppes Cottee’s, NSW, installed a tank, piping and a pump on a
cordial line for the collection of final rinse water for the first wash in the
next cleaning cycle. It has been estimated that this has halved the
mains water consumption (UNEP 2004).
)
Taw Valley Creamery, UK, utilised two redundant tanks to collect
used acid solutions and final rinse waters from evaporators and
finishers. They installed a conductivity probe to detect interfaces
between cleaning steps. The annual savings were reported to be:
56 m
3
of 60% nitric acid
2.75 ML water
The payback was estimated to be just over 1 year. The overall benefits
include cost savings, improved effluent quality, and a more reliable
cleaning (ETBPP 1998). The only drawback reported is the need to
control the tanks to avoid any deposits.

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
44
)
Dairy Farmers have implemented two types of multi-use systems
(Price 2004):
The reuse of rinse waters for less critical areas (outside CIP
systems)
The reuse of cleaning solutions from pasteurisers to be used for
pre-rinse on tanks. The pay-back period is only a few months.
6.4 CIP Reuse systems
6.4.1 General remarks
CIP reuse systems enable the collection of used cleaning solutions and
their reuse in subsequent cleaning cycles. Prior to reuse, the cleaning
solutions can be treated to remove parts of the soil, thus further
extending the life of the cleaning chemicals. The types of treatments
performed include:
Gravity separation (sedimentation and centrifugation)
Physicochemical methods (coagulation/precipitation)
Membrane separations
Before going into any more detail about these treatments, their benefits
and related case studies in the following sections, a few common
remarks on CIP reuse systems are described below.
1. In most CIP systems in the food and beverage industry, soil is mainly
present in soluble form. Thus, it should not be expected to remove the
majority of the soil using gravity or coagulation.
2. For any reuse system, whether straight reuse or following treatment,
it is important to perform a good pre-rinse to extend the life of the
cleaning solution. It has been shown that pre-rinsing can remove a
large portion of the soil, while the main cleaning step will remove the
more resistant soiling compounds.
3. It is also important is to prevent dilution of the cleaning solution with
rinsing water. To isolate the different cleaning steps (see section 4.1.3 in
relation to the cleaning steps), the use of air blows has been trialled but
was proven to be very difficult to implement in factories (Davis 1980).
Most systems are now defining interfaces between cleaning steps using
electrical conductivity. This method, however, is not the best as the
electrical conductivity does not vary linearly with the strength of the
cleaning solution.
4. In many reuse applications, it will be necessary to re-dose cleaning
chemicals to adjust their strength, and to add additives to compensate
for those that have reacted in the previous cleaning cycle and/or have

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
45
been removed by the treatment process (Trägårdh and Johansson 1998;
Bhave et al. 2001; Bolch 2005).
5. While the reuse of CIP solutions is often reported as being operated
within a production area of a factory, CIP solutions from one area can
also be reused in another area of the factory. An example of this
configuration is the use of dilute used caustic solutions for evaporators
in the dairy industry (Bhave et al. 2001).
6. Alkaline and acid cleaning agents are the chemicals which are mostly
reused. Disinfectants can also be collected and reused although the
strength and contamination should be controlled (ETBPP 1998).
7. Recovery of cleaning solutions should be done on fresh streams to
avoid chemical alteration of the contaminants. However, this leads to
technical difficulties as the CIP flows are variable (Dresch et al. 1999).
For membrane technologies, benefits of treating cleaning solutions
without extended delays have also been reported by Henck (1995), as a
cooling down of the solutions reduces membrane performances.
8. The maximum acceptable level of soil in a cleaning solution should
be determined on a case by case basis. In study on evaporators in the
dairy industry conducted by DPEC (2005), it was shown that a NaOH
solution with 1% total alkalinity and a COP of up to 45,000mg/L could
still satisfactorily clean. However, due to foaming problems, the authors
recommended to keep the COD < 10,000mg/L for this application
(DPEC 2005).
6.4.2 Straight reuse vs. treatments
Straight reuse consists in the direct reuse of used cleaning chemicals
without any treatment. In bottle washing plants, it has been shown that
there are better economic benefits for a straight reuse system compared
to the inclusion of any treatment prior to reuse (Novalic et al. 1998).
However, in most cases, some form of soil removal will be applied,
whether by gravity separation, chemical means or membrane
separation. In these systems, the used cleaning solution is collected
after the cleaning process and delivered to a storage tank. It is then
treated in batches or in a continuous process. The aim of any of these
treatments is to remove the soil from the used cleaning solutions to
enable the reuse of the cleaning solution for subsequent cleaning
applications before discharging it. This can result in energy, water and
chemical savings, while maintaining cleaning efficiency (Trägårdh and
Johansson 1998; Dresch et al. 1999).
6.4.3 Reuse after gravity separation
6.4.3.1 Reuse following sedimentation
In tanks where used CIP solutions are collected, sedimentation usually
occurs and large suspended particles settle. This is beneficial because it

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
46
provides some treatment of the solution before reuse. However, the
limitations of this technique are that (Bhave et al. 2001):
(1) the recovered caustic is not very clean, and
(2) large amounts of sludge can accumulate of at the bottom of the
settling tank.
Decantation trials were conducted at room temperature for 3.5h
with caustic soda from a dairy standardisation process (Dresch et al.
1999). A 22-35% reduction in total COD was measured, while soluble
COD remained unchanged (Dresch et al. 1999). It should be noted that
the effect of temperature on CIP solutions is important as gels form at
lower temperatures, while nothing can be observed at process
temperature of 50-60ºC. Therefore, these observations might not be
replicable in industrial scale as used cleaning solutions would rarely
have time to cool down.
6.4.3.2 Reuse following centrifugation
To improve the separation of suspended solids, the use of centrifugal
forces can be used, either in hydrocyclones or in separators/clarifiers.
Hydrocyclones create centrifugal forces by moving the liquid. They can
typically remove particles larger than 5µm if the density difference
between the particles and the medium is more than 100 mg/kg
(Prendergast 2005). Hydrocyclones operate better at higher temperature
(due to reduced viscosity) and at higher flow rates (Prendergast 2005).
Advantages of hydrocyclones (Spinifex 2004; Bolch 2005; Prendergast
2005):
simple and reliable operation
low maintenance
no chemicals required
no moving parts
small footprint
low capital costs
slightly better recovery than sedimentation due to higher gravity
forces
Disadvantages of hydrocyclones (Spinifex 2004; Bolch 2005;
Prendergast 2005):
removes suspended solids only
does usually not perform as well as a centrifuge
removes particles with density sufficiently different from that of
the medium
)
In addition to the previous successes detailed above, Pauls Limited is
using a hydrocylone system (by Spinifex, $32,000), in which the CIP
solution is pumped tangentially into the separator. Heavy suspended
solids are accelerated against the outside of the hydrocyclone and

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
47
removed, while the cleaned solution can be reused. A removal efficiency
of 80% for 10 micron particles is reported, increasing the lifespan of the
cleaning fluid in the reuse system (DEH 2001; Spinifex 2004).
)
Ultraspin installed a hydrocyclone unit at a Nestlé dairy factory to
clean their caustic solution. The unit was installed in an external loop
off the already existing caustic recovery tank. This installation extended
the life of the cleaning chemical from 10-14 days to beyond 40 days by
removing suspended matter. The quality of the treated caustic solution
was improved compared to solutions reused without treatment, leading
to significant cost savings. It was found that an increasing amount of
suspended matter in the solution increased the efficiency of the
hydrocyclone (Prendergast 2005).
In a study conducted by DPEC (2005) on CIP solutions from
evaporators in the dairy industry, the suspended solids were removed
by up to 20% in a hydrocyclone, while total COD was reduced by up to
16%.
In centrifugal clarifier, the centrifugal force is created by rotation of the
machine.
Advantages of centrifuges (Bhave et al. 2001; Bolch 2005):
no chemicals required
slightly better recovery than hydrocyclones due to larger
centrifugal forces: removes suspended particles down to 5 µm
Disadvantages of centrifuges:
high capital cost
high energy requirements
removes suspended solids only
removes particles with density sufficiently different from that of
the medium
Trials were conducted with caustic soda from a dairy
standardisation process using a lab centrifuge at 3000g for 20min, at a
temperature of 20ºC. The total COD reduction ranged between 26 and
36%. No soluble COD reduction was measured (Dresch et al. 1999).
An industrial centrifuge skimmer was also used for the same
solution of caustic soda from a dairy standardisation process. The
centrifuge was operated continuously, with 6000g at 80ºC. The results
differed significantly from the lab centrifuge tests, as less than 4% of
the total COD was removed (Dresch et al. 1999).
)
A Victorian dairy factory upgraded their evaporator CIP system by
separating dirty and clean caustic solutions. The company also retro-
fitted an old milk-separator to improve the quality of the recovered CIP

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
48
solution. While suspended solids were removed by the centrifugal
forces, no reduction in soluble COD was observed (DPEC 2005).
6.4.4 Reuse following physicochemical treatments
An alternative approach to remove soil from cleaning solution is to use a
combination of chemical and physical processes, including:
Precipitation
Coagulation
Conventional filtration after chemical reaction
)
The Cedilar dairy factory in France has been using a physical-
chemical process to clean their used CIP solutions. The process involves
the addition of precipitation agent, coagulants, adsorbents and oxidants
to the tank holding the used CIP solutions. As a result, dissolved
impurities are precipitated, adsorbed or chemically transformed into
compounds not affecting the cleaning process. The treated CIP solution
is then passed through a belt-filter press and a fixed-bed reactor, before
being reused in the factory (Jung and Niederhünigen 1996). According
to the authors, the benefits of such process are:
Constant efficiency of cleaning solution due to COD and SS
remaining constant
Reuse of solution for 10 weeks without discharging it. This results
in:
o
Reduced consumption in cleaning chemicals
o
Reduced environmental impact
According to the CEO of the company, the reuse could be
extended for up to 24 weeks
Reduced water consumption
Reduced energy consumption as warm cleaning solution is reused
Chemical processes are strongly affected by pH variations and
temperature. Because of the nature of the cleaning solutions, extreme
pH values and temperatures limit the use of some chemical processes:
Flocculation processes suggested for CIP recovery typically operate
below 30ºC (DPEC 2005). Furthermore, coagulation can only remove
particles, not soluble compounds (Dresch et al. 1999). The neutral
density of the flocs formed can also be a limitation as flocs do not settle
easily (DPEC 2005).
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
49
6.4.5 Reuse following membrane separation
6.4.5.1 Membrane selection
The pore size and physical characteristics of the various membranes
define their ability to separate specific compounds from the cleaning
solution (see Figure 3).
Figure 3: Membrane categories
It is important to select the membrane type and characteristics
according to the CIP requirements, such as composition of the cleaning
solution and type of soil present in the used cleaning solution. This
would be different for a bottle washing plant, a cheese plant or a
brewery (Jung and Niederhünigen 1996; Dresch et al. 1999). Extreme
pH values of the cleaning solutions (highly acidic or highly basic), as
well as elevated temperatures (50 - 80ºC) limit the choice of membranes
(Bhave et al. 2001). For alkaline cleaning solutions, the available
membrane types are summarises in Table 10.
MF enables recovery and reuse of cleaning solution for a number of
cycles only, because soluble COD is not removed and builds up rapidly
in a few CIP cycles. On the other hand, the flux through UF and NF
membranes is smaller but this is outweighed by the longer life of the
CIP solution and the lower cleaning requirements for the membrane
themselves (Jung and Niederhünigen 1996). In terms of choosing
between UF and NF, the decision depends on the compounds to be
retained. UF retains all suspended solids, colloids and high molecular-
weight compounds, and some bacteria but tensides and low molecular-
weight compounds pass through: sugars, salts and colour-causing
compounds. NF will additionally remove colour and almost all COD.
Microfiltration
Ultrafiltration
Nanofiltration
Reverse Osmosis
Suspended solids
Macromolecules, e.g.
proteins
Sugars and colour
Salts
Water
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
50
Table 10: Membranes available for used cleaning solutions (adapted from
Bolch (2005) and KMS (2005))
Membrane
type
Material
Characteristics
Membrane
life
Ceramic
MF/UF
Inorganic
membrane
constructed of
alumina
Robust
Handles particles
Provides coarse to medium
filtration only
5+ years
Tubular NF
Polymeric tubes
Handles particles
Provides finest filtration
2 years
Spiral NF
Polymeric sheets
Will not handle particles
Provides finest filtration
Example of application:
Treatment of used acid
solutions with low
suspended solids
1 year
Performance versus cost of various types of membranes for sodium
hydroxide recovery is summarised in Table 11.
Table 11: Performance and costs of various membranes for sodium
hydroxide recovery (Bolch 2005)
Membrane
type
Percentage of
cleaning solution
recovered
Life of cleaning
solution
Cost
Ceramic UF
60%
2 weeks
High cost but
longer life:
$2100 per m
2
Tubular NF
95%
2.5 – 4 months
Medium cost:
$600 per m
2
Spiral NF
95%
2.5 – 4 months
Low cost:
$260 per m
2
It was reported that 20 MF, 5UF and 8NF processes were used
worldwide in 1997 to clean cleaning solution in the dairy industry alone
(Horton 1997).

