IRON
1
Iron
Fe
[7439-89-6]
Fe
(MW 55.85)
InChI = 1/Fe
InChIKey = XEEYBQQBJWHFJM-UHFFFAOYAG
(efficient catalyst for a wide variety of organic transformations)
Physical Data:
mp 1535
◦
C; d 7.860 g cm
−
3
.
Solubility:
insol water, alkalis, alcohols, ethers; sol acid (attacked
or dissolved).
Form Supplied in:
chip, foil, filings, powder, wire.
Analysis of Reagent Purity:
atomic absorption methods can be
used.
Handling, Storage, and Precaution:
moisture sensitive.
General. Iron is used to catalyze a wide variety of organic
transformations. The nature of the iron catalyst depends on the re-
action conditions (solvent, cocatalysts, etc.) employed for a spe-
cific transformation. The reaction descriptions included in this
entry will be limited to those utilizing iron powder and to some
reactions in which iron(0) is generated in situ, serving as the active
catalyst during the reaction.
Iron Powder.
Formation of Lactones. The lactonization of γ-bromo-α,β-
unsaturated carboxylic methyl esters and acids is promoted by
heating the substrate in the presence of iron powder (eq 1).
Iron apparently facilitates the elimination of methyl bromide and
rotation about the double bond.
1
R
1
CO
2
R
R
2
Br
O
O
R
1
R
2
iron powder
120–150 °C
(1)
~40–66%
Preparation of Nonconjugated Dienes.
Nonconjugated
dienes are conveniently prepared from allylic halides via an iron-
promoted coupling reaction in DMF (Table 1).
2
Excellent yields
are obtained in many cases and some catalytic effect of added
halide salts is also observed. The iron salts in the DMF residue
were analyzed as 70% Fe
2
+ and 30% Fe
3
+ (via oxidation of Fe
2
+).
Similar types of reaction are observed with a variety of transition
metal catalysts, including Pd- and Ni-based catalysts.
Preparation of Ketones from Carboxylic Acids. Carboxylic
acids react with hydrogen-reduced iron powder to give an iron
dicarboxylate intermediate which, upon thermolysis, decomposes
to give the condensed ketone, CO
2
, and FeO (eq 2).
3
CO
2
H
CO
2
2
+
Fe
2
+
H
2
(2)
O
thermolysis
60–75%
+
FeO
+
CO
2
Fe
Table 1
Coupling of allyl chlorides with iron in DMF (150
◦
C, 1 h)
Conversion
Crude
Allyl chloride
(%)
Product
yield (%)
Allyl chloride
65
1,5-Hexadiene
61.5
β
-Methallyl chloride
77
2,5-Dimethyl-1,5-
hexadiene
60
Allyl chloride +β-
methallyl chloride
(1:1)
98
1,5-Hexadiene
21
2-Methyl-1,5-
hexadiene
44.8
2,5-Dimethyl-1,5-
hexadiene
24.1
Reduction. The reduction of nitroaromatics to anilines with
iron powder is carried out in a mixture of methanol and concen-
trated Hydrochloric Acid (eq 3). In the absence of methanol, only
partial reduction is observed.
4
(3)
Cl
CN
NO
2
Cl
CN
NH
2
Fe
MeOH, HCl
89%
The reduction of optically active nitroalkanes to active amines
is accomplished with iron in Acetic Acid. The reaction proceeds
with ≥82% optical purity. Other reducing agents, such as Lithium
Aluminum Hydride, gave a completely racemic mixture of
amines.
5
Iron Cluster Compounds.
Gif Catalyst–Air Oxidation of Saturated Hydrocarbons. The
Gif catalyst, Fe
2
FeO(OAc)
6
py
35
, is a triiron cluster compound
that is generated from the reaction of iron dust, acetic acid, and
pyridine. The use of this and a reducing agent in acetic acid and
pyridine promotes the air oxidation of saturated hydrocarbons. It
is a selective reagent in that least hindered, secondary positions
are preferentially attacked, leading to the formation of ketones. It
does not epoxidize simple alkenes.
The Gif catalyst is but one of a number of (µ
3
-oxo) triiron com-
plexes, some of which have been used as a catalyst for the epox-
idation of alkenic alcohol acetates with molecular oxygen (see
µ
µ
µ
3-Oxohexakis(µ
µ
µ
-trimethylacetato)tris(methanol)triiron(III)
Chloride).
6
Iron(0) Catalysts: Reduction of Fe
III
Compounds.
Deprotonation of Aldehydes and Ketones: Preparation of
Silyl Dienol Ethers.
Grignard reagents have been shown
7
to
reduce Fe
III
compounds to air sensitive Fe
0
and it is this re-
duced species that is responsible for catalyzing a number of
useful organic reactions.
