
Genistein, EGCG, and capsaicin inhibit adipocyte differentiation
process via activating AMP-activated protein kinase
Jin-Taek Hwang
a
, In-Ja Park
b
, Jang-In Shin
b
, Yun Kyoung Lee
b
, Seong Kyu Lee
c
,
Haing Woon Baik
c
, Joohun Ha
a
, Ock Jin Park
b,*
a
Department of Biochemistry and Molecular Biology, Medical Research Center for Bioreaction to Reactive Oxygen Species,
Kyung Hee University College of Medicine, Seoul 130-701, Republic of Korea
b
Department of Food and Nutrition, Hannam University, Daejeon 306-791, Republic of Korea
c
Department of Biochemistry, College of Medicine, Eulji University, Daejeon 301-832, Republic of Korea
Received 20 September 2005
Available online 11 October 2005
Abstract
Phytochemicals such as soy isoflavone genistein have been reported to possess therapeutic effects for obesity, diabetes, and cardiovas-
cular diseases. In the present study, the molecular basis of selective phytochemicals with emphasis on their ability to control intracellular
signaling cascades of AMP-activated kinase (AMPK) responsible for the inhibition of adipogenesis was investigated. Recently, the
evolutionarily conserved serine/threonine kinase, AMPK, emerges as a possible target molecule of anti-obesity. Hypothalamic AMPK
was found to integrate nutritional and hormonal signals modulating feeding behavior and energy expenditure. We have investigated the
effects of genistein, EGCG, and capsaicin on adipocyte differentiation in relation to AMPK activation in 3T3-L1 cells. Genistein
(20–200 lM) significantly inhibited the process of adipocyte differentiation and led to apoptosis of mature adipocytes. Genistein, EGCG,
and capsaicin stimulated the intracellular ROS release, which activated AMPK rapidly. We suggest that AMPK is a novel and critical
component of both inhibition of adipocyte differentiation and apoptosis of mature adipocytes by genistein or EGCG or capsaicin further
implying AMPK as a prime target of obesity control.
Ó 2005 Elsevier Inc. All rights reserved.
Keywords: AMP-activated protein kinase; Obesity; Genistein; EGCG; Capsaicin; Apoptosis
Obesity is a complex multifactorial chronic disease that
increases the risk for developing hypertension, type 2 dia-
betes, and coronary heart disease, and remains a major
health obstacle in the industrialized world. Obesity arises
from the imbalance between energy intake and energy
expenditure that may lead to a pathologic growth of adipo-
cytes. It is known that the amount of adipose tissue can be
regulated by the inhibition of adipogenesis from precursor
cells as well as the control of adipocyte size. Obesity is in-
duced by the hypertrophy of adipocytes and to the recruit-
ment of new adipocytes from precursor cells and these two
processes are dependent on the regulation of adipocyte dif-
ferentiation
Genistein, a soybean derived bioactive polyphenol, has
been the subject of numerous researches as a chemothera-
peutic agent
. This phytoestrogen has been implicated
in cancer control primarily because of its strong anti-prolif-
erative and apoptotic potential
. Also, it has been report-
ed that genistein exhibits anti-adipogenic effects in several
adipocytes, although its precise mechanism of action is
not known
.
We have investigated the molecular events leading to the
inhibition of adipogenesis by genistein, specially focusing
on the role of AMP-activated protein kinase (AMPK).
AMPK represents a metabolite-sensing protein kinase that
shares amino acid sequence homology with yeast SNF1
.
AMPK is known to play a major role in energy homeostasis
by coordinating a number of adaptive responses in ATP-de-
pleting metabolic states such as ischemia/reperfusion,
0006-291X/$ - see front matter
Ó 2005 Elsevier Inc. All rights reserved.
doi:10.1016/j.bbrc.2005.09.195
*
Corresponding author. Fax: +82 42 629 7490.
E-mail address:
(O.J. Park).
www.elsevier.com/locate/ybbrc
Biochemical and Biophysical Research Communications 338 (2005) 694–699
BBRC

hypoxia, heat shock, oxidative stress, and exercise
AMPK is sensitively regulated by allosteric binding of
AMP under pathological or physiological conditions of
ATP depletion
. The persistent activation of AMPK
showed to be connected to p53-dependent cellular senes-
cence suggesting its role as an intrinsic regulator of the cell
cycle in mammalian cells
. Moreover, AMPK cascades
have emerged as novel targets for the treatment of obesity
and type 2 diabetes
. AMPK is known to be activated
with 5-amino-imidazole-4-carboxamide riboside (AICAR),
which is converted to a nucleotide that mimics the effect of
AMP, and the long-term treatment with AICAR has pre-
vented the development of diabetes in animal models
Also the pro-apoptotic potential of the activated AMPK
was observed in the AMPK over-expressed conditions of
various cells
We have hypothesized that genistein mediates the inhibi-
tion of adipocyte differentiation and induces apoptosis of
mature adipocyte through the activation of AMPK signal-
ing. Our results show that genistein activates AMPK,
blocks adipocyte differentiation comparable to AICAR,
and induces apoptosis of adipocytes through the genera-
tion of ROS.