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
51
6.4.5.2 Case studies
This section presents various industry case studies as well as scientific
work published in the literature in relation to the use of membrane
processes to remove soil from cleaning solutions.
The AlkaSave® process is based on a NF-membrane separation, which
can operate at a pH range of 1-14 and at temperatures of up to 70ºC.
The process is used in the dairy industry and enables a 95% recovery of
the cleaning solution, leading to a 95% reduction in water consumption.
AlkaSave® is reported to remove 95% of COD and colour, and 80-90%
carbonate (Jung and Niederhünigen 1996; KMS 2005).
)
The AlkaSave® process is used in the brewing industry, where it can
remove 90% of the total COD and 80% of the carbonates present in
used caustic solutions. This leads to significant reductions in caustic
usage, water consumption and effluent volumes. According to the
membrane manufacturer, the use of additives, such as antifoaming
agents, can also be reduced or eliminated in most cases (KMS 2005).
)
The AlkaSave® process can also be used for the recovery of acid
solutions by removing 80-90% of the calcium (KMS 2005). The reject
stream can be cleaned of the remaining salts by diafiltration and
subsequently used in stockfeed. The return on investment is reported to
be 2 years (Jung and Niederhünigen 1996).
Although not directly relevant to this project, it is worth noting that the
AlkaSave® process is also successfully used to clean ion exchanger
regeneration solutions from food and beverage processing as well as
spent mineral acids in the metal processing and finishing industry
(KMS 2005).
)
Sunkist Growers, a producer of juice in the US, has been using a
ceramic Membralox® membrane, from GEA Filtration since 1994 for the
treatment of used alkaline cleaning solutions. The daily caustic usage
has dropped by more than 40% and “essentially eliminated spent
caustic as a waste disposal issue”. The system has been operating
reliably for seven years, with no membrane replacement (Bhave et al.
2001).
)
Another juice and juice by-products producer, Southern Gardens
Citrus, USA, also installed a Membralox® membrane (Ultrafiltration).
Recovered caustic solution was successfully reused in the plant, after
its strength had been adjusted, leading to a 30% reduction in annual
caustic consumption. Benefits are also reported in terms of reduced
effluent treatment costs and energy consumption. In comparison with
the centrifuge previously employed to recover used caustic solutions,
the solution is much cleaner (Bhave et al. 2001).
)
The application of nanofiltration in the dairy and brewing industry
has been reported by Bolch (2005). The rejection of active compounds
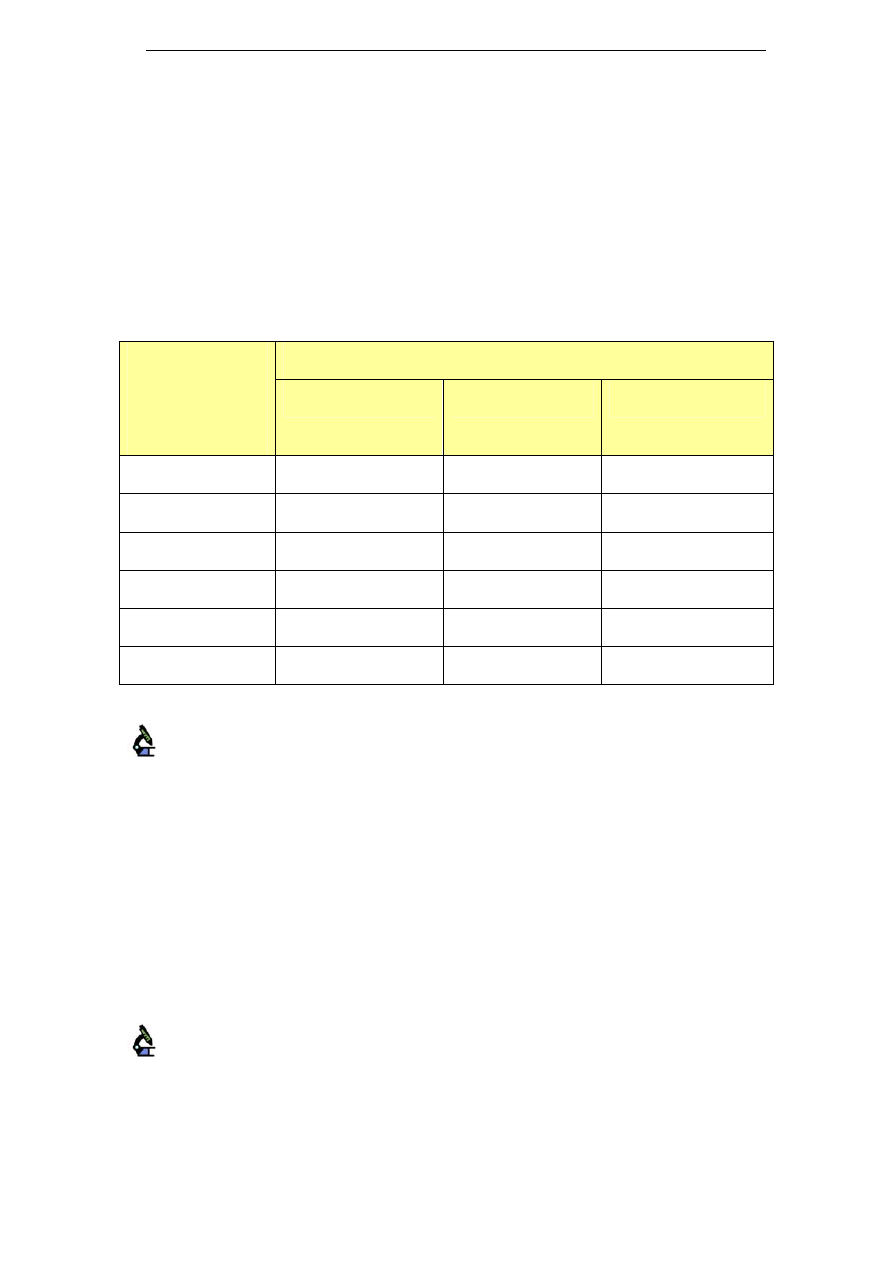
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
52
(available NaOH) is very low as can be seen in Table 12. On the other
hand the NaOH, which is bound to organic soil and thus not longer
active, is significantly removed (>85%). Similar trends are seen for the
total COD. Additives such as sequestrants are large molecules and are
fully removed by nanofiltration, thus the need to re-dose them prior to
subsequent cleaning cycles. Nitrogen and calcium, are usually well
removed, which is critical to prevent foaming and fouling in subsequent
cleaning cycles (Bolch 2005).
Table 12: Rejection of various compounds from sodium hydroxide
cleaning solution using an NF plant (Bolch 2005)
CIP system in following applications
Rejection of
following
compounds
Dairy powder
Dairy fat
Brewery
Available NaOH
<5%
<5% <5%
Bound NaOH
>85%
>85% >85%
Sequestrant
100%
100% 100%
COD
>70%
>75% >90%
Nitrogen
>60%
>35% >75%
Calcium
>85%
>95% >95%
A comparison of MF and UF ceramic membranes for the treatment
of cleaning solutions from the dairy industry was preformed using a
pilot-plant (Henck 1995). All results showed better organic matter
retention with UF than with MF. For protein contaminated cleaning
solutions, no decline in flux was observed when using UF as compared
to MF. On the other hand, fat contaminated cleaning solutions showed
a strong decline in flux when using tighter membranes. An increase in
trans-membrane pressure above 2bar or an increase in flow velocity
only increased membrane performance for fat containing solutions or
when strongly hydrolysed proteins were present in solution (stored or
highly heated cleaning solutions). From these results, a 70% reduction
in cleaning chemicals was estimated for factories.
Trägårdh and Johansson (1998) used various types of ceramic
membranes to investigate the soil removal from used cleaning solutions
from the dairy industry. The ceramic membranes used were:
UF membranes with nominal pore size of 20 nm, operated at a
pressure of 0.15-0.2MPa

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
53
for dairy evaporators, new nanofiltration membranes with
molecular cut-off of 5000 and 1000 Dalton, operated at
pressures 0.5-0.7MPa, were also tested
The results obtained using caustic detergents from various sources
showed that all sodium hydroxide passed through, while 20% of the
complexing agents were retained. High and stable fluxes were observed
through the membranes although some scaling issues occurred with
high hardness waters and low levels of complexing agents. Some
cationic surfactants are problematic for membranes and these solutions
could not be filtered.
Overall, the COD retention was between 30-70%, depending on the
origin of the cleaning solution and the cleaning agents used. No
advantage was found in using smaller pore size membrane as compared
to UF. It should be noted that the cleaning efficiency of recovered
solution was not tested in this study.
A further comparison of membrane technologies in the dairy
industry investigated 0.1µm MF, 300 and 15kDalton UF, Sol-gel and
organic NF (Dresch et al. 1999). Both caustic and nitric acid solutions
of various strengths (SS between 5 and 1000 mg/L, COD from 100 to
18,600 mg/L, of which 60-83% was soluble COD) from different parts of
the plant were tested.
The results show that UF removed 9 to 50% of total COD and more than
99% of SS leaving a clear but coloured cleaning solution after
treatment. However, it was found that only a small amount of soluble
COD was removed. Short-chained proteins, amino-acids, soaps and
lactose by-products were not retained by UF. Some irreversible fouling
was observed (Dresch et al. 1999).
Nanofiltration removed more soluble COD than ultrafiltration. It was
also found that more than 99% of SS was removed at satisfactory and
stable fluxes. Only slight irreversible fouling was observed. The NF
permeate was clear and uncoloured. Calcium and phosphorous were
efficiently removed (Dresch et al. 1999). The hardness removal is an
additional benefit of NF versus MF or UF as it reduces subsequent
mineral fouling on equipment (Novalic et al. 1998). A MF-pre-treatment
prior to NF was not recommended (Dresch et al. 1999).
With the aim of recovering as much cleaning solution (permeate) as
possible and of producing the lowest amount of by-product (retentate),
it is recommended to operate the membrane system at high volume
retention ratio. The volume retention ratio (VRR) is defined as volume of
retentate over volume of solution treated. In the study by Dresch et al.
(1999), the VRR could be increased up to 50, leaving 2% of sludge on
the retentate side. The downsides of operating high VRR are the
increased fouling (still moderate) and increased COD in permeate.
According to the authors, a volume retention ratio of 100 should give

