
Amygdalin mediates relieved atherosclerosis in apolipoprotein E deficient mice
through the induction of regulatory T cells
Deng Jiagang
, Chunyang Li
, Hailian Wang
, Erwei Hao
, Zhengcai Du
, Chuanhong Bao
, Jianzhen Lv
,
Yi Wang
,
a
Guangxi Traditional Chinese Medical University, Nanning, Guangxi 530001, China
b
Institute of Organ Transplantation, Sichuan Academy of Medical Science/Sichuan Provincial People’s Hospital, Chengdu, Sichuan 610072, China
c
School of Life Science, Sichuan University, Chengdu, Sichuan 610064, China
a r t i c l e
i n f o
Article history:
Received 7 June 2011
Available online 2 July 2011
Keywords:
Atherosclerosis
Amygdalin
Regulatory T cells
Foxp3
Transforming growth factor-beta
Interleukine-10
a b s t r a c t
Objective: Regulatory T cells (Tregs) play a critical role in the regulation of T cell-mediated immune
responses in atherosclerosis, a chronic autoimmune-like disease. Therefore, in this study, we aimed to
investigate the therapeutic effect of amygdalin on atherosclerosis of apolipoprotein E deficient
(ApoE
/
) mice, and to explore its immune regulatory function by stimulation of Tregs. Methods and
results: To evaluate the anti-atherosclerotic effect of amygdalin and for in vivo Treg expansion/activation
analysis, ApoE
/
mice received intraperitoneal injections of amygdalin, and this therapy resulted in a
comparatively 2-fold decrease in triglyceride (TG), 1.5-fold decrease in total cholesterol (TC) and low den-
sity lipoprotein (LDL). By comparing the vessel areas, lumen areas, plaque areas, and aortic plaque cov-
erage percentage, the effects of amygdalin on pre-existing lesions were assessed. Studies on IL-10 and
TGF-b indicated that mice treated with amygdalin had increased expression of Treg-related cytokines.
Meanwhile, flow cytometry and real-time PCR data showed that mice treated with amygdalin had higher
percentage of CD4
+
CD25
+
Foxp3
+
T cells than untreated mice and increased expression of forkhead box P3
(FOXP3) gene. Conclusion: Our data showed amygdalin could attenuate the development of atherosclero-
sis by suppressing inflammatory responses and promoting the immunomodulation function of Tregs. The
effects of amygdalin ultimately resulted in the enlarged lumen area and the loss of atherosclerotic plaque.
All these data indicated the therapeutic potential of amygdalin in preventing and/or treating of
atherosclerosis.
Ó 2011 Elsevier Inc. All rights reserved.
1. Introduction
Atherosclerosis is an inflammatory disease of the arterial wall.
Hypercholesterolemia, smoking, male gender, hypertension, diabe-
tes and age were traditionally considered as risk factors. However,
recent research with accumulating evidence suggests that the in-
nate and adaptive immune responses play a critical role in the
pathogenesis of atherosclerosis
. In this multifactorial process,
endothelial cells, macrophages, smooth muscle cells and lympho-
cytes are involved and interacted. As a result of their interaction,
atherosclerotic plaque is formed, that influences on organ perfu-
sion and ultimately results in cerebrovascular events and acute
coronary syndromes. It has been shown that cytokine product of
CD4
+
T cells (IFN-
c
, IL-10, IL-4, etc.) influence the extent and nature
of the atherosclerotic plaque. Thus studies on the immune system
in atherosclerosis have received considerable interest in recent
years, especially on naturally Tregs. Previous studies have shown
that Th1 cells were detrimental to the atherosclerotic process
, and Th2 cells or natural killer T cells were also contributed
to the development of atherosclerosis
. Two major counter reg-
ulatory cytokines as IL-10
and transforming growth factor-
beta (TGF-b)
are required for the immunoregulatory functions
of natural or adaptive antigen induced Tregs, which play an impor-
tant role in the progression of atherosclerosis
. Moreover, it
was found that transcriptional regulator forkhead winged helix
transcription Foxp3 controlled mouse CD4
+
CD25
+
Treg function
. It is well known that Tregs play a protective role in the
progression of atherosclerosis
and they are considered a ther-
apeutic target for the treatment of atherosclerosis. Therefore, in or-
der to determine whether amygdalin treatment attenuate plaque
progression and alleviate the symptom of atherosclerosis, we fo-
cused our study on the immune regulatory function of amygdalin.