Materials and methods
Cell culture and reagents. The 3T3-L1 pre-adipocyte was purchased
from ATCC (Gaithersburg, MD). Cells were cultured in DulbeccoÕs
modified EagleÕs medium (DMEM) containing 10% FBS under normoxic
conditions (20% O
2,
5% CO
2
, and 75% N
2
) in a CO
2
incubator at 37
°C.
Insulin was obtained from Eli Lilly (Indianapolis, IN, USA). IBMX and
dexamethasone were purchased from Sigma (St. Louis, MO, USA).
Hoechst 33342 and AICAR (5-aminoimidazole-4-carboxamide-ribose)
were also purchased from Sigma. The anti-phospho-specific antibodies
that recognize phosphorylated ACC-Ser
79
and AMPK antibodies were
from Cell Signaling Technology. Antibodies for b-actin were purchased
from Santa Cruz Biotechnology (Santa Cruz, CA).
Adipocyte differentiation. The cells were plated in 6-well plates, and at
day 0 (usually 2 days after the cells had achieved confluence), and adi-
pocyte differentiation was induced with hormone cocktail containing 1 lM
dexamethasone, 5 lg/ml insulin, and 0.5 mM IBMX for 2 days. After 2
days, the medium was changed with the regular medium, and at day 8, the
cells were treated with various stimuli.
Protein extract and Western blotting. Cells were rinsed twice with ice-
cold PBS and scraped with lysis buffer (50 mM Tris–HCl, pH 7.4, 1% NP-
40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM
PMSF, 1 mM sodium orthovanadate, 1 mM NaF, 1 lg/ml aprotinin,
1 lg/ml leupeptin, and 1 lg/ml pepstatin) and subjected to Western blot
analysis.
Oil-Red O staining. On day 8 of adipocyte differentiation induction, the
cells were stained with Oil-Red O dye. The cells were fixed with 70%
ethanol and dehydrated with 100% propylene glycol. The cells were
stained with Oil-Red O and with HarriÕs hematoxylin. Fat droplets in
adipocytes were stained red.
Chromatin staining with Hoechst 33342. Apoptosis was observed by
chromatin staining with Hoechst 33342, as previously described
.
Cells were incubated with each stimulus. Termination of incubation,
the supernatant was discarded and cells were fixed with 3.5% form-
aldehyde in PBS for 30min at room temperature, washed four times
with PBS, and exposed to Hoechst 33342 (10 lM) for 30 min at room
temperature. Stained cell preparations were examined under ultraviolet
illumination with a fluorescence microscope (Olympus Optical, Tokyo,
Japan).
Cell proliferation by MTT assay. Cells were seeded on 96-well micro-
plates at 4000 cells/well and incubated with each test compound for the
indicated time period. Supernatant was discarded and then cells were
incubated with 100 ll MTT solution (2 mg/ml MTT in PBS) for 4 h.
Absorbance was measured using an autoreader (Spectra Max 360,
Molecular Device, Minnesota, USA).