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
54
the best outcomes to industries (1% sludge). However, the cleaning
efficiency of the recovered cleaning solution would need to be proven
(Dresch et al. 1999).
Further work was conducted in the dairy industry on the
comparison between a straight reuse system and various configurations
of nanofiltration (Dresch et al. 2001). The use of nanofiltration
maintained much lower COD levels in the cleaning solution than
straight reuse systems. Furthermore, the process configuration where
cleaning solution is treated continuously in an external loop attached to
the CIP reuse tank was found to be easier to set-up and less expensive
than batch systems. This configuration gave good COD removal and
slow fouling.
Merin et al. (2002) studied the cleaning performance of reused
NaOH solutions after they had been reused a number of times, in some
cases up to 400 times. The authors compared:
newly prepared NaOH solutions
untreated used NaOH solutions
used NaOH solutions after MF + NF treatment
The used cleaning solutions had been reused for one week at a dairy
plant at 70-80°C. An ultrafiltration membrane fouled with reconstituted
whey proteins was used to test the cleaning efficiency of the various
solutions.
Merin et al. (2002) found that the cleaning efficiencies of the NaOH
solutions that had been reused in the CIP processes were higher than
the newly prepared NaOH solutions. In addition the cleanliness of the
UF membrane after the cleaning tests had been performed was better
for the reused NaOH solutions than the clean NaOH solution. Despite
these promising results, the authors recommend further testing to gain
further understanding of the physical and chemical phenomena (Merin
et al. 2002).
6.4.5.3 Costs of membrane technologies and pay-back periods
Payback periods have been reported by various authors and the results
show large fluctuations. Indications of the range are given below but
return on investment must be calculated case by case (Novalic et al.
1998). The major influencing parameters are:
Price of caustic, which fluctuates strongly depending on world
market demand
Size of the plant
Industry and process type in which the membrane system is
installed (including the concentration of the cleaning chemicals
in solution)
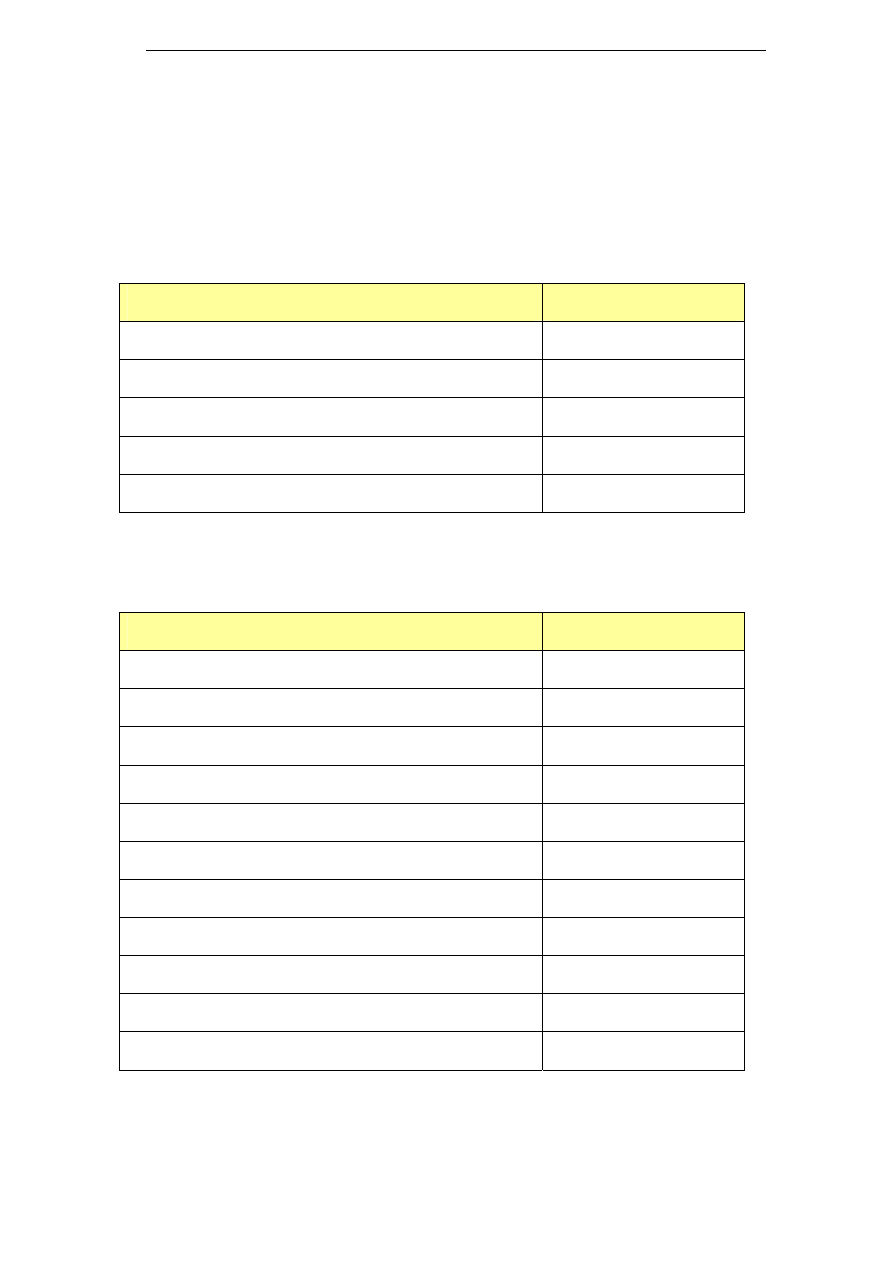
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
55
A cost calculation has been performed by Bolch (2005) for a plant with a
CIP capacity of 50m
3
/d. The single use system (Table 13) is compared
with a recovery system using a nanofiltration, which enables a reuse of
the cleaning solution for 3 months (Table 14). The payback period is
reported with 3.1 years.
Table 13: Operating costs for a 50m
3
/d caustic CIP plant – Single use
(Bolch 2005)
Item
A$ p.a.
Caustic cost (@ $0.40/L)
150,000
Caustic make-up water (@ $0.50/m
3
)
6,000
Heating cost (@ $2.39/m
3
)
32,000
Trade waste disposal costs (@ $1.00/m
3
)
13,000
Total annual costs for single use system
201,000
Table 14: Operating costs for a 50m
3
/d caustic CIP plant – with NF
membrane recovery of used caustic solution (Bolch 2005)
Item
A$ p.a.
Caustic cost (@ $0.40/L and with 90 days reuse)
10,000
Caustic make-up water (@ $0.50/m
3
)
400
Make-up heating cost (@ $2.39/m
3
)
2,000
Power cost (@ $0.065/kWh)
8,000
Membrane replacement cost
9,600
Additive re-dosing cost
25,000
Total annual costs for NF reuse system
55,000
Total Plant capital cost
410,000
Typical pay-back time
3.1 years
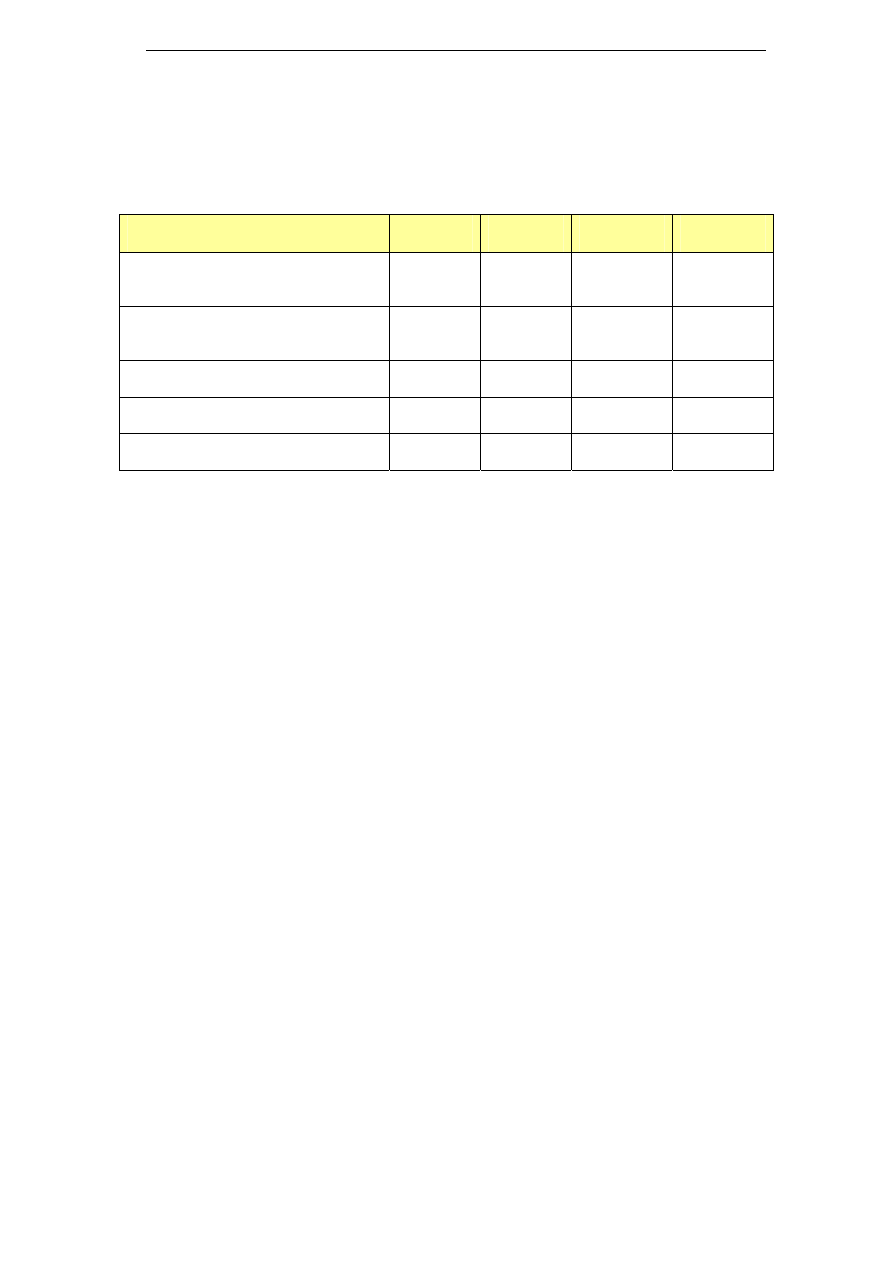
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
56
Due to the economy of scale, the return on investment becomes more
favourable for larger CIP systems, as shown in Table 15.
Table 15: Cost analysis summary for various size CIP systems (Bolch
2005)
30m
3
/d
50m
3
/d
100m
3
/d
150m
3
/d
Total annual costs for single CIP
($)
121,000 201,000 403,000 604,000
Total annual costs for NF reuse
system ($)
34,000 55,000 102,000 152,000
Savings ($)
87,000
146,000
301,000
452,000
Total Plant capital cost ($)
260,000
410,000
690,000
880,000
Typical pay-back time (years)
3.3
3.1
2.5
2.2
A wide range of payback periods have been reported in the literature
and have been listed below:
In the study conducted by Dresch et al. (2001), which was
mentioned above, a 14-year pay-back period was estimated for an
NF plant.
Henck (1995) estimated payback periods for an 8m
3
/d plant
using ceramic MF or UF. For 1.5% NaOH solutions, the payback
period was 8 years, while for more concentrated solutions (such
as from dairy evaporators, 5% NaOH), the payback was reduced
to 1.5 year. As discussed above, the authors also expected shorter
payback periods for larger plants.
In the case study presented earlier with Sunkist, the annual
savings have been estimated at US$135,000, and the payback
period was 2 years. For Southern Gardens Citrus, the savings
were approximately US$90,000, leading to a payback period of
1.5 year (Bhave et al. 2001)
Bonlac Foods in Cobden, Victoria, upgraded the cleaning solution
regeneration plant, leading to $83,000 savings p.a., with a
payback period of 2.3 years (Prasad 2004a).

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
57
6.5 Review of possible implementation of CIP recovery
technologies
6.5.1 Single use vs. reuse systems
To facilitate the selection of processes that could be implemented in
factories, the following section presents a comparison of single use
versus reuse systems. While reuse systems require additional pipes and
tanks, the benefits of reuse systems are numerous.
Table 16: Comparison between single and reuse systems (Adapted from
(Davis 1980; Hamblin 1990; Romney 1990b))
Criteria
Single use
Reuse
Space
Less required
Large floor and plant areas
required. Long supply and return
headers
Simplicity
Equipment and control are simpler Equipment and control are
complex
Running
costs
Water: higher costs
Trade waste: higher costs
Heating: higher
Chemicals: higher
Water: lower, especially if both
the main CIP solutions and the
post-rinses are reused
Trade waste: lower costs
Heating: lower costs as heat
recovery from warm used CIP
solution (particularly beneficial
for hot CIP)
Chemicals: lower costs.
NB: If a reuse system is installed
and centralised across an entire
plant, the chemical concentration
needs to meet the requirements
of the most stringent applications
and will therefore exceed the
minimum requirements in some
parts of the plants. Overall
however, the chemical needs will
be lower than in a single-use
system.
Capital
costs
Lower Higher
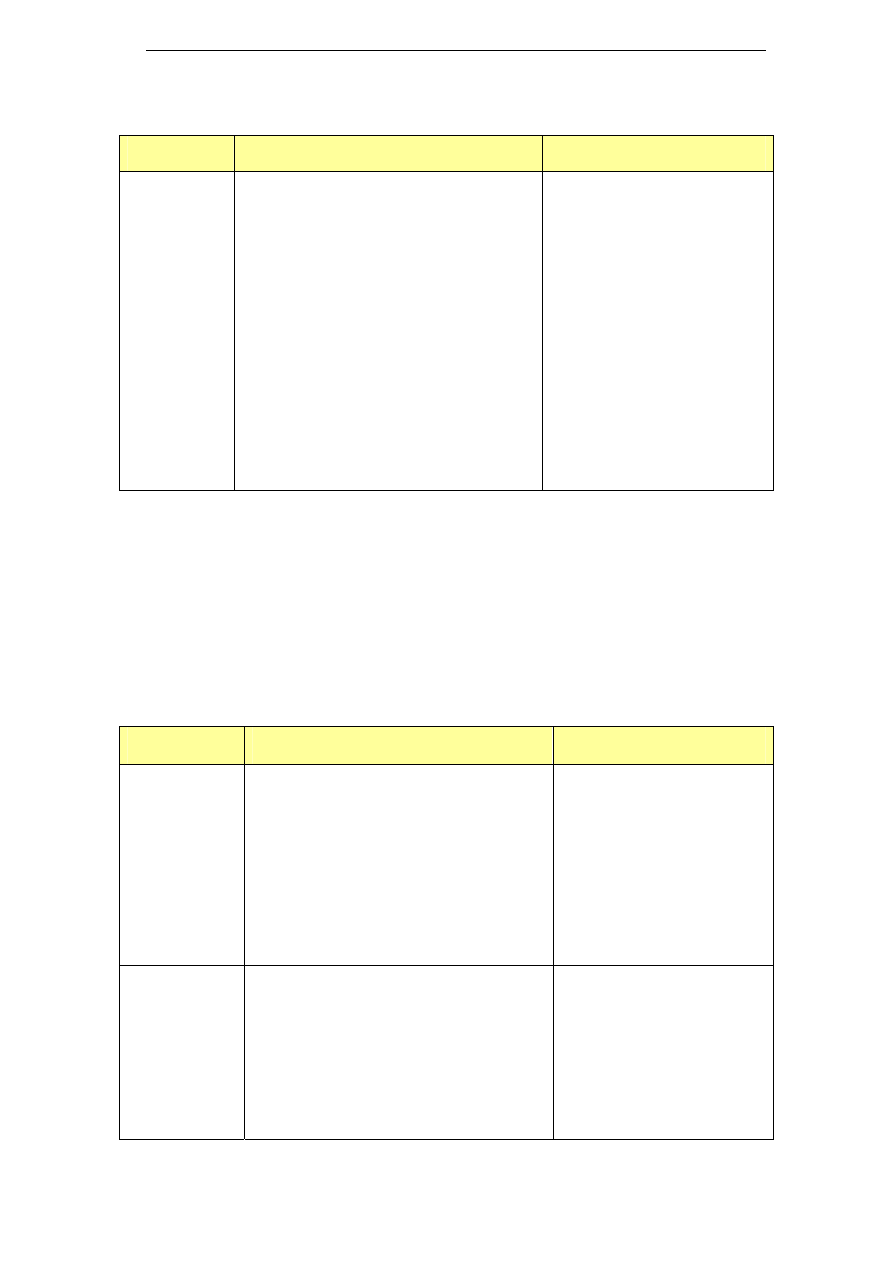
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
58
Table 17 continued
Criteria
Single use
Reuse
Type of
applications
Heavy soil loads (e.g. butter or first few
minutes of main cleaning step)
Areas where cross-contamination is a
high risk
Plants with large variety of processes
and CIP requirements, unless various
tanks are used to collect solutions of
various strengths and temperatures
Plants with decentralised CIP systems
Applications where blends of chemicals
are used with large amounts of
additives, which get use in the first
cleaning cycle (NB: chemicals can be
re-dosed in a reuse system)
Recommended for most
applications where
chemical balance of
compounds in cleaning
solution can be
maintained
soil removed during each
cycle is reasonably low
6.5.2 Selection summary of reuse treatment technologies
Table 18 summarises the advantages and disadvantages of the various
technologies described in the previous sections, as a tool for selection
for specific applications. Not mentioned in the table is the fact that for
all of these technologies successful applications in large scale systems
have been reported.
Table 18: Selection of treatment technology for CIP recovery and reuse
Technology
Advantages
Disadvantages
No technology:
straight reuse
Simple
Cost-effective
Number of CIP cycles before full
discharge to sewer: 7 (DPEC 2005)
a
Life of NaOH: 3.7 cycles (DPEC
2005)
a
Reduction in sodium discharges
from CIP: 73% (DPEC 2005)
a
No removal of soil from
the cleaning solution
Sedimentation Increased cleaning solution quality
Reduced plant downtime
Short payback period
Does not remove soluble
COD
Low quality of recovered
cleaning solution
Removes only particles
with density sufficiently
different from that of
the medium
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
59
Table 19 continued
Technology
Advantages
Disadvantages
Hydrocyclone
Simple and reliable operation, low
maintenance
No chemicals required
Small footprint
Low capital costs
Very good removal of heavy
suspended solids
Slightly better recovery than
sedimentation
Does not remove
soluble COD
Removes only particles
with density
sufficiently different
from that of the
medium
Centrifuge
No chemicals required
Slightly better recovery than
hydrocyclones: removes suspended
particles down to 5 µm
Number of CIP cycles before full
discharge to sewer: 7 (DPEC 2005)
a
Life of NaOH: 3.5 cycles (DPEC 2005)
a
Reduction in sodium discharges from
CIP: 71% (DPEC 2005)
a
Does not remove
soluble COD
High capital cost
High energy
requirements
Removes particles with
density sufficiently
different from that of
the medium.
Chemical
separation
Better recovery than with
sedimentation
Lower capital costs than centrifuge
Coagulation does not
remove soluble COD
Extreme pH values
and temperatures limit
the use of some
chemical processes
Microfiltration
Better soil removal than any of the
above systems: removes all
suspended solids thus longer life of
CIP solution
Usually higher flux than UF and NF
Lower pressure required than for NF
Less loss of cleaning chemical
compounds than NF
Ceramic MF are robust, with life
spans of 5y+ and can handle
particles
Number of CIP cycles before full
discharge to sewer: ∞ (DPEC 2005)
a
Life of NaOH: approx. 4 cycles (DPEC
2005)
a
Reduction in sodium discharges from
CIP: ~75% (DPEC 2005)
a
Does not remove
soluble COD
Extreme pH values
and temperatures limit
the use of some
membrane materials
High costs of ceramic
MF
Higher membrane
cleaning requirements
than UF or NF