Amygdalin (vitamin B17, also called Laetrile), is extracted
from Semen Persicae, the seed of Prunus persica (L.) Batsch.
Amygdalin is also abundant in the seeds of apricots, almonds, pea-
ches and other rosaceous plants. Besides the antitumor activity
0006-291X/$ - see front matter Ó 2011 Elsevier Inc. All rights reserved.
doi:
⇑
Corresponding authors. Address: School of Life Science, Sichuan University,
Wuhou District, Chengdu, Sichuan 610064, China. Fax: +86 28 8541 5171.
E-mail addresses:
(J. Lv),
(Y. Wang).
Biochemical and Biophysical Research Communications 411 (2011) 523–529
Contents lists available at
Biochemical and Biophysical Research Communications
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / y b b r c

, amygdalin has also been used for the treatment of asth-
ma, bronchitis, emphysema, leprosy and diabetes
. Semen
Persicae is a major component of Xuefu Zhuyu decoction, which
could relief the symptom of atherosclerosis according to Tradi-
tional Chinese Medicine (TCM) theory and clinical practice. Be-
cause amygdalin is the major component of Xuefu Zhuyu
decoction, we focused our study on whether amygdalin has anti-
atherosclerotic effect in vivo. Could amygdalin therapy decrease
TG, TC, and LDL levels of ApoE
/
mice? In particular, as amygdalin
may stimulate the immune system and has some immune modu-
lator function
, does the function of amygdalin rely on Tregs?
Studies have shown coronary atherosclerosis was associated
with coronary constriction during cold pressor stimulation
. Carrageenan induced inflammation contributes to the
progression of atherosclerosis too. In TCM, cold pressor is also
called cold factor, while carrageenan induced inflammation is
called hot factor. After we established atherosclerotic models by
either cold factor
or hot factor, we delivered amygdalin
to these mice in order to investigate its anti-atherosclerosis effect.
We hypothesized that studies of amygdalin on the immune modu-
latory perspective shed light on unraveling the pharmacological
function of amygdalin in atherosclerosis.
2. Materials and methods
2.1. Animals
ApoE
/
mice on a C57BL/6J background
and their wild-
type littermates were purchased from Jackson Laboratories and
kept in 12 h light/dark cycle with free access to water and food,
which is in accordance with IVC requirement in Sichuan Univer-
sity. Animal handling was in accordance with Ethics Committee
of Sichuan University.
2.2. Study design
Amygdalin was purchased from Changsha Staherb Natural
Ingredients Co., Ltd.
Eight-week-old male mice were divided into six groups:
C57 control group: Eight-week-old male C57BL/6J mice were
given a standard laboratory diet (10% fat, 15% protein and 75%
carbohydrate).
ApoE
/
control group: Eight-week-old male ApoE
/
mice were
given High Fat Diet (HFD, containing 40% fat, 14% protein and
46% carbohydrate) for 12 weeks.
Cold control group: Eight-week-old male ApoE
/
mice were
given HFD for 12 weeks. Everyday mice were frozen at
20 °C
for 2 h.
Cold + amygdalin group: Atherosclerotic mice were established
as cold control group. Amygdalin (1 mg/kg) was intraperitone-
ally injected.
Hot control group: Eight-week-old male ApoE
/
mice were
given HFD for 12 weeks. Carrageenan (5 mL/kg, Sigma) was
intraperitoneally injected.
Hot + amygdalin group: Atherosclerotic mice were established as
hot control group. Amygdalin (1 mg/kg) was intraperitoneally
injected.
All groups of mice were sacrificed after 4 weeks of drug
delivery.
2.3. Lipid profile and cytokine measurements
For measuring cholesterol content, blood samples were col-
lected at the beginning and end of amygdalin treatment. Total plas-
ma cholesterol and triglyceride levels were determined by
commercial kits (Applygen, Beijing, China). Murine TGF-b and
IL-10 levels were assayed by ELISA kits using paired antibodies
according to the manufacturer’s instructions (R&D Systems,
Minneapolis, Minn.).
2.4. Assessment of aortic sinus atherosclerosis
Hearts and upper sections of the aorta were removed and fixed,
embedded and sectioned. Atherosclerotic lesions were quantified
by calculating the lesion size in the aortic sinus as previous de-
scribed
. Sections were stained with hematoxylin and eosin,
and vessel areas were measured by ImageJ software (NIH) from
images obtained with Nikon 80i microscope. For comparisons of
plaque areas between amygdalin groups and control groups, 100
and 200
l
m distant sites were used.