8
For example, aldehydes and ketones
are smoothly converted to the corresponding, thermodynami-
cally favored, trimethylsilyl enol ethers upon treatment with Fe
0
,
Chlorotrimethylsilane, and Triethylamine (eq 4). When a sto-
ichiometric amount of Fe
0
catalyst is reacted (no additional
Grignard reagent) with cycloalkenones, the exocyclic through-
conjugated dienol ether is predominantly formed.
9
When a stoi-
chiometric amount of Grignard reagent is reacted with the cyclo-
hexenone in the presence of a catalytic amount of Iron(III) Chlo-
Avoid Skin Contact with All Reagents
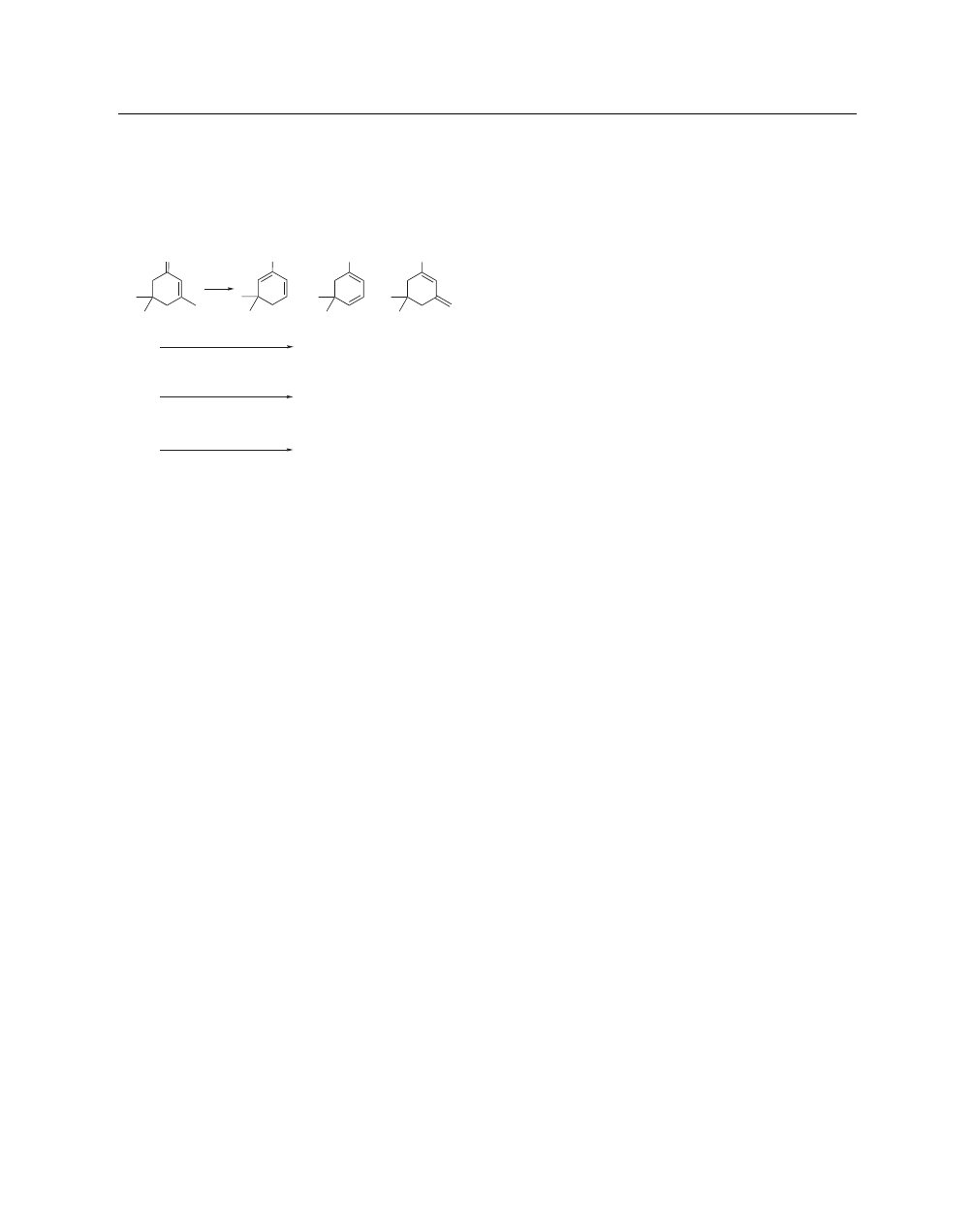
2
IRON
ride (Kharasch reagent),
10
the endocyclic through-conjugated
dienol ether is formed. Likewise, when 0.5–1 equiv of Fe
0
catalyst and 1 equiv of Grignard reagent is reacted with the
same cyclohexenone (oxygen excluded), the endocyclic through-
conjugated dienol ether is again the predominant dienol ether
formed.