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
60
Table 20 continued
Technology
Advantages
Disadvantages
Ultrafiltration
Better soil removal than MF as some
soluble compounds are removed,
thus longer life of CIP solution
Higher flux than NF
Lower pressure requirements than
NF
Less loss of cleaning chemical
compounds than NF
Ceramic UF are robust, with life
spans of 5y+ and can handle
particles
Removes only part of
soluble COD
Extreme pH values
and temperatures limit
the use of some
membrane materials
High costs of ceramic
UF
Smaller flux than MF
Nanofiltration
Usually much better retention of low
molecular organic compounds
present in used cleaning solution as
compared to UF. No colour remaining
in the treated solution.
NF removes most of nitrogen,
phosphorous and calcium, which
reduces foaming and mineral fouling
during subsequent cleaning
Higher percentage of cleaning than
with MF or UF = lower volume of
waste (retentate)
Lower initial capital costs than
ceramic MF/UF
Number of CIP cycles before full
discharge to sewer: ∞ (DPEC 2005)
a
Life of NaOH: between 10 and 30
cycles (DPEC 2005)
a
Reduction in sodium discharges from
CIP: 89-96.6% (DPEC 2005)
a
Extreme pH values
and temperatures limit
the use of some
membrane materials
Loss of all additives
present in cleaning
solution: need to re-
dose them prior to
reuse of the cleaning
solution
Smaller flux than MF
and UF
Shorter lifetime of NF
membranes compared
to ceramic MF or UF
membranes
a:
Figures from DPEC (2005) are related to CIP solutions from evaporators used in the
dairy industry
7
OPTIMISATION OF CLEANING TOWARDS REDUCED
CHEMICAL USAGE
Although this was not directly part of the project brief, a third approach
to reduce the impact of cleaning chemicals on trade waste discharges is
to optimise CIP cycles. Information found during the literature review
that was considered to be relevant to trade waste customers is

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
61
presented below. This is not a comprehensive review as it was not the
main focus of the work.
There may be opportunities to improve the efficiency of CIP systems by
reviewing (Prasad 2004b):
chemicals and blends (refer to section 5)
chemical concentrations
cleaning cycle length
in-line monitoring instrumentation
temperatures
opportunities to recover more rinse water and spent solution
water treatment effectiveness
operator training and supervision
equipment operation and maintenance
7.1 Frequency of cleaning
While many food and beverage plants clean on a daily basis,
investigations and site trials have shown the benefits of increased
intervals between cleans, without negative impact on product quality
and hygienic requirements.
Holm et al. (2002) investigated the efficacy of a 62h cleaning
frequency in the manufacturing of ice-cream. Samples were taken from
various products and product contact surfaces progressively
throughout the time period between cleaning cycles and analysed for
microbial growth (Holm et al. 2002). Samples were collected from a silo,
fillerhead, flavour vat and liquifier.
Coliform loads in product samples were found to be consistently low
over the entire time period. However, standard plate count (SPC) levels
increased slightly over time after CIP. There were no significant
differences in microbial counts (coliform and SPC) of the product
contact surfaces at the various times that the samples were collected (0,
24, 48 and 62h). However, there were significant differences in the SPC
microbial counts at the different locations the samples were collected
from. They also found that production variables influenced microbial
growth during the manufacturing process. A greater number of flavours
manufactured in the 24h time interval were beneficial at decreasing
SPC microbial counts. However, by the 48h time interval, the number of
flavours was at a threshold, and more flavours manufactured increased
SPC microbial counts. Holm et al. (2002) concluded that there were no
differences in the microbial growth over time at 0, 24, 48, or 62h from

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
62
CIP. Therefore, food safety was not compromised by the utilisation of a
62h CIP cycle (Holm et al. 2002).
7.2 Mechanical action to support cleaning
7.2.1 High pressure spray and mechanical scrubber
Gibson et al. (1999) evaluated other cleaning methods, including a
high pressure spray and mechanical floor scrubber. The efficacy of
factory cleaning and disinfection programmes were assessed by
swabbing and total viable count (TVC) analysis of surfaces before
cleaning, after cleaning and after disinfection. Biofilms of Pseudomonas
aeruginosa and Staphylococcus aureus were used in the cleaning trials.
The authors found that the high pressure spray and mechanical floor
scrubber were more effective cleaning methods, but warned that both
methods can be responsible for the spread of contaminants by aerosols
(Gibson et al. 1999).
7.2.2 Pigging systems
It is important to remove as much product as possible from pipes before
wet cleaning commences to avoid increasing wastewater loads and
wasting product (UNEP 2004). ‘Pigging systems’ or low-pressure blowers
have shown to be effective at cleaning pipes. A ‘pig’ (solid material plug)
is propelled along the pipe to push out the product. Pigs are very useful
for the removal of viscous liquids, but usually require specially designed
or modified pipe work because the pig cannot get trough pumps or
values (UNEP, 2004). Several companies have used pigging systems to
great effective.
)
A jam processing plant in the United Kingdom installed a pigging
system to clean its sumps, gulleys and food traps (UNEP, 2004). The
amount of water used to flush the pipeline fell from 2020 kL/year to
only 310 kL/year, while 173 tonnes of saleable product is recovered
annually. It was also found that the COD of the plant’s effluent was
reduced by 76%.
)
Food Spectrum in Queensland, which produces stabilised fruit
product, modified their pasteuriser to introduce a new silicon rubber
pig that better adhered to the pipe work than the starch pig that was
previously used (UNEP, 2004). The company saved approximately 10
minutes of water rinsing per product batch. This amounted to a saving
of about $700 per year in water supply and discharge costs. The new
system was also responsible for the recovery of $14,600 of product
every year.

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
63
7.3 CIP Monitoring
Dodd (2003) described the CIP monitoring system ShurLogger which
was developed by JohnsonDiversey. ShurLogger has up to 16 analogue
inputs which enables the flow, temperature and conductance to be
recorded and displayed. These three variables are considered to be the
main variables that show a clean process is operating. The 16 available
analogues enable 4 CIP channels to be monitored simultaneously.
ShurLogger is very flexible in its set-up, enabling JohnsonDiversey’s
technical specialists to configure the device to monitor exactly the
operation of vales and pumps on a CIP unit (Dodd 2003). Dodd (2003)
reported that several customers in a range of food sectors, including
dairy factories, have benefited from using this technology.
7.4 Case studies
Optimisation of CIP systems can lead to significant reductions in
chemical usage. Prasad (2004b) briefly presented several case studies
about the optimisation of CIP systems at different dairy plants. The case
studies include the reuse of water by a CIP system, validation and fine-
tuning of a CIP system, and burst rinsing.
)
For example, a Victorian milk processing plant assessed 15 separate
CIP wash cycles (UNEP, 2004). Modifications were made to the
Programmable Logic Controller (PLC) programs, pipework/valving and
return pumps for each cycle to maximise recovery and reuse of caustic
soda. It was reported that caustic usage was reduced by 50%.
)
In another example, the Taw Valley Creamery reduced cleaning times
and detergent consumption by 15% after installing conductivity sensors
on all its CIP systems (UNEP, 2004).
)
The dairy manufacturer Pauls Limited incorporated a central
computerised system to manage the CIP systems. The new system
enables sensitive measuring and control of CIP and through
automation, led to optimisation of CIP cycles (DEH 2001).
)
Prasad (2004c) reported that National Foods in Morwell, Victoria,
identified that cleaning times were above recommended levels after
conducting an audit of dosing equipment. Reduction of caustic and acid
timer settings did not compromise product quality. The plant also
managed to reduce caustic and acid usage by utilising automatic dosing
systems and optimising the concentration of chemicals for each
different task. This resulted in a saving of $100,000 per year.
)
Dairy Farmers also conducted an audit of all its CIP processes
(Prasad, 2004c). They installed optical sensors to fine-tune water and
milk interfaces as well as conductivity and turbidity meters to improve

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
64
cleaning operations. The savings for the plant was estimated to be
$211,500 per year.
8
RECOMMENDATIONS FOR FUTURE WORK
Further recommendations for future work are discussed below. These
are mainly focusing on the following aspects:
testing identified and promising alternative chemicals,
testing of reuse and recovery systems
optimisation of existing CIP systems to reduce chemical usage
technology transfer to factory representatives
cost-evaluation of the various options
Collection of further information and selection of sites for factory
trials
Using information made available from this project and from further
data collection, it is recommended to select some food and beverage
industrial sites, discharging high salt loads to sewer as a result of their
CIP systems and presenting high potential for TDS reduction through
identified approaches.
Testing the efficiency of alternative cleaning chemicals
This project has identified a range of alternative chemicals, from low
sodium chemicals to enzymes or plant-based products. The use of any
of these chemicals by factories is highly improbable unless trials have
shown their efficiency on the specific equipment and products used in
factories. It is therefore recommended to evaluate the efficiency of
alternative cleaning chemicals. This should be carried out in several
phases: lab trials followed by pilot-scale trials and large scale trials in
factories.
The first task would be to short-list some chemicals that appear to be
the most promising for a selected factory. The selection should be based
on data collected as part of this report but also on the type of product
manufactured and processing equipment used. Consultation with
chemical suppliers and factory sites is strongly recommended.
To evaluate the cleaning performance in the laboratory, a piece of
equipment representative of factory processes should be used. It should
then be fouled with product originating from the factory or with a model
product. The efficiency of selected alternative cleaning chemicals should
then be tested. A preliminary evaluation of costs and environmental

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
65
benefits could then be performed in terms of reduced TDS and sodium
loads. The results should be made available to the industry.
Based on the results of these laboratory experiments, a restricted
number of chemicals should be selected for factory trials. An
experimental protocol should be developed in collaboration with
operational staff from the selected factory site, while the factory area
should be prepared for the investigations (addition of valves, sampling
points, flow meters etc.).
The cleaning efficiency of alternative cleaning chemicals would have to
be tested in comparison with conventional products used on site. The
parameters to consider in the performance evaluation are:
anticipated operational/capital cost-benefit
reduction of trade waste TDS (and sodium) load
reduction of water consumption (compared with existing on-site
practices)
reuse potential of treated effluent
residue risk (including product and environmental impacts)
regulatory risk
OH&S of factory and sewer workers
Corrosion issues: in-factory, sewer infrastructure and treatment
processes
Once the trials are completed and the performance evaluated, the
results should be communicated to industry operational staff and other
factories that could benefit from the outcomes of these investigations.
Reuse of cleaning chemicals (with or without recovery
technologies)
According to the case studies presented in this report, the reuse of used
cleaning solutions (with or without recovery technologies) can lead to
significant reduction in chemical usage. The possibilities for straight
reuse of used cleaning solutions should be explored first.
The possibility to collect used cleaning solutions for reuse in other “less
demanding” process sections with lower hygienic requirements should
be considered too. The notion of “fit-for-purpose” can lead to chemical
savings too. Furthermore, the use of recovery technologies presented in
this report should be considered for some sites.
Following these considerations, an experimental protocol for testing of
these technologies in selected factory sites should be developed in
collaboration with operational staff. After preparation of the site,
experimental trials should be conducted. The performance evaluation of
the trials should be based on the following criteria:

Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
66
anticipated operational/capital cost-benefit
reduction of trade waste TDS load
reduction of water consumption
OH&S of factory and sewer workers
Corrosion issues: in-factory, sewer infrastructure and treatment
processes
Once the trials are completed and the performance evaluated, the
results should be communicated to industry operational staff and other
factories that could benefit from the outcomes of these investigations.
Optimisation of CIP cycles through engagement and training of
industry operational staff
Engaging factory staff has proven to be a key element to improve CIP
operation. It is recommended to conduct a training program for
interested industry operational staff, focusing on techniques to optimise
CIP cycles. The factory staff should be encouraged to identify and
conduct small trials relevant to their site between the training sessions.
Site visits or demonstrations could also be a useful tool to promote best
practice in CIP management.
Outcomes and findings from this report should be disseminated to the
wider industry. For this reason, a cut-down version of this report has
been prepared focusing on case studies from industry. It is
recommended to circulate this document widely.
9
ACKNOWLEDGMENTS
The project team would like to thank
City West Water, and in particular Mr Nigel Corby, for initiating and
supporting this project
The companies that provided input into the project
The chemical suppliers for supplying information on chemicals,
including low sodium chemicals, and
Mr Craig Bolch and Mr George Lech for making information available
for this project
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
67
10
REFERENCES
ABS (2005). Manufacturing Industry, Victoria. Reference No. 8221.2.
Australian Bureau of Statistics.
www.abs.gov.au/Ausstats/abs@.nsf/Lookup/168980EF2D554A0CCA2
568A900139427
24 October 2005
ADHS (2005). Food Equipment Cleaning and Sanitizing.
www.azdhs.gov/phs/oeh/fses/fecs_wcq.htm
9/9/2005
Allie, Z., E.P. Jacobs, A. Maartens and P. Swart (2003). "Enzymatic
cleaning of ultrafiltration membranes fouled by abattoir effluent."
Journal of Membrane Science 218: 107-116.
Argüello, M.A., S. Álvarez, F.A. Riera and R. Álvarez (2003). "Enzymatic
cleaning of inorganic ultrafiltration membranes used for whey protein
fractionation." Journal of Membrane Science 216: 121-134.
Argüello, M.A., S. Álvarez, F.A. Riera and R. Álvarez (2005). "Utilization
of enzymatic detergents to clean inorganic membranes fouled by whey
proteins." Separation and Purification Technology 41: 147-154.
Australian Standards, AS (2001). Guide to cleaning and sanitizing of
plant and equipment in the food industry - AS 4709-2001.
Bhave, R., G. Jung and R. Sondhi (2001). Food processing: Potential
savings through ceramic membrane caustic reclaim.
http://www.pall.com/pdf/foo_pdf_causticrecovery-2001.pdf
21/09/2005
BIO-WISE (2001a). Biological solution for cheaper metal cleaning.
www.dti.gov.uk/biowise
BIO-WISE (2001b). Biotechnology cuts cleaning costs by 90%.
www.dti.gov.uk/biowise
BIO-WISE (2002). Engineering Bulletin 3.
www.dti.gov.uk/biowise
Bolch, C. (2005). Personal communication.
Bremer, P.J., S. Fillery and A.J. McQuillan (2005). "Laboratory scale
Clean-in-Place (CIP) studies on the effectiveness of different caustic and
acid wash steps on the removal of dairy biofilms." International Journal
of Food Microbiology (in press).
Chisti, Y. and M. Moo-Young (1994). "Clean-in-place for industrial
bioreactors: design, validation and operation." Journal of Industrial
Microbiology 13: 201-207.
Costerton, J.W., B. Ellis, K. Lab, F. Johnson and A.E. Khoury (1994).
"Mechanism of electrical enhancement of efficacy of antibiotics in killing
biofilm bacteria." Antimicrobial Agents and Chemotherapy 38: 2803-
2809.
Davies, C.P., N. Wagle, M.D. Anderson and M.M. Warren (1991).
"Bacterial and fungal killing by iontophoresis with long-lived
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
68
electrodes." Antimicrobial Agents and Chemotherapy 35: 2131-2134.
Davis, P.F. (1980). Single use, re-use and multi-use CIP systems. A
seminar on cleaning-in-place, melbourne, Australian Society of Dairy
Technology.
DEH (2001). Cleaner production - Multiple use Clean-In-Place (CIP)
system in milk processing - Pauls Limited (NT).
www.deh.gov.au/industry/corporate/eecp/case-studies/pauls1.html
22/4/2005
DEH (2003). Cascade brewery company: Using technology modifications
to reduce energy and water use and enhance materials recycling.
www.deh.gov.au/industry/corporate/eecp/case-studies/cascade-
brewery.html
22/4/2005
Dodd, T. (2003). "Cleaning records and CIP optimization." International
Journal of Dairy Technology 56(4): 247.
DPEC (2005). Open Day Forum, Werribee.
Dresch, M., G. Daufin and B. Chaufer (1999). "Membrane processes for
the recovery of dairy cleaning-in-place solutions." Lait 79: 245-259.
Dresch, M., G. Daufin and B. Chaufer (2001). "Integrated membrane
regeneration process for dairy cleaning-in-place." Separation and
Purification Technology 22-23: 181-191.
DSE (2004). Securing Our Water Future Together. Victorian
Government White Paper. Department of Sustainability and
Environment. Melbourne.
D'Souza, N.M. and A.J. Mawson (2005). "Membrane cleaning in the
dairy industry: A review." Critical Reviews in Food Science and Nutrition
45: 125-134.
Dufour, M., R.S. Simmonds and P.J. Bremer (2004). "Development of a
laboratory scale clean-in-place system to test the effectiveness of
"natural" antimicrobials against dairy biofilms." Journal of Food
Protection 67(7): 1438-1443.
Eide, M.H., J.P. Homleid and B. Mattsson (2003). "Life cycle assessment
(LCA) of cleaning-in-place processes in dairies." Lebensmittel-