2.5. Immunohistochemistry (IHC)
Serial paraffin sections at 100 and 200
l
m distant of aortic sinus
(n = 6 mice of each group) were used for IHC staining. TUNEL (ter-
minal deoxynucleotidyl transferase dUTP nick end labeling) kit
(Roche) was applied to assess the apoptotic percentage. Slides
were counterstained by DAPI (sigma). At least four sections per
mouse were examined for each immunostaining and appropriate
negative controls were used. Measurements were carried out
blinded by two assessors.
2.6. Western blotting analysis of Foxp3
T cells from spleen were isolated from mice in each group. Wes-
tern blotting was performed with rabbit anti-Foxp3 (polyclone
antibody, Abcam) at a dilution of 1:1000. A secondary antibody,
peroxidase-conjugated anti-rabbit IgG (Jackson Laboratories), was
applied. The levels of b-actin served as the loading control.
2.7. Real-time PCR analysis
Total RNA from splenocytes and lymphocyte cells was isolated
using Trizol reagent (Invitrogen). We chose the oligonucleotide
primers and probe previously described
. The real-time PCR
was performed on an ABI 7900 using Taqman Universal PCR maser
mix (TAKARA) in triplicates. C
T
for GAPDH was used to normalize
the samples.
2.8. Cell separation and flow cytometry
For fluorescence-activated cell sorting (FACS) analysis, mouse
regulatory T cell staining kit (Becton–Dickinson) was used. Fluores-
cein isothiocyanate-labeled rat IgG2a isotype (eBioscience) and
phycoerythrin-labeled rat IgG1 isotype (eBioscience) were used.
Stained cells were analyzed on a FACScan flow cytometer with
CellQuest software (Becton–Dickinson). The analysis gate was set
on the forward and side scatters to eliminate cell debris and dead
cells.
2.9. Statistical analysis
The effects of treatment on lesion areas, plaque compositions,
IL-10 and TGF-b levels, mRNA levels and TUNEL percentages were
calculated by ANOVA and Bonferroni/Dunn’s test or Student’s t
test. A value of p < 0.05 was considered to be statistically
significant.
524
D. Jiagang et al. / Biochemical and Biophysical Research Communications 411 (2011) 523–529

3. Results
3.1. Decreased blood lipid levels induced by amygdalin
Judged from blood lipid changes (TG and TC), atherosclerosis in
aortic sinus increased with the prolonged dietary of HFD in
ApoE
/
mice. However, situation was exacerbated when mice
experienced with either cold factor or hot factor. TG increased
7- to 8-fold compared with normal control fed with normal diet
(
). The increase of TC was about 9- to 10-fold. In order to as-
sess the anti-atherosclerotic effect of amygdalin, TG and TC levels
were compared between drug delivery groups and corresponding
control groups. We found that mice treated with amygdalin had
decreased TG and TC levels (p < 0.05). Meanwhile, amygdalin treat-
ment could also decrease LDL-c levels (p < 0.05). But cold factor
and hot factor had no influence on high density lipoprotein choles-
terol (HDL-c) compared with ApoE
/
mice (p > 0.05). Thus the
treatment of amygdalin did not induce decreased HDL-c level
(p > 0.05).