Results
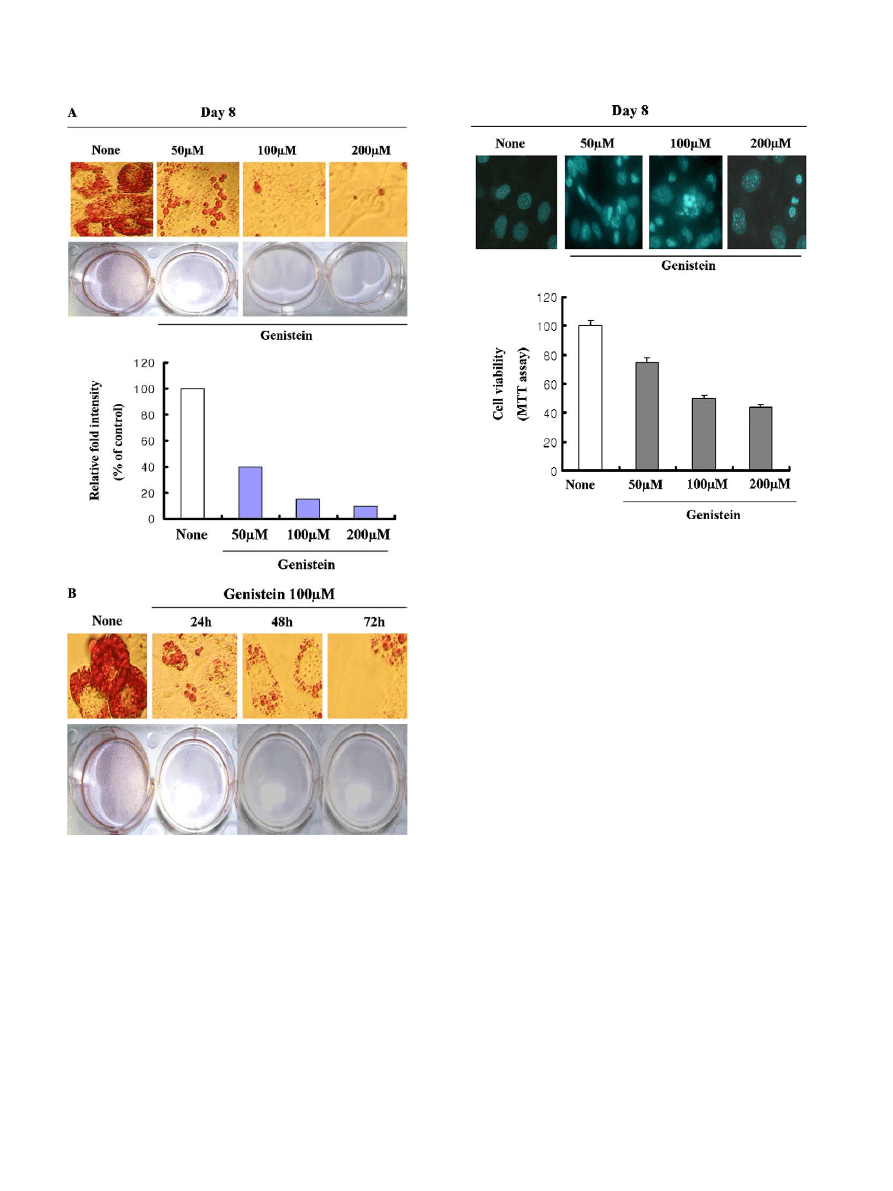
Genistein inhibits adipocyte differentiation
We first examined anti-obesity potential of genistein by
determining pre-adipocyte differentiation into adipocytes.
Cultured 3T3-L1 adipocytes were exposed to genistein at
different doses (at day 0), and cell differentiation was
performed with a differentiation medium. At day 8, differ-
entiations were terminated and fat drops were detected by
Oil-Red O staining. As shown
A, treatment of 3T3-
L1 cells with genistein markedly inhibited adipocyte differ-
entiation dose-dependently, and genistein (100 lM) also
abrogated adipocyte differentiation in a time-dependent
manner. These results indicated that genistein may have
been efficiently blocking adipocyte differentiation and have
potential of anti-obesity effects in 3T3-L1 cells.
Genistein also induces apoptosis of mature adipocyte
Several reports indicated that certain naturally occur-
ring compounds have been shown to promote loss of body
fat by inducing apoptosis
. Thus, inducing apoptosis of
mature adipocytes can be important for the treatment of
obesity with the naturally occurring compounds. There-
fore, we next examined the apoptotic possibility of geni-
stein in mature adipocyte. 3T3-L1 cells were fully
differentiated at day 8, and matured adipocyte was exposed
to genistein for indicated concentrations. After stimulation,
cell apoptosis was detected by either MTT assay or Hoe-
chst33342 dye. These results indicated that genistein effec-
tively induced apoptosis in mature 3T3-L1 adipocyte
(
A and B).