Wissenchaft und -Technologie 36(3): 303-314.
EPA, QLD (2003). A spectrum of environmental initiatives - Food
Spectrum case study.
http://www.epa.qld.gov.au/publications?id=375
01/09/2005
ETBPP, Environmental Technology Best Practice Program (1998).
Reducing the cost of cleaning in the food and drink industry.
http://www.envirowise.gov.uk
28/07/2005
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
69
Forday, W.L. (2005). Clean in place technology.
http://www.np.edu.sg/~dept-bio/microbiology/cip.html
21/09/2005
Gan, Q., J.A. Howell, R.W. Field, R. England, M.R. Bird and M.T.
McKechinie (1999). "Synergetic cleaning procedure for a ceramic
membrane fouled by beer microfiltration." Journal of Membrane Science
155: 277-289.
Garrick, C. and Schiekowski (1980). Cleaning effectiveness of CIP and
product quality. A seminar on cleaning-in-place, Melbourne, Australian
Society of Dairy Technology.
Gibson, H., J.H. Taylor, K.E. Hall and J.T. Holah (1999). "Effectiveness
of cleaning techniques used in the food industry in terms of the removal
of bacterial biofilms." Journal of Applied Microbiology 87: 41-48.
Hamblin, R. (1990). Design and control of CIP. CIP: Cleaning in Place.
A. J. D. Romney: 153-168.
Henck, M. (1995). Recycling of used caustic cleaning solutions in the
dairy industry by cross-flow filtration. Fouling and cleaning in pressure
driven membrane processes. I. D. Federation. Brussels: 175-183.
Holm, S., R.B. Toma, W. Reiboldt, C. Newcomber and M. Calicchia
(2002). "Cleaning frequencing and the microbial load in ice-cream."
International Journal of Food Science 53: 337-342.
Horton, B.S. (1997). "Water, chemical and brine recycle or reuse -
Applying membrane processes." Australian Journal of Dairy Technology
52: 68-70.
Jass, J., J.W. Costerton and H.M. Lappin-Scott (1995). "The effect of
electrical currents and tobramycin on Pseudomonas aeruginosa
biofilms." Journal of Industrial Microbiology 15: 234-242.
Jass, J. and H.M. Lappin-Scott (1996). "The efficacy of antibiotics
against Pseudomonas aeruginosa biofilms." Journal of Antimicrobial
Chemotherapy 38: 987-1000.
Jung, C. and CH. Niederhünigen (1996). "The recovery of washing
caustic from CIP waste water." European Dairy Magazine 5: 32-34.
KMS (2005). KMS Alkasave recovery system - Products.
http://www.kochmembrane.com/alkasave.html
22/09/2005
Kumar, C.G. and S.K. Anand (1998). "Significance of microbial biofilms
in food industry: a review." International Journal of Food Microbiology
42: 9-27.
Kumar, C.G., R.K. Malik and M.P. Tiwari (1998). "Novel enzyme-based
detergents: An Indian perspective." Current Science 75(12): 1312-1318.
Lagrange, F., W. Reiprich and M. Hoffmann (2004). "CIP-cleaning and
desinfection with ozoned water." Fleischwirtschaft 84(2): 112-114.
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
70
Langsrud, S., B. Baardsen and G. Sundheim (2000). "Potentiation of the
lethal effect of peroxygen on Bacillus cereus spores by alkali and enzyme
wash." International Journal of Food Microbiology 56: 81-86.
Leaver, G., W. Stewart and J.S. Deans (1995). "Containment aspects of
couplings and connections for biotechnology plant." BioSafety 1(1).
Lech, G. (2005). Personal communication.
Loghney, C. and P. Brougham (2005). How to Clean For Best Results in
the Food Processing Industry, The Society of Food Hygiene Technology.
Luo, M. and Z. Wang (2001). "Complex fouling and cleaning-in-place of
a reverse osmosis desalination system." Desalination 141: 15-22.
Madaeni, S.S. and Y. Mansourpanah (2004). "Chemical cleaning of
reverse osmosis membranes fouled by whey." Desalination 161: 13-24.
Merin, U., G. Gésan-Guiziou, E. Boyaval and G. Daufin (2002).
"Cleaning-in-place int he dairy industry: criteria for reuse of caustic
(NaOH) solutions." Lait 82: 357-366.
Muñoz-Aguado, M.J., D.E. Wiley and A.G. Fane (1996). "Enzymatic and
detergent cleaning of a polysulfone ultrafiltration membrane fouled with
BSA and whey." Journal of Membrane Science 117: 175-187.
NFESC (1999). Evaluation of Bio-Based Industrial Products For Navy
and DoD Use. Phase 1. Citra-Solv Natural Citrus Cleaner & Degreaser.
Port Hueneme, CA, Naval Facilities Engineering Service Center.
Novalic, S., A. Dabrowski and K.D. Kulbe (1998). "Nanofiltration of
caustic and acidic cleaning solutions with high COD. Part 1 - Recycling
of sodium hydroxide." Journal of Food Engineering 38: 125-132.
Parkar, S.G., S.H. Flint and J.D. Brooks (2004). "Evaluation of the effect
of cleaning regimes on biofilms of thermophilic bacilli on stainless
steel." Journal of Applied Microbiology 96: 110-116.
Prasad, P. (2004a). Eco-efficiency for Australian dairy processors - Fact
sheet 10: Membranes.
http://www.geosp.uq.edu.au/emc/CP/Dairy%20Project%20Factsheet/
Fact%20Sheet%2010(3).pdf
16/09/2005
Prasad, P. (2004b). Eco-efficiency for Australian dairy processors - Fact
sheet 8: Optimisation of CIP systems.
http://www.geosp.uq.edu.au/emc/CP/Dairy%20Project%20Factsheet/
Fact%20Sheet%208(3).pdf
16/09/2005
Prasad, P. (2004c). Eco-efficiency for Australian dairy processors - Fact
sheet 9: Chemical use.
http://www.geosp.uq.edu.au/emc/CP/Dairy%20Project%20Factsheet/
Fact%20Sheet%209%20(4).pdf
16/09/2005
Prasad, P., B. Pagan and M. Renouf (2004). A critical analysis of Cleaner
Production in Queensland's food industry - past efforts and future
Clean in Place – A Review of Current Technology and its Use in the Food and Beverage Industry
71
opportunities. Asia Pacific Roundtable for Cleaner Production, Kuala
Lumpur, Malaysia.
Prendergast, G. (2005). Separators in the dairy industry - Personal
communication.
Price, N. (2004). Cleaner production case studies: Dairy Farmers.
http://www.geosp.uq.edu.au/emc/cp/Res/ydairy_farmers.htm