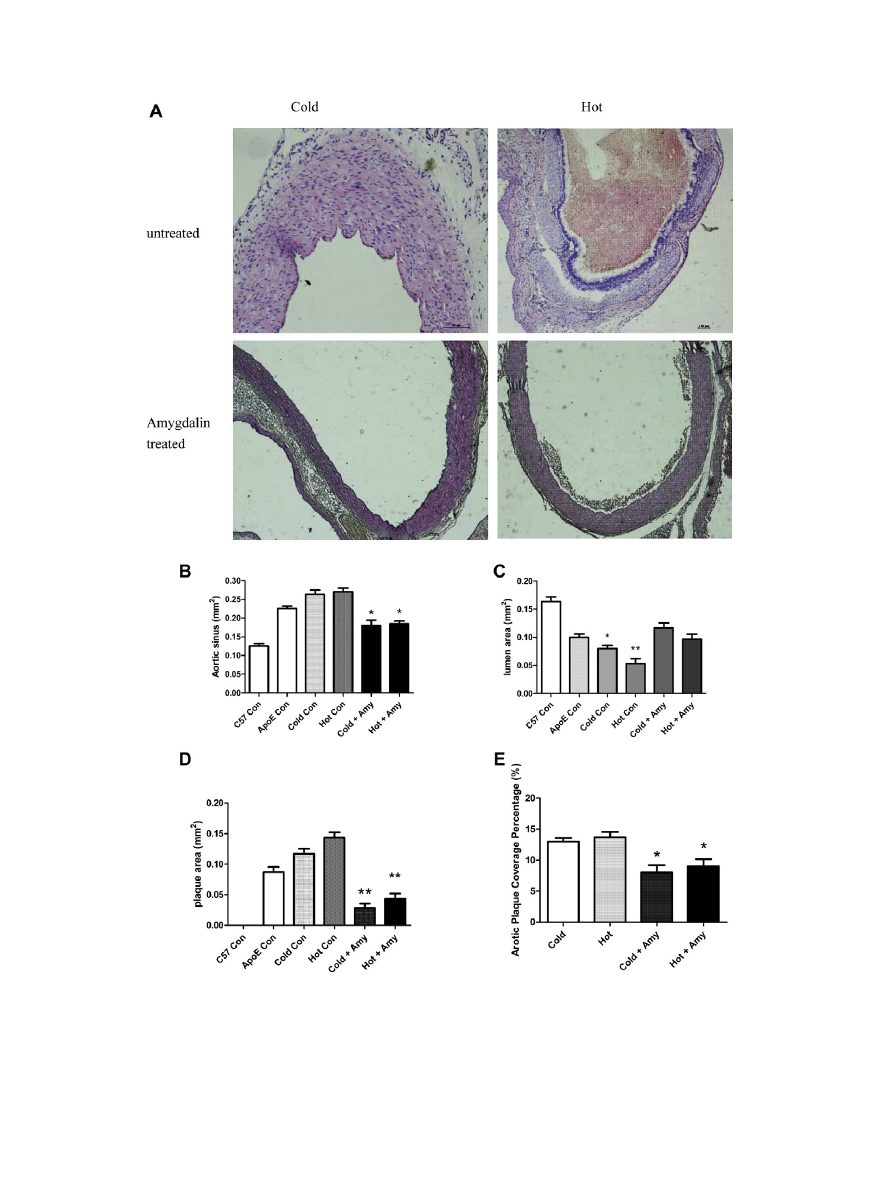
3.2. Retarded atherosclerosis development
In order to further investigate the pathological changes of
amygdalin at the presence of cold and hot factors, we analyzed ves-
sel areas, lumen areas and plaque areas (
A). With the progres-
sion of atherosclerosis, the diameters of aortic sinus increased with
time (
B), combined with decreased lumen areas (
C) and
increased plaque areas (
D). So both cold factor and hot factor
would exacerbate the atherosclerosis situation. However, mice
administered amygdalin exhibited a significantly reduced extent
of atherosclerosis as evident by smaller aortic sinus plaques, less
enlarged aortic sinus and the expanded lumen areas, compared
with those of in ApoE
/
mice under cold or hot pressure
(p < 0.05, treated vs. untreated,
D). This finding was validated
by analysis of atherosclerosis surface coverage areas, showing de
creased lesions in amygdalin treated ApoE
/
mice with cold or
hot pressure (p < 0.05, treated vs. untreated).