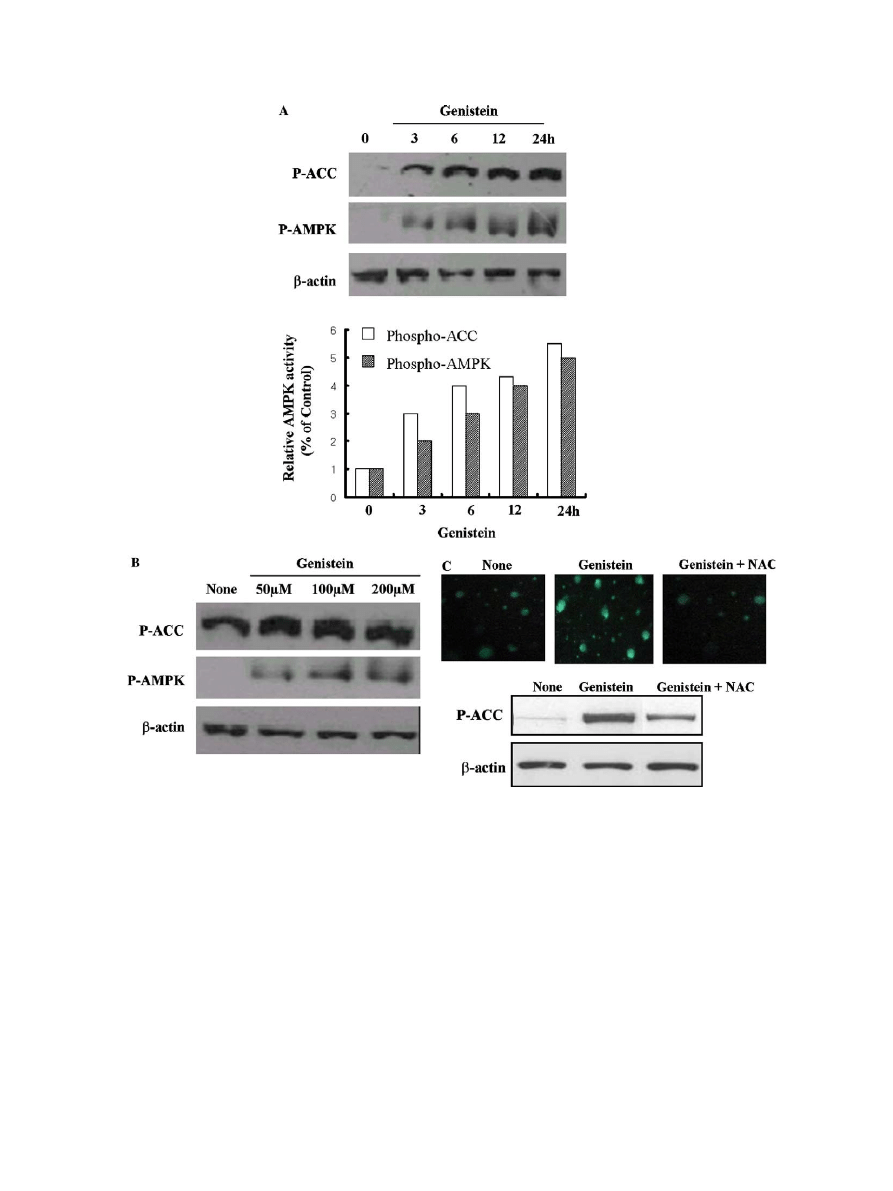
Genistein significantly activates AMP-activated protein
kinase in 3T3-L1 cells via ROS generation
Recent report shows that hypoxic suppressions of adipo-
genesis are associated with AMPK activation and can in-
duce the failure of mitotic clonal expansion at the early
phase of adipogenesis
. We next tested whether there is
AMPK activation in the inhibitory process of adipocyte dif-
ferentiation by treating genistein. 3T3-L1 cells were cultured
with normal medium and then exposed to genistein
(100 lM) and differentiation medium for the indicated time
periods. AMPK activation and its substrate acetyl-CoA car-
boxylase (ACC) phosphorylation were detected by Western
blot analysis. As shown in
A, AMPK phosphorylation
increased 2.4-fold in a time-dependent manner, and its sub-
strates, ACC-Ser
79
phosphorylation showed enhancement.
Also AMPK and ACC were significantly activated by
J.-T. Hwang et al. / Biochemical and Biophysical Research Communications 338 (2005) 694–699
695
genistein in a concentration-dependent manner (
B).
One of the AMPK activation mechanisms was suspected
to be ROS, since it was recently reported that various ther-
apeutic effects of natural occurring compounds involve re-
lease of ROS
. We tested the activation of AMPK via
ROS release in inhibition of genistein-inhibited adipocyte
differentiation. As shown in
C, genistein significantly
induced ROS generation, which led to AMPK activation,
and these effects were abolished by NAC (5 mM) treatment.
These results indicate that ROS is necessary for the AMPK
activation in the inhibitory process of adipocyte differentia-
tion by genistein in 3T3-L1 cells.