(4)
O
OTMS
OTMS
OTMS
+
+
1. stoic. Fe
0
, no MeMgBr
1. MeMgBr
+
cat. FeCl
3
1. stoic. Fe
0
+
MeMgBr
2:96:2
7:1:92
2:96:2
2. TMSCl, Et
3
N
2. TMSCl, Et
3
N
2. TMSCl, Et
3
N
Iron(0) compounds have been proposed to be the active catalyst
in a number of other organic reactions. For example, cross cou-
pling reactions between Grignard reagents and alkyl, vinyl, allyl,
and phenyl halides are catalyzed by various iron(III) complexes,
such as Tris(dibenzoylmethide)iron(III).
11
It is proposed that the
reaction between the Grignard reagent and the iron(III) compound
produces the ‘active’ iron(0) catalyst.
1.
Loffler, A.; Norris, F.; Taub, W.; Svanholt, K. L.; Dreiding, A. S., Helv.
Chim. Acta 1970
, 53, 403.
2.
Hall, D. W.; Hurley, E., Jr., Can. J. Chem. 1969, 47, 1238.
3.
(a) Davis, R.; Granito, C.; Schultz, H. P., Org. Synth. 1967, 47, 75.
(b) Davis, R.; Schultz, H. P., J. Org. Chem. 1962, 27, 854.
4.
Koopman, H., Recl. Trav. Chim. Pays-Bas 1961, 80, 1075.
5.
Kornblum, N.; Fishbein, L., J. Am. Chem. Soc. 1955, 77, 6266.
6.
Ito, S.; Inoue, K.; Mastumoto, M., J. Am. Chem. Soc. 1982, 104, 6450.
7.
Krafft, M. E.; Holton, R. A., J. Org. Chem. 1984, 49, 3669 and references
therein.
8.
(a) Felkin, H.; Swierczewski, G., Tetrahedron 1975, 2735. (b) Tamura,
M.; Kochi, J. K., Synthesis 1971, 303. (c) Karasch, M. S.; Lambert, F.
L.; Urry, W. H., J. Org. Chem. 1945, 10, 292, 298. (d) Corey, E. J.;
Yamamoto, H.; Herron, D. K.; Achiwa, K., J. Am. Chem. Soc. 1970,
92
, 6635. (e) Corey, E. J.; Posner, G. H., Tetrahedron Lett. 1970, 315.
(f) Ashby, E. C., Pure Appl. Chem. 1980, 52, 545.
9.
(a) Krafft, M. E.; Holton, R. A., J. Am. Chem. Soc. 1984, 106, 7619.
(b) Also see Ref. 7.
10.
Kharasch, M. S.; Tawney, P. O., J. Am. Chem. Soc. 1941, 63, 2308; 1945,
67
, 128.
11.
(a) Kochi, J. K.; Neumann, S. M., J. Org. Chem. 1975, 40, 599.
(b) Molander, G. A.; Rahn, B. J.; Shubert, D. C.; Bonde, S. E.,
Tetrahedron Lett. 1983
, 24, 5449. (c) Fiandanese, V.; Miccoli, G.; Naso,
F.; Ronzini, L., J. Org. Chem. 1991, 56, 4112. (d) , J.-L.; Julia, M.;
Verpeaux, J.-N., Bull. Soc. Chem. Fr. 1985, 5, 772. (e) Grichey, H.;
Wilkins, G. W. Jr., Tetrahedron Lett. 1976, 723. (f) Molander, G. A.;
Etter, J. B., Tetrahedron Lett. 1984, 25, 3281. (g) Kochi, J. K.; Smith,
R. S., J. Org. Chem. 1976, 41, 502.
Mark W. Zettler
The Dow Chemical Company, Midland, MI, USA
A list of General Abbreviations appears on the front Endpapers
Wyszukiwarka
Podobne podstrony:
iron III chloride eros ri054
iron II chloride eros ri055
benzyl chloride eros rb050
Iron Maiden Power Slave
hydrobromic acid eros rh031
chloroform eros rc105
magnesium eros rm001
oxalyl chloride eros ro015
Iron Kingdoms Lock & Load Errata
potassium permanganate eros rp244
peracetic acid eros rp034
p toluenesulfonic acid eros rt134
hexamethylenetetramine eros rh019
copper II chloride eros rc214
glyoxylic acid eros rg009
Nano zero Vilent Iron
więcej podobnych podstron