16/09/2005
Romney, A.J.D. (1990a). Principles of cleaning. CIP: Cleaning in Place.
A. J. D. Romney: 1-6.
Romney, A.J.D. (1990b). Management of cleaning operations. CIP:
Cleaning in Place: 202-210.
Sakiyama, T., T. Toyomasu, A. Nagata, K. Imamura, T. Takahashi, T.
Nagai and K. Nakanishi (1998). "Performance of protease as a cleaning
agent for stainless steel surfaces fouled with protein." Journal of
Fermentation and Bioengineering 85(3): 297-301.
Spinifex (2004).
http://www.spinifex-group.com.au/index.htm
16/09/2005
Takehara, A., H. Urano and S. Fukuzaki (2000). "Effect of ozone
pretreatment on alkali cleaning of alumina fouled with Bovine serum
albumin." Journal of Bioscience and Bioengineering 89(3): 267-270.
Trägårdh, G. (1989). "Membrane Cleaning." Desalination 71: 325-335.
Trägårdh, G. and D. Johansson (1998). "Purification of alkaline cleaning
solutions from the dairy industry using membrane separation
technology." Desalination 119: 21-29.
UNEP, Working group for cleaner production in the food industry
(2004). Eco-efficiency toolkit for the Queensland food processing
industry, Australian Industry Group.
Wagner, J. (2001). Membrane filtration handbook - Practical tips and
hints.
http://gewater.com/pdf/1229223-%20Lit-
%20Membrane%20Filtration%20Handbook.pdf
July 2005
Walker, S.P., A. Demirci, R.E. Graves, S. Spencer and R.F. Roberts
(2005). "Response surface modelling for cleaning and disinfecting
materials used in milking systems with electrolysed oxidizing water."
International Journal of Dairy Technology 58(2): 65-73.
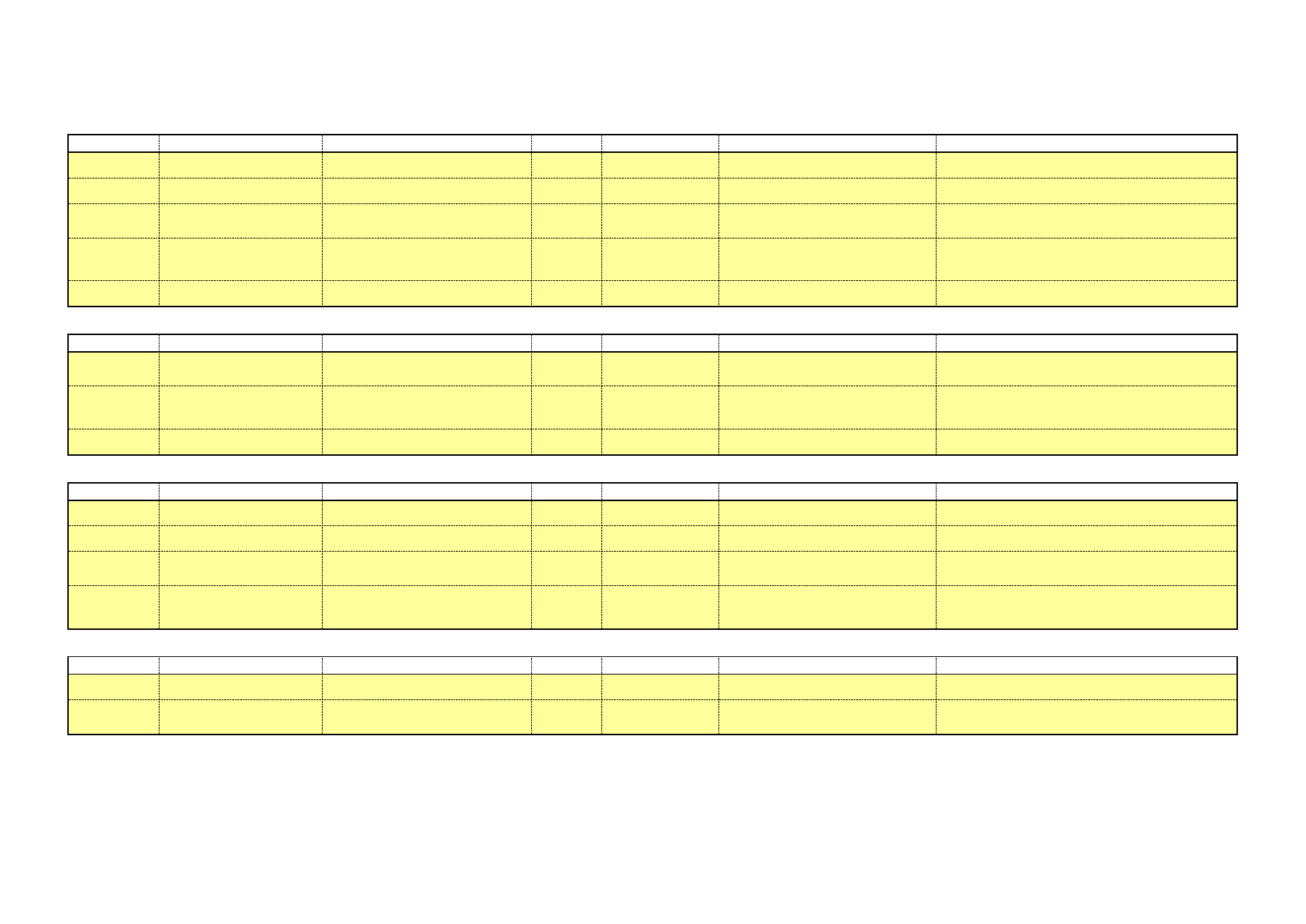
!
" #
$ %
& '
&
(
"
'
!
'
)
)
*+*
,
$
- .
- .
/)
&
0
& *
$
&
!
& & %
123 42 5 6
7 &
7
(
$ 7
3 & $ &
8
& 5 7
&
&
5
(
&
&
& &
&
$7
&
(
& " 0
-
) ")
*+*
2
& $
(
& $
&
*
& $
4
%
$
*
(
%% $ &
&
&
7
&
%
&
& 7 $
& &
9 *&
&
: * %
&
$
(
&
'
)- ");
*+*
1 &$ 1 8
&
(
7 $
<
$
;
*
8
& < $
(+(
- .
$
&
& & < $
) ")
(+( %
"
&
2
3 7 (
3
&
& $
& & 3
& 5
$ &
&
=$
&
>
(
%
%
5
&
: * %
&
7
(
&
%
$
$
$
-
)
)
*+* %
"
&
# 0 .
)
*+* &
$%%
&
1
7
7
& 8
*
&
%
$
%
$
&
& &
&
8
&
=$
& &
%
?
$
$&
'
6
&
7 &
*+* < ;
&
%
&
&
$ %
&
"
$ &
"
:+:
& $
4
&
=$
& 3
3 &8 3 7
3
& &
<
(
&
%
3 7
7 3
7
(
/
3
3 &
&
$
& /
&
%
&
3 & &
& & & &
$ %
&
&
8 &
& )
6
&
7 &
*+* < #
%
)
"
$ &
"
:+:
2
& $
4
&
=$
& 3
3 &8 3 7
3
& &
<
(
&
%
3 7
7 3
7
: * %
&
(
/
3
3 &
&
$
>&(
3 &
$ '"
:
'"
$ %
& '
2
&
$
7
3
* 83
/
&
& *
$77
$
3
3
&
3
& $
$&
@
%
& 3 & &
/ 3 & &
A
$ 3 7
7
$
& =$
& *
&
& <
(
$
*&
3
%
$
&3 7 $
!"#
B
&
'
& &
$ %
&
!(
7 B
& '
!
& C
&
7
& %
&
* %
& & 5 7
$ &
&
D
$
& + &
3
&
%
8
&
E
!
F$
G
F$
& $
$&
G
6
&
G
&
1 8
&
(
&
&
7
H
5
( & < & &
%
7 *
&
&
(
+
&
( &
%$
"
&
& &
(
&
(
&
-
!& &
$ %
&
)
)-
*+*
4
% &
&
%
B
&
)
(+( %
8
&
2
&
&
&
*
&
(
&
!
& C
&
7
&
*
(
& )
' "
$ %
& '
)
)" *+*
&
&
$
)
)
(+( %
7 &
# . 3
$
%
& 5
& + &
< $
)
)
- # .
& *
8
3
$
%
& 5
&
!
&
&
&
4
& + &
3
&
%
8
&
(
%
3
8
& 5
*
!"#
>&( *
&
)"
(+( *
*
&
$
- # . %
&$
!
*
A (
&
&
& (
& &
&
!
& C
&
&
5 $77
$ %
/
8
B&
*
&
& *
$
%
&
&
B
& ,
#
B
&
B
B
&
& &
& *
" #
(
7
&
2
& A &
$ B
&
!7
: (
8+
$
2
H &
!
& C
&
>/
&
& &
7
D
% 3 $
% &
2
7
&
(
&
$
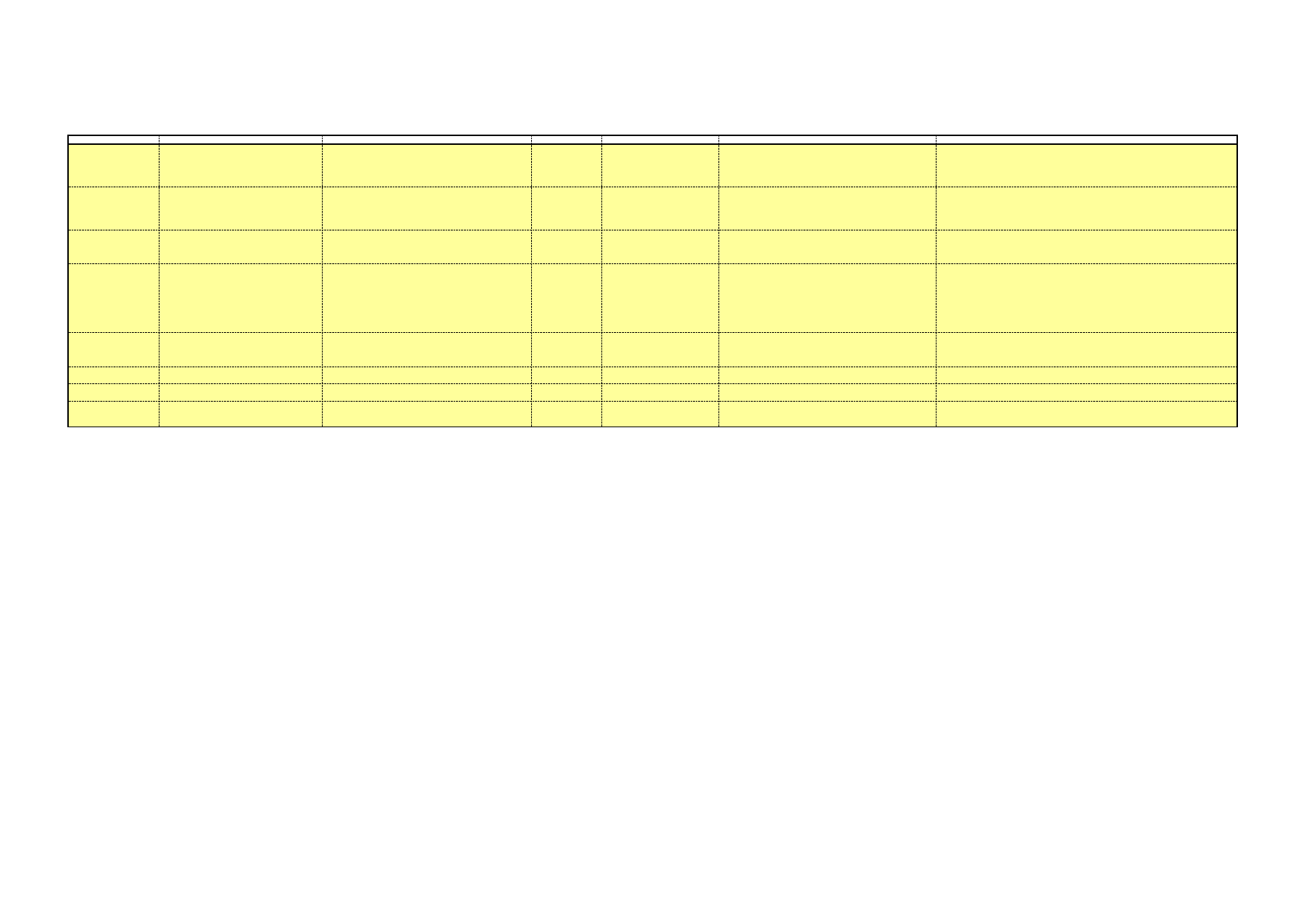
!
"
!
" #
$
%
! &
!
'
'
(
'
' #
'
!
#
#
!
'
!!
(
! &
)
)
!
!
*
#
+
#
#
&
!
# #
,
##
(
%
! &
!
#
#
#
#
#
-
#
)
(
#
&
(
!
(
. #
. #
/ $
(
) 0 12
&*&
2$ 12 &*&
! " #
0 1 3*32
0 $, 3*3
4
(
!
&
#
0 1
3*3
5
# & !
6 !!
(
'
' ! '
#
(
) !
!
!
74
3 &
89 * 9
&
(
!
7
#
4
#
: $
) 0 %$
&*&
21 2
&*&
,
8
' 8 &
8 (
#
'
;
' 4
!
#
5
#
'
#
-
9
!
#
#
# (
!
# #
!!
(
(
#.
#
!
2
)
<
,$
4
#
/:
, %##
=
(
<
%$
( '
!
!!
(
(
#.
>$ $ ?
)
<
%$
(
@
6 ,2 ,2,
) /$21
&*&
, %##
4 !
4
.
#
@
$
9@
(
4 @
4
/$
.
#
@
/1
/$
2, $2,
&*&
8 &
#
8 (
#
#
##
6
#
A B
&
'
(
#
7
!
&
9, # #
(
' (
' #
'
2
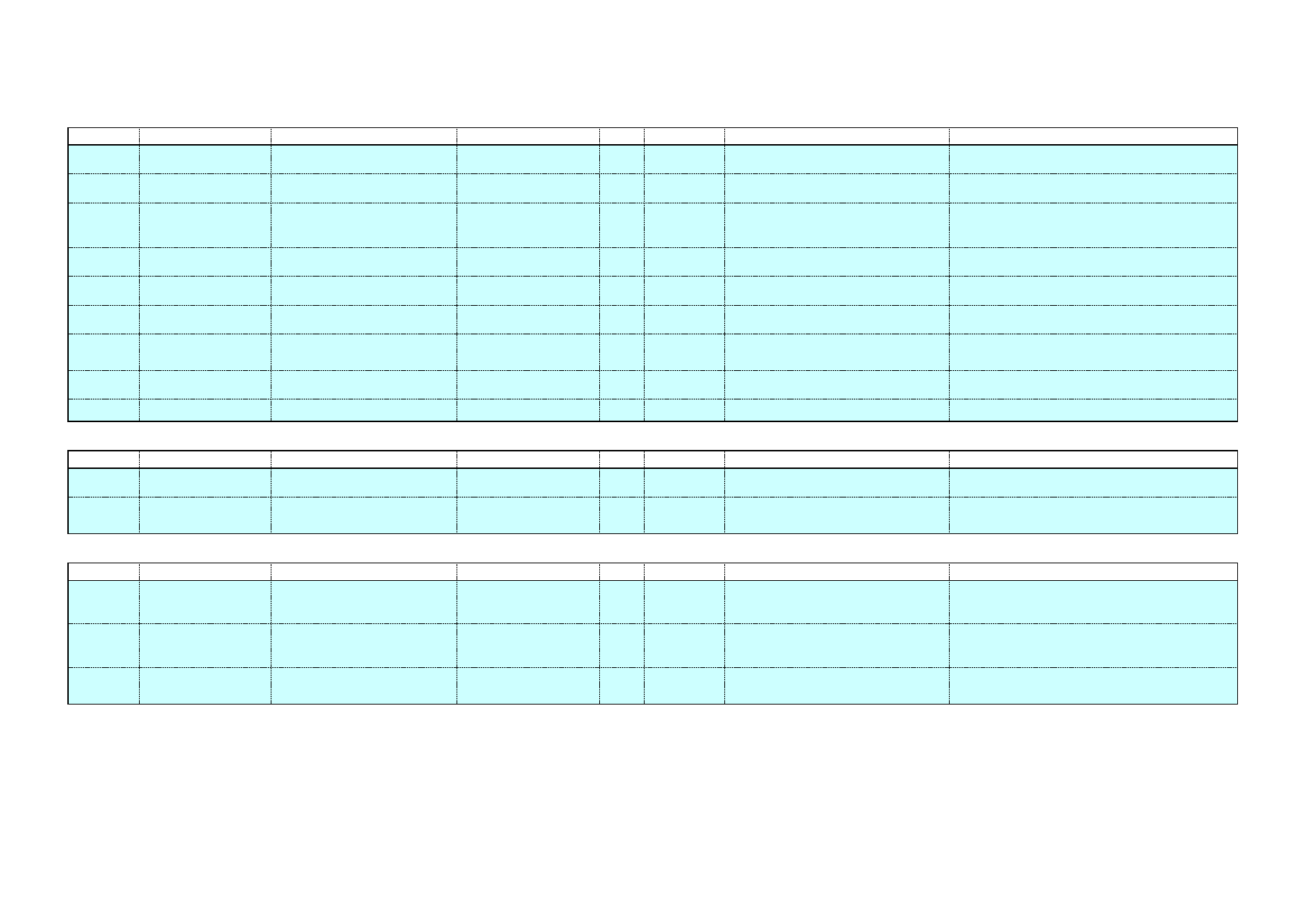
!
" "
! #
$ % $ # "&' '(
!$ )
$(
*)
+,) !$ )
$(
-
("
"( #&
! #
$ !
!
" #$% #
!
&
' (
#
)
*#$+#,&
"#$"
- . ( ' (
# $" #
)
#$%#
/ 0
( ' (
"$"
)
"-
$ +
0
(
1
(
1
.
(
2
((
(
3
4
5
. '
&62 7 (0
(- . 00
(
82
*#$"##
9
! :
;
( "$"#
2- (0-
( ' # +*
( 2
# +$"
!
% * $ *
0
(
1
.
(
<
0
(( .
(
<
.
= 4
. 7
0
(( . > 0
=
-
-
( (
- . ( 7
> 0
3
4
/
( 4
=
.
<
0
(
4
$ 0=
(
.
((
. 7
-
.
3 >
(
( (
(
(
.
/
%#
"+
!
.-
( > (
?
0
.
(( ( 7
( $
! 7
@ *#$ #,&
#$%#
/ 0
( %
! 7
.
0(
%#$
*#
2 (
( (
! 7
*#$ #,&
" $ #
&
# $# *
! 7
*#$ #,&
" $ #
# * $ * A
0
(
1
(
1
.
(
2
((
(
4
-
(
3
4
.- ( > (
'
.
( = 0
-
.
( (
.-
' (
( 0
4
0
(
( .= (
.
5
.
?-
%#$*#
!
;
( %#$*#
-
(
#$"#
# %$"
!
" %+ $ *
0
(( .
&62
( 7
0
(( . > 0
&
.
=
. (-
> 0
-
-
(
(
3
4
&
( ( > (
. .
0
(
-
4
(
(
(
<
2
.
(
-
9
: B
(
-
( 9
: B
CB
5 .- (
( ' "# "
-
D
(
( ' "
-
(
( ' (
.-
$
E
0
(( .
0
0
(( .
<
(
(
= (0
= (
-
( (
= 0
0
(( . ( 4 (-
(
"##
4
.
4
?
(
4
&
(
- F
( .
(
&
(
F
(
<
2
.
(
G
G
-
9
:
#
(
-
( 9
:
#
"A
5 .- (
( '
" 0
"# 0
(
D
(
( '
" 0
. (
0
(
(
( ' ( (
.-
"A $ "A E
4
(
0
(
0
0
(( (
4
. 0
(
<
(
4
(=
0
(= 0
0
(( (= 4
. 0
(
&
4
(
0
(( .
00 .
-
(
(
(- 00 .
( (
&
-
.
<
2
.
(
&
(
F
(
/
%#$*#
"+
!
.-
( > (
# $ #
!
/ 0
( %
! 7
.
0
%#$
*#
2 (
( (
! 7
+#$ #,&
%#
&
# $# *
! 7
*#$ #,&
" $ #
# * $ * A
0
(
4
-
(
/ 0
(= 0 (
( ( 7 -
-
- . (
(
3
4
.- ( > (
'
.
( = 0
-
.
( (
.-
' (
( 0
4
0
(
( .= (
.
5
.
0
2
*#$A#
9
! :
# %$"
!
1
(- . '
(
= ( %
-
(
$
-
7
- (
$-
-
(
" * $ *
<
0
(( .
(
.
0
(( .
(
3
4
-
4
(
-
(
(
H"A% & 0
%#$*#
(
D
(
#$"#
#
!
" #$ #
!
$
3
4
.-
5
. 7
4
(
&62 ( (
(
!
" "
! #
$ % $ # "&' '(
!$ )
$(
*)
+,) !$ )
$(
-
("
"( #&
! #
$ !
&-
F
#
!
( (
-
;
-
4
-
.
(0 ( . .
(
CB ""
# %$#
!
) 9" **$" # ! : 7
#$*#,& 9
4
+#,&:
" + $ A
( 7
(
D
(
<
(
(
H(
. (
(( (
0
(
7
(
(
<
0
(( . > 0
= ( (= 0 0
( 7 -
- . (
2
.
.$
0
.
(
(( (
0
5
/
0
((
. 0
1
0 0
(
/
(
6
5"
%#
0 -
4
-
" *
!
- 4
(=
(
7 -
4
-
# $"
!
# *% $ " A
(-
(- .
3
4
6
(
.
0
(= (
- 7 0
(
(
.
!
" "
! #
$ % $ # "&' '(
!$ )
$(
*)
+,) !$ )
$(
-
("
"( #&
! #
$ !
(- I&
"
!
?
. .
(= 4
. .
(= ( > (
( 7