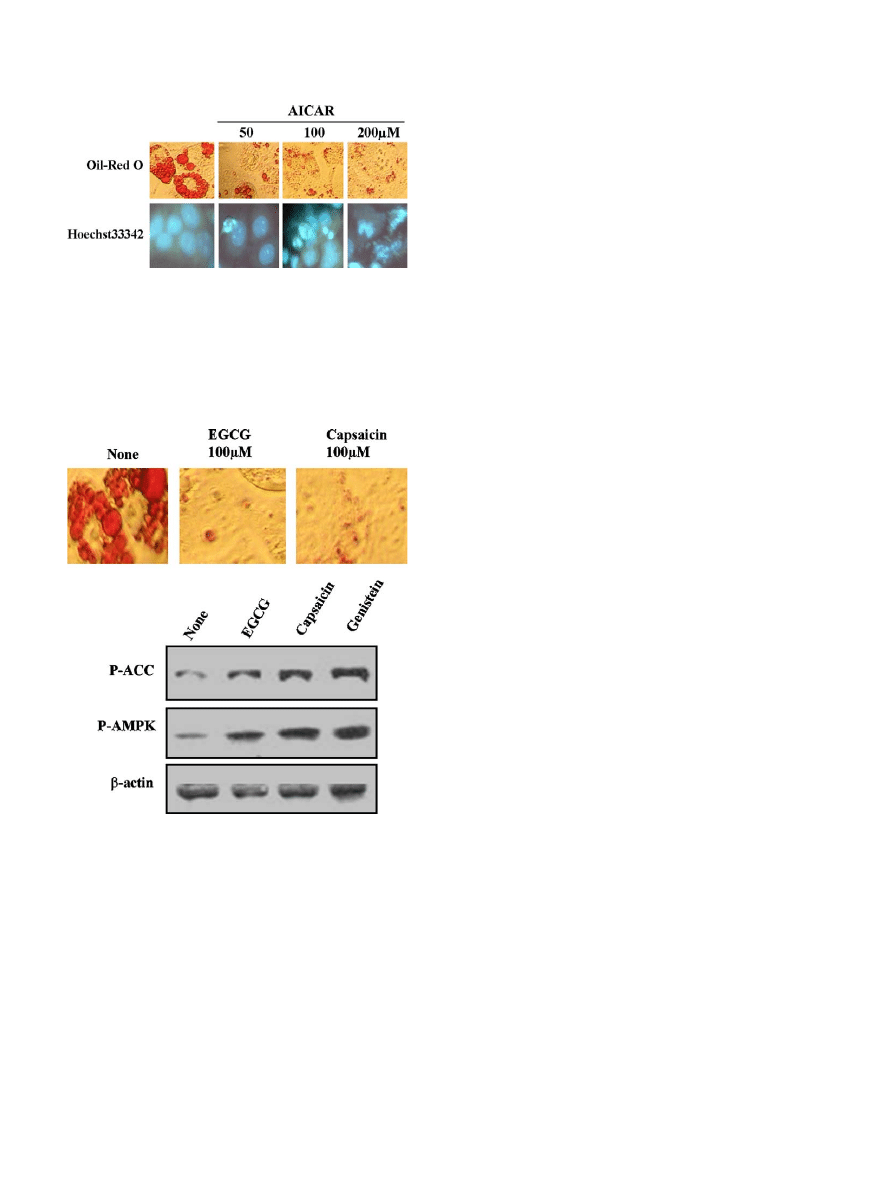
AICAR, an AMPK activator, also inhibits adipocyte
differentiation and induces apoptosis of mature adipocyte
To evaluate the involvement of AMPK in lipogenesis
accurately, we next tested the effects of AMPK activation
with AICAR (an AMPK activator) on adipocyte differen-
tiation. Treatment with 500 lM–2 mM AICAR significant-
ly phosphorylated either AMPK or ACC, and at the same
time, adipocyte differentiations were inhibited (
These results indicate that AMPK plays a critical role in
adipogenesis and is essential for the blocking process of
adipocyte differentiation blocking.
Other naturally occurring compounds also activate AMPK
and inhibit adipocyte differentiation
Several reported suggest that several naturally occurring
compounds have potential of anti-obesity effects, and
therefore we tested the effects of EGCG or capsaicin on
AMPK activation as well as adipocyte differentiation pro-
cess. 3T3-L1 pre-adipocyte was pretreated with EGCG or
capsaicin and then immediately incubated with differentia-
tion medium. After each time period, AMPK activation
Fig. 1. Effects of genistein on adipocyte differentiation in 3T3-L1 cells.
Confluent 3T3-L1 cells were exposed to differentiation cocktail (1 lM
dexamethasone, 5 lg/ml insulin, and 0.5 mM IBMX) for 2 days, and cells
were changed into normal DMEM in the presence or absence of genistein
for the indicated concentrations (A) or cells exposed to genistein for the
indicated time period (B). These cells were fixed with 3.5% formaldehyde
in PBS and fat drops were stained by Oil-Red O dye staining.
Fig. 2. Genistein also induced apoptosis of mature 3T3-L1 cells. Cells
were fully differentiated at day 8 and exposed to genistein for indicated
concentrations. After inducing apoptosis by genistein, apoptosis was
detected either by MTT assay or Hoechst33342 dye.
696
J.-T. Hwang et al. / Biochemical and Biophysical Research Communications 338 (2005) 694–699
and adipocyte differentiation were detected either with
Western blot or Oil-Red O staining. As shown
, either
EGCG or capsaicin can activate AMPK and also inhibit
adipocyte differentiation in 3T3-L1 cells. These results
strongly indicate that AMPK activation is necessary for
inhibition effect of adipocyte differentiation by EGCG
and capsaicin.
Discussion
A variety of naturally occurring flavonoids have been
found to possess beneficial effects on health, and these com-
pounds have drawn attention because of their relative safe-
ness and accumulated evidence of anti-obesity and anti-
diabetic effects in animals and humans
. We report
the evidence that genistein exerts the inhibition of adipo-
cyte differentiation and the induction of adipocyte apopto-
sis through the activation of AMPK paralleled with the
generation of ROS. We also confirmed that green tea pol-
yphenol EGCG and red pepper polyphenol capsaicin
blocked the adipocyte differentiation in 3T3-L1 adipocyte
cultures. The anti-proliferatory and lipolytic effects of these
phytochemicals have been attributed to their ability to
modulate various signaling pathways, specially, the control
Fig. 3. The effects of AMPK activation by genistein. 3T3-L1 cells were fully differentiated by differentiation cocktail, cells were exposed to genistein
(100 lM) for the indicated time periods (A) or various concentrations, respectively, (B) and AMPK activation and its substrate acetyl-CoA carboxylase
(ACC) phosphorylation were detected by Western blot analysis. Also differentiated cells were exposed to genistein for 12 h in the presence or absence of
NAC (5 mM). After additional incubation for 30 min in the presence of 10 lM DCFH-DA the changes in fluorescence intensity were measured by
fluorescence-activated cell scanning analysis. Under the same conditions, the phosphorylation level of ACC-Ser
79
(P-ACC) were examined (C).
J.-T. Hwang et al. / Biochemical and Biophysical Research Communications 338 (2005) 694–699
697
of cell proliferation and survival
. However, the precise
target of their anti-proliferatory effect has remained unre-
solved. Here, we introduce AMPK as a possible main tar-
get of these phytochemicals in their anti-obesity activity.
AMPK is activated by various stimuli including exercise,
heat shock, and ROS
. Furthermore, activated AMPK
blocks anabolic pathways and promotes catabolic path-
way, and thus activation of AMPK is linked to inhibition
of cell proliferation and apoptosis
. Genistein,
EGCG, and capsaicin activated AMPK in a dose-depen-
dent manner. The mechanism by which affects AMPK reg-
ulation with physiological stimuli or anti-obesity agents
might present a promising target for the development of
strategies for the treatment of obesity. AMPK cascades
have been postulated to respond to the intracellular level
of AMP or AMP:ATP ratio
and to be highly sensitive
to the oxidative stress. ROS have been suggested to be up-
stream molecules of AMPK activated signals. We suggest
that the generation of ROS generated by the phytochemi-
cals is one of the responsible elements for the activation
of AMP kinase.
The exact mechanism to stimulate pre-adipocyte mito-
sis and differentiation in vivo remains exclusive. Howev-
er, it is proposed that hypertrophy of fat cells grown
beyond a certain size might propagate to differentiate
by sending specific signals
. Adipocyte inducers stim-
ulate pre-adipocytes to undergo mitotic clonal expansion
before transcriptional activation of adipocyte genes be-
fore anchoring adipocyte phenotypes. The balance be-
tween positive and negative signals of adipogenesis
determines the fate of differentiation of pre-adipocytes.
It is not clear from the present study whether the activa-
tion of AMPK is mediated by one of the negative signals
or acting directly on adipocyte differentiation as a nega-
tive signal.