(0 (
(
# " $ "
!
# # $ # %
<
(
1
.
(
D
(
(
&
(
;
(
(
&62
(0
(- . 00
(
3
4
(
(
(= .
7 .
(
(
5
. 7 -
(
4
(
(
- .-
4
7 - .- 0 ((
<
(
-
$"
- 0 -
$"
&-
$4 (
4
- . .
( $"
2
4
(
% "
!
# $"
!
# " $ # *
1
7 -
4
.
(
(
2
((
H(
0
(
. .
4
#,&
1
. 7 4
.
00
(
(= ( . !(
0
(= 0
(( (=
(
-
. 7
> 0
. ( .
.
0
(
&62
F (
.
0
((
3
4
5
.
- .-
4
.
= (
( (0 (
7 (
0 0
(
(0
(- . 7 &62
;
(
#
0 -
(
%
!
# $" *
! @ #,&
%#$
(
3
-
( ( ( " *
!
3
.- ( ( ( #
# $ #
1
0
(
;
4
(= 4
-
(=
( 7 ((
> 0
7 0 0
0
=
7 .
(
0 0
(
( (
.
. J( 0K
4
. (
(L
( ( 0
-
- (
(
. (
> 0
7
0
0
. 0 0
(
(
-
(
(
.
=
- 4
-
.-
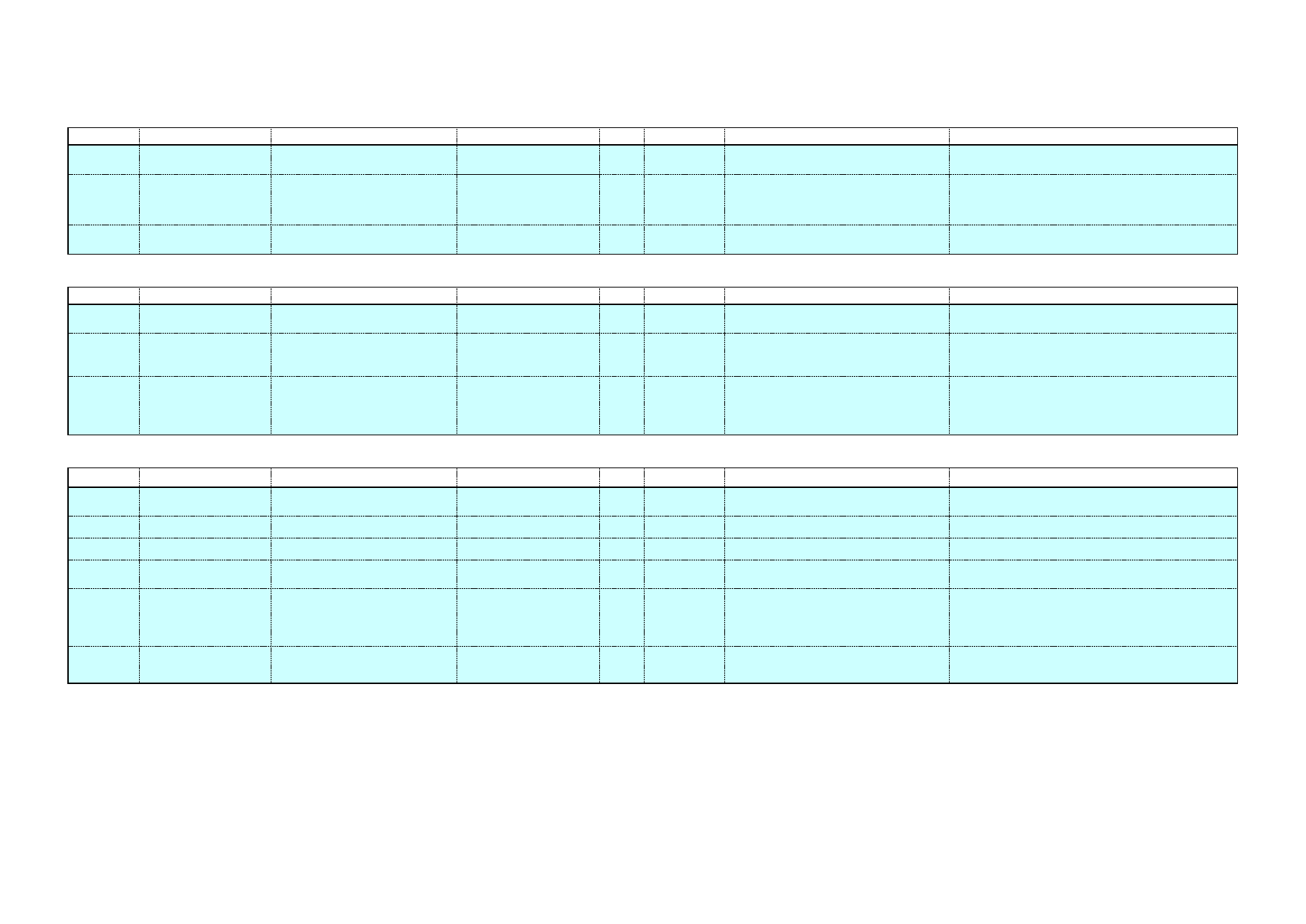
!
"#
#$ %
##
&"
" ' ( '
& " )* * %
' "
+ % '
,+ " -+ ' "
+ % '
.
"%
%
% &) %
&
$$' "
%
##
%
!
"
"
#
!
$ $
!
%
&
&
!
#
!
#
!
'( ) #
!
* + ,* -
"
&
&
# #
.
!
!
/ #
/
/
/ .
0
1
#
&
##
$
!
&
!
2
##
.
#
$
#
!
$
/
&$ &$
#
0##
%
3
4
5
%
!
5
+
6
#
7
#
6
/
/
* +
8
#
#
8
$ !
#
9 $
&&
&&
. 0
.
:
% &
$
&
/
/
&
/
/
.
! $
!
$
!
$
$
% &
.
'(
!
1 &
&
(
#
#
!
!
! /
"#
#$ %
##
&"
"%
%
% &) %
&
$$' "
%
##
%
;
(
$
2
7 ,-
)
-
)
$ & $
* -
*
-
"
:
&
/
/
+
!
.
&
.
#
! $
!
"
!
( #
(
$
2
< -
)
$ & $
< -
)
$
2
< -
($ &$
/ $
.
.
$ ! !
< -
= > *8-
*
* -
*6 *,-
.
#
&&
?
#
8
#
'(
/
$ !
&
!
$
@
&
!
:
.
#
&&
:
#
'(
&
$ ! &&
:
6
#
'( #
&
!
$
:
&
!
#
$
! ?
$ !
5
&&
1
.
02
&
& &
#
!
$ !$
.
1
&
< -
)
$ & $
< -
($ &$
, -
$
.
.
$ ! !
< -
= > 6*6-
*, *8-
*
-
.
#
&&
#
'(
&
$ ! &&
"
.
!
:
.
#
&&
:
#
'(
&
$ ! &&
:
6
#
'( #
&
!
$
'(
&
$ ! &&
$ #
? .
!
:
&
!
#
$
!/
#
#
&
$ ! ? '( # #
3
(
!
$
@
1
.
#
!
$ !$
.
02
&
& &
:
&
.
? (09 .
/
?
!
! /
"#
#$ %
##
&"
"%
%
% &) %
&
$$' "
%
##
%
$
)
(
(
$
2
A -
) #
< -
$
!
< -
) %
<8 -
*8 * -
) #
#
$
59
B
(
5
2
)
(
5
2
>
-
) %
< -
) #
< -
* ,-
&
) #
#
=
&
$
$
! !
?
#
* 8* -
:" ? 1C
.
&
!
&
=
=
& .
$
#
$
3
=
(
5
2
< -
*8 8*6-
"
* &5
.
+
8-
7
#
8 6
/
$ 8 &&
$
&&
!
&&
($
&&
! $
. :" ? @"
.
= )
&
.
5 !$
##
# &
? # .
&/ ? &
.
&
9
.
#
$
. :" ? @"
.
&
)
(
$
2
, -
9
&
$
, -
$
&
&
< -
0 9
-
#
/ &$ &$
/
#
/ &$ &$
< -
* 8* -
*6 *B-
&
! ! #
D
"
!
E !
. &
!
:
&
! ! #
D
&
!
$
&&
#
> 59)9/ $
#
/ $
!
/
! /
2 ! ? !
!
#
F
!
# &&
#
? .
!
/ *!*
!
.
&
&
#
&
1
.
5 !$
##
#
$ !$
# &
?
!
#
!/ )
. #
'(
# $ !$
.
) & %
(
$
2
AG, -
9
$
-
0 9
-
($ &$
< -
* ,* -
*6 8* -
&
! ! #
B
:
&
! ! #
B
&
!
$
&&
5 !$
##
! $
!
?
!
!
!
'(
1
.
#
! ?
. #
'(
# $ !$
.
.
#
$ !
#
/ $
$ !$
%
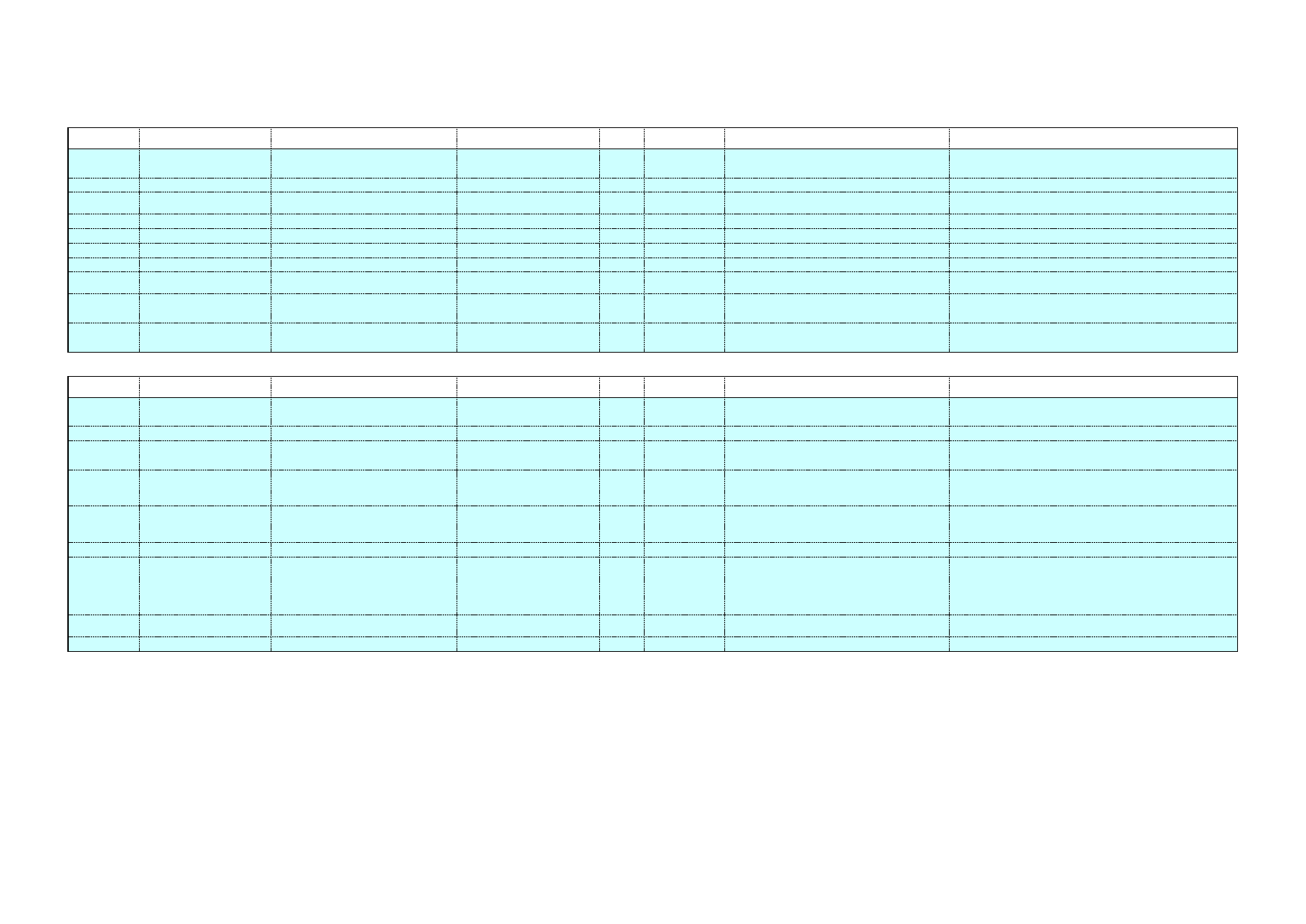
!
"
!
!
! "# !
"
$
!
!
!
"
#
$
%
!
%
&
$
$
' %
%
! %%
# #
#
(
) *+
,
*
-
.
%
/
$
-" 0 12 3
!
/
$
4 5
!
6
!
#
)
7
8
7 9
9
:
; &
<
' !
; *
<
-
$
#
-1 = ,> ?
:
" #
; &
<
; *
<
-
$
#
%
+
,
;#
<
' !
; *
<
-
$
#
'@
:
A
$ *
; &
<
B*
; *
<
-
$
.
1 % . , 7
,
6(
C
' !
; ' 8 +* * < D
E
!
$ .
# #
@ #
'
!
%
; *9
<
'
!
;?* 9 <
'
$
$
;9 *+
<
;
# :<
"
-
&
4
%
!
4
%
# &
4 ! #4
4
&
4
5 4
A
#
!
!
1
$
"
#
F
. ,
,
#
&
.
%
%
E
$ ;D
<
@
$
;D
<
%
% &
!
"
!
!
! "# !
"
$
!
!
+ ? @1E@26 =*
$
$ 4 4+*
.
;B<
, $
.
$
#
@
=*
>
#
E
%
# #
4
$
4
4
!
4 %
4
$
*'
*
!
) *8
%
*
$ .
$
8*
*
$
8
3
%
#
#
A
#
!
%
!
6 *
#
6 *
%
4
$
7*
$ .
@
%
2 #
!
4
*
-
%
$
$
5
5 4
%
#4
#4
$
4
4
!
4
% #
$
"
%
4
$
6 *
4
G2
1
A
#
!
7
,
0 -(@
#H
* 9
'
0
H
* 7
'
4 :
0 @ 5 H
* )
7
!
.
9
# $ $
% '
(
.
5
I
$
4 $
%
!
!
$
$
'
%
0
E
%%
%
6 2(
, 'J* :, 6 1 # K $
5
%
0 !
6 $
!
;
<
3
#
%
#
%
0
&
"
, $
>
3
!
, $
;
< D
>
#
7
(
*
#
+
' 5
5
$
#
$ 7 *
! 5
4
%
#
!
#
#
#
!
>
%
A
#
!
#
#
L
%
%
%
#
!
(
' %
D
@
%
D
'
D
%
"
#
# #
4
%
4
3 5
%
5
1
!
#
!
1
%
#
'
@
%
1
#
! %
%
&
1
!
%
!
6 *$
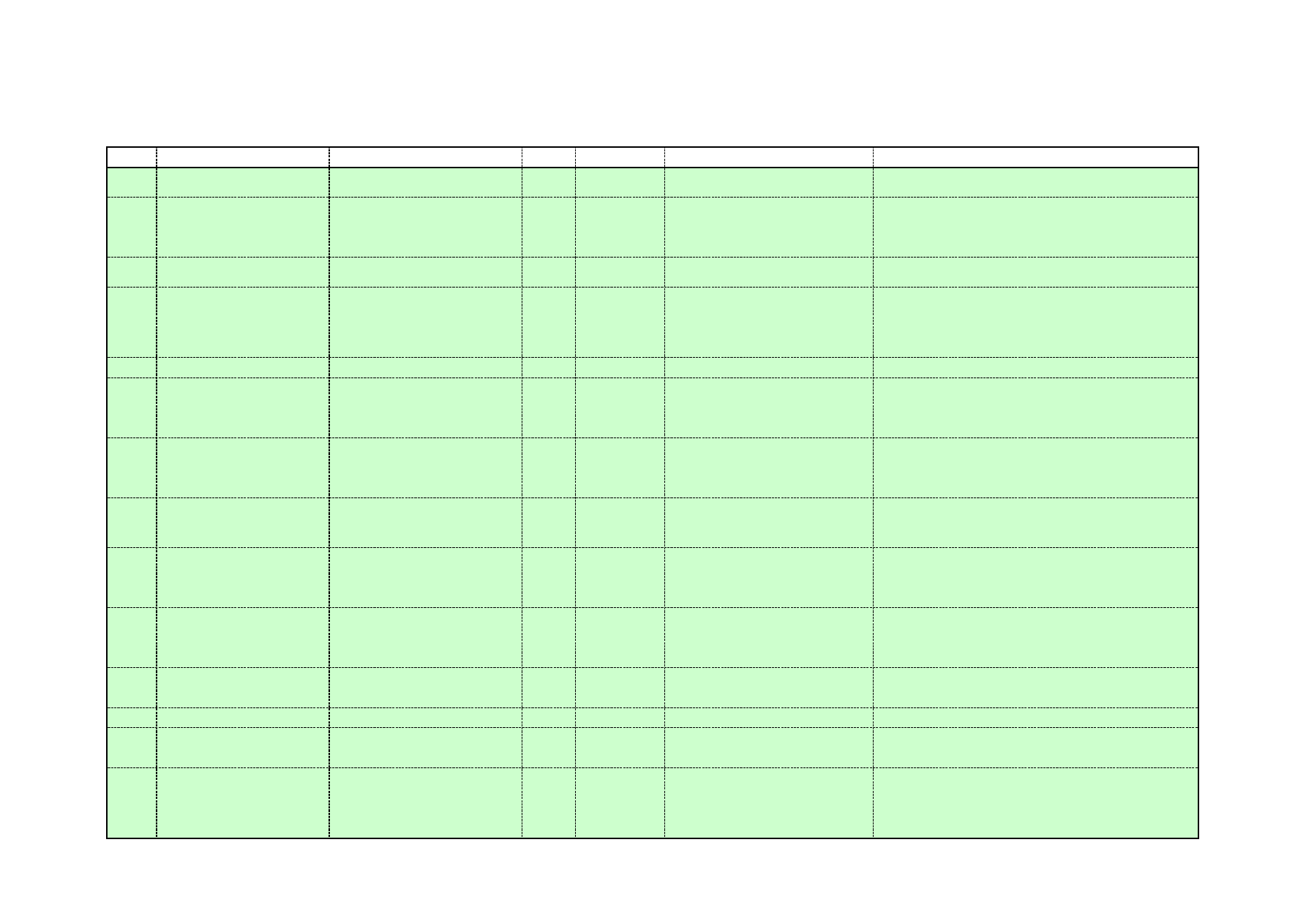
! !"
#
#
#
$%
& '(
)
%
%
#
& '(
*
%
+
#
, %
!
-
%
( %
.
#%
%
/
.
#%
%
/
0
#% %
.
#%
/1
1/
! !"
,
.
#
#
/1
"
#
(23
!
4
5.6.7
/
8
!
7 /1
8
#
!
#
0
6
(23
!
% ,
-
!
9
5.6.
#
(23,
% !
% #
% !
)
#
-
# -
-
: %
*
%
;
- %
# ,
9
--
!
% !
- % 4
0 - %
$ 4
# -
<
/=
<
>
%%
,
-
1
% #
0
!
1/?
8
#
!
#
) -#
@# #
#
!
?'(
8
#
#
9 (23
#
-
9
!
#
*
%
0 !
?
6
#%
> 7
=/ 1
%
A #
;
;
#
"
- 0 !
?
=
#
"
%
A #
11;
;
#
"
- 0 !
?
#
"
(23
*
%
0 ! ?
>
/ B
= /
3 /
-
47
/
! !
'(
(
#
#
'(
"
8
*
%
0 ! 5<
-
@#
,
- % # -
9
#
$
%
9
! !
> 7 B B
/1
! !
*
%
6 @#
9
A
9
% !
%#
#
2%
!
A
!
#
#
#
#
2%
!
A
--
!
- % # -
%
#
% ,
!
9 -- #
0 ! C*
.
#%
%
.
#%
6
#%
<0.$
/
>
/
B/ B ! !"
,
8
#
!
#
0
#
)
-
9
!
#
! -
9 (23
*
%
6#
-
% !
-
#%
" 9
#%
% 4
"
5
--
!
% !
% # , -#
9
!
6
-
, %
!
- %
0 ! 3
4
, %
-
/
/
# -
/
/ ? ! !"
#
#
#
& /B '(
4
#
@# %
0
3
-
)
%
#
& /B '(
*
%
+
#
, %
!
-
%
0 !
$00
$ 4
:
6# -
$
6# -#
$
6# -# 0
/
)
/
%
0 !
B
>8 9 *D % %
)
/
%
0 !
)8 9 C8 % %
0
8
#
!
#
C8, )8, >8
*D % %
0
-
%
5
--
!
0 !
-
C8 % %
,
)8 % %
9
%
#
)8 % %
(
7
#
0 !
#
%
!
% ! 9
- % % %
,
#
#%
-
9
!
%
>
%
< %
-
9
@#
"
#
#
#
/1
! !"7
1 / ? ! !
1"
4
%
7
;
- < %
!
1; -
#
#
0
8
#
C
#
;
%
#%
9 -
,
% 4
,
9
(23 #
,
,
#
,
%
9
% 4
4
6
- % 4
-
D
(23
!
<
% ! - %
# -
* #
#%
,
#
,
% 9 -- #
! #%
<
/
! !
#
#
(23
#
/B '(
0
% 4
% !
* % !
-
#%
;
- %, $ !
- %
D
!
2
(23
- %
6 -
7 >
-
#
8 %
0
(
#
%
#
- % ?/B '(
*
%
(
#
!
- %
6
(23
( %
-
%
,
,
/- %
,
!
%# -
E
#
% 4
A
/
= '(/
B '(
5 !
#
#
A
/
& '(/
B '(
0
,
,
!
, - #
#
)
4
, -
%
,
#
@# %
,
#
<
% !
F
#
-
#
#
* /#
#
>
-
-
4
6
5$
(
- # -
, #
$
4 6
5$
1 4
-
#
(
@# %
, -
4 , (23
#
* % !
%
4
<%# -
-
<
-
#
#
0
;
- %
;
#
* #
%
%
#
Wyszukiwarka
Podobne podstrony:
Cleaning In Place Technology(1)
Cleaning In Place Systems
Cleaning in Place
making tea in place experiences of women engaged in a japanese tea ceremony
Cleaning In Place Technology(1)
Laurence M Janifer Agent in Place
[7] Core Strength Variation of In Place Concrete tcm77 1305833
Jerry Olton Abandon in Place
Cleaning In Place Technology(1)
Applications and opportunities for ultrasound assisted extraction in the food industry — A review
Resuscitation Current?vances in intraosseous infusion – A systematic review
Clean Well Lighted Place by Hemingway
Grzegorz Ziółkowski Review of MEN IN BLACK
[Mises org]Raico,Ralph The Place of Religion In The Liberal Philosophy of Constant, Toqueville,
więcej podobnych podstron