We have tested whether AICAR has similar effect on
adipocyte differentiation and AMPK activation in compar-
ison with genistein. Both AICAR and genistein similarly
blocked the differentiation and the early clonal expansion
of pre-adipocytes. The present study strongly suggests that
the activation of AMPK is necessary for the inhibition of
adipogenesis in 3T3-L1 cells by phytochemicals such as
genistein, EGCG, and capsaicin, and AMPK as a primary
target of adipogenesis control.
References
[1] I. Shimomura, R.E. Hammer, J.A. Richardson, S. Ikemoto, Y.
Bashmakov, J.L. Goldstein, M.S. Brown, Insulin resistance and
diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in
adipose tissue: model for congenital generalized lipodystrophy, Genes
Dev. 12 (1998) 3182–3194.
[2] F.H. Sarkar, Y. Li, Soy isoflavones and cancer prevention, Cancer
Invest. 21 (2003) 744–757.
[3] M.J. Messina, C.L. Loprinzi, Soy for breast cancer survivors: a
critical review of the literature, J. Nutr. 131 (2001) 3095S–3108S.
[4] K. Szkudelska, L. Nogowski, T. Szkudelski, Genistein affects
lipogenesis and lipolysis in isolated rat adipocytes, J. Steroid
Biochem. Mol. Biol. 75 (4-5) (2000) 265–271.
[5] D.G Hardie, D. Carling, M. Carlson, The AMP-activated/SNF1
protein kinase subfamily: metabolic sensors of the eukaryotic cell?
Annu. Rev. Biochem. 67 (1998) 821–855.
[6] B.E. Kemp, D. Stapleton, D.J. Campbell, Z.P. Chen, S. Murthy, M.
Walter, A. Gupta, J.J. Adams, F. Katsis, B. Van Denderen, I.G.
Jennings, T. Iseli, B.J. Michell, L.A. Witters, AMP-activated protein
kinase, super metabolic regulator, Biochem. Soc. Trans. 31 (2003)
162–168.
[7] R.J. Shaw, M. Kosmatka, N. Bardeesy, R.L. Hurley, L.A. Witters,
R.A. DePinho, L.C. Cantley, The tumor suppressor LKB1 kinase
directly activates AMP-activated kinase and regulates apoptosis in
Fig. 5. Similar effects of capsaicin and EGCG on AMPK activation and
adipocyte differentiation interference. 3T3-L1 pre-adipocyte were pre-
treated with EGCG or capsaicin (100 lM) for 30 min and rapidly
incubated with differentiation medium. After each time period, AMPK
activation and adipocyte differentiation were detected with either Western
blot analysis or Oil-Red O staining.
Fig. 4. Effects of AICAR on adipocyte differentiation and apoptosis of
mature adipocytes. Confluent 3T3-L1 cells were exposed to differentiation
cocktail (1 lM dexamethasone, 5 lg/ml insulin, and 0.5 mM IBMX) for 2
days, and cells were changed into normal DMEM in the presence or
absence of AICAR for the indicated concentrations (A). These cells were
fixed with 3.5% formaldehyde in PBS and fat drops were stained by Oil-
Red O dye staining or apoptosis was detected with Hoechst33342 dye.
698
J.-T. Hwang et al. / Biochemical and Biophysical Research Communications 338 (2005) 694–699

response to energy stress, Proc. Natl. Acad. Sci. USA 101 (10) (2004)
3329–3335.
[8] R.G. Jones, D.R. Plas, S. Kubek, M. Buzzai, J. Mu, Y. Xu, M.J.
Birnbaum, C.B. Thompson, AMP-activated protein kinase induces a
p53-dependent metabolic checkpoint, Mol. Cell 18 (3) (2005) 283–
293.
[9] Z. Luo, A.K. Saha, X. Xiang, N.B. Ruderman, AMPK, the metabolic
syndrome and cancer, Trends Pharmacol. Sci. 26 (2) (2005) 69–76.
[10] X.M. Song, M. Fiedler, D. Galuska, J.W. Ryder, M. Fernstrom, A.V.
Chibalin, H. Wallberg-Henriksson, J.R. Zierath, 5-Aminoimidazole-
4-carboxamide ribonucleoside treatment improves glucose homeosta-
sis in insulin-resistant diabetic (ob/ob) mice, Diabetologia 45 (1)
(2002) 56–65.
[11] D. Meisse, M. Van de Casteele, C. Beauloye, I. Hainault, B.A. Kefas,
M.H. Rider, F. Foufelle, L. Hue, Sustained activation of AMP-
activated protein kinase induces c-Jun N-terminal kinase activation
and apoptosis in liver cells, FEBS Lett. 526 (1-3) (2002) 38–42.
[12] A. Carriere, Y. Fernandez, M. Rigoulet, L. Penicaud, L. Casteilla,
Inhibition of pre-adipocyte proliferation by mitochondrial reactive
oxygen species, FEBS Lett. 550 (1–3) (2003) 163–167.
[13] K.H. Kim, M.J. Song, J. Chung, H. Park, J.B. Kim, Hypoxia inhibits
adipocyte differentiation in a HDAC-independent manner, Biochem.
Biophys. Res. Commun. 333 (4) (2005) 1178–1184.
[14] S. Qanungo, M. Das, S. Haldar, A. Basu, Epigallocatechin-3-gallate
induces mitochondrial membrane depolarization and caspase-depen-
dent apoptosis in pancreatic cancer cells, Carcinogenesis 26 (5) (2005)
958–967.
[15] S.J. Bhathena, M.T. Velasquez, Beneficial role of dietary phytoestro-
gens in obesity and diabetes, Am. J. Clin. Nutr. 76 (6) (2002) 1191–1201.
[16] T. Murase, A. Nagasawa, J. Suzuki, T. Hase, I. Tokimitsu, Beneficial
effects of tea catechins on diet-induced obesity: stimulation of lipid
catabolism in the liver, Int. J. Obes. Relat. Metab. Disord. 26 (11)
(2002) 1459–1464.
[17] S. Horman, G. Browne, U. Krause, J. Patel, D. Vertommen, L.
Bertrand, A. Lavoinne, L. Hue, C. Proud, M. Rider, Activation of
AMP-activated protein kinase leads to the phosphorylation of
elongation factor 2 and an inhibition of protein synthesis, Curr. Biol.
12 (16) (2002) 1419–1423.
[18] G.J. Browne, S.G. Finn, C.G. Proud, Stimulation of the AMP-
activated protein kinase leads to activation of eukaryotic elongation
factor 2 kinase and to its phosphorylation at a novel site, serine 398, J.
Biol. Chem. 279 (13) (2004) 12220–12231.
[19] J.E. Jung, J. Lee, J. Ha, S.S. Kim, Y.H. Cho, H.H. Baik, I. Kang, 5-
Aminoimidazole-4-carboxamide-ribonucleoside enhances oxidative
stress-induced apoptosis through activation of nuclear factor-kappaB
in mouse Neuro 2a neuroblastoma cells, Neurosci. Lett. 354 (3) (2004)
197–200.
[20] A. Sorisky, From pre-adipocyte to adipocyte: differentiation-directed
signals of insulin from the cell surface to the nucleus, Crit. Rev. Clin.
Lab. Sci. 36 (1) (1999) 1–34.
[21] J.T. Hwang, J. Ha, O.J. Park, Combination of 5-fluorouracil and
genistein induces apoptosis synergistically in chemo-resistant cancer
cells through the modulation of AMPK and Cox-2 signaling
pathways, Biochem. Biophys. Res. Commun. 332 (2) (2005) 433–440.
J.-T. Hwang et al. / Biochemical and Biophysical Research Communications 338 (2005) 694–699
699
Document Outline
- Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase
- Materials and methods
- Results
- Genistein inhibits adipocyte differentiation
- Genistein also induces apoptosis of mature adipocyte
- Genistein significantly activates AMP-activated protein kinase in 3T3-L1 cells via ROS generation
- AICAR, an AMPK activator, also inhibits adipocyte differentiation and induces apoptosis of mature adipocyte
- Other naturally occurring compounds also activate AMPK and inhibit adipocyte differentiation
- Discussion
- References
Wyszukiwarka
Podobne podstrony:
1 s2 0 S0006291X07005785 main
1 s2 0 S0006291X11011752 main
1 s2 0 S0020025512001946 main
1 s2 0 S0378382002000085 main
1 s2 0 S0304397599001000 main
1 s2 0 S0040603111000104 main 2
1 s2 0 S0944501312001358 main
1 s2 0 S0166218X96000583 main
1 s2 0 S0005273614000303 main
1 s2 0 S0304397502001342 main
1 s2 0 S0377221798003622 main (1)
1 s2 0 S0022169496031423 main
1 s2 0 S1046592814002101 main
1 s2 0 0166218X93E0153P main
1 s2 0 S0022000006001474 main
1 s2 0 S000925099800520X main
1 s2 0 S0022283610008843 main
1 s2 0 S0960852409006385 main
więcej podobnych podstron