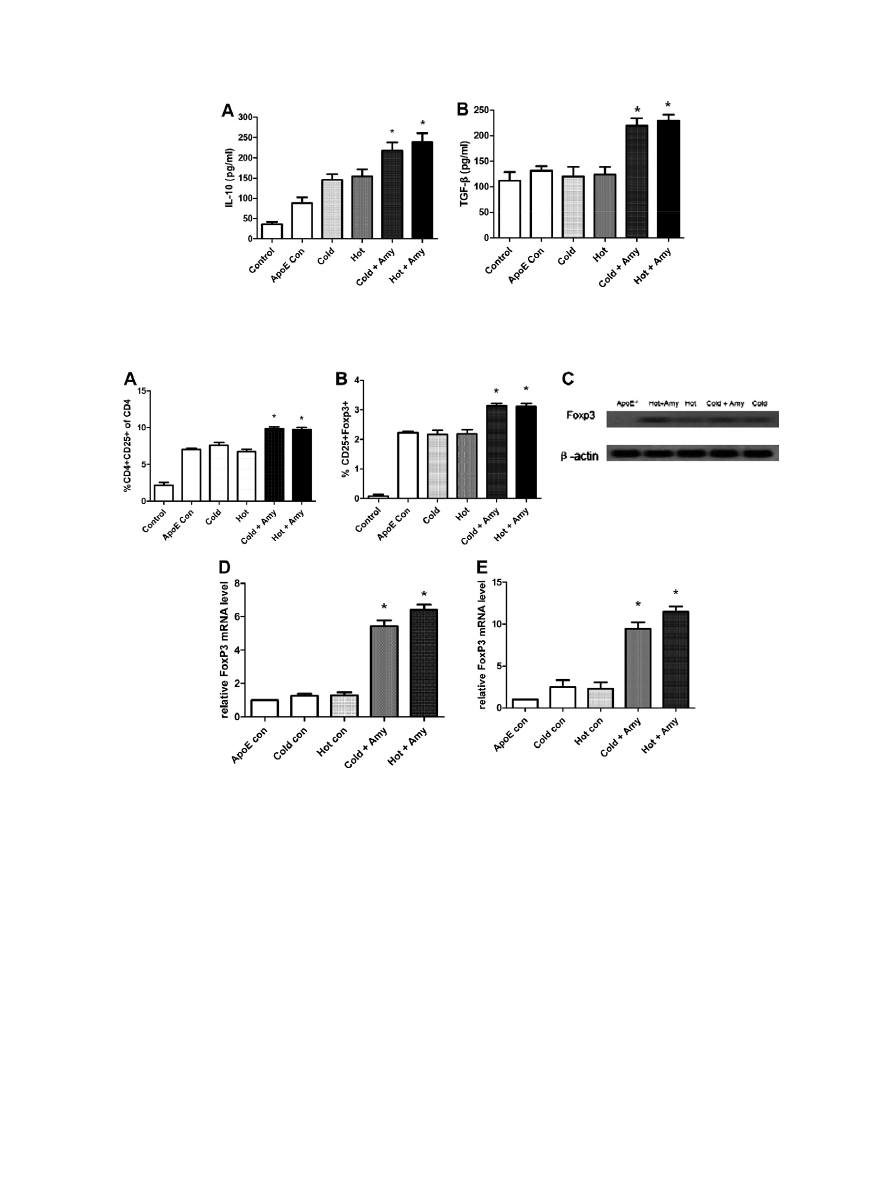
3.3. Inhibited atherosclerosis progression via induction of Treg-cell-
associated cytokines
To pursue the question of whether the presence of Treg is asso-
ciated with a change in amygdalin administration of atherosclero-
sis in ApoE
/
mice under cold or hot factor, we compared the
abundance of Treg-related cytokines in blood. Serum samples from
amygdalin treated or untreated mice were separated and analyzed
for Treg-related cytokines as IL-10 and TGF-b, both of which are
crucial for the immunoregulatory function of Tregs. IL-10 levels
(
A) were increased after the drug delivery. TGF-b (
B) is
required for the peripheral maintenance of Tregs and mediates
their suppressive function in vivo. We found the levels of TGF-b
were also significantly increased in amygdalin treated groups. High
abundance
of
IL-10 and TGF-b suggested
that the
anti-
atherosclerosis function of amygdalin was relied on Tregs.
3.4. Induction of Foxp3
+
Tregs
As forkhead/winged helix transcription factor Foxp3 is specifi-
cally expressed in Tregs, which control T cell homeostasis by sup-
pression of both Th1 and Th2 to pathogenic immune responses, we
reasoned that the anti-atherosclerotic effect of amygdalin could be
mediated by Foxp3 positive Tregs. Initially, we measured the per-
centage of CD4
+
CD25
+
T cells in total CD4 population by FACS anal-
ysis (
A, n = 6 in each group), and found that there were
significantly increased activated T cells in amygdalin treated mice
compared with untreated groups (p < 0.05). Then we performed
double staining for CD25 and Foxp3 (
B). Amygdalin treated
groups have obviously more CD25 and Foxp3 double positive cells
compared with untreated groups (p < 0.05). To further analyze the
influence of amygdalin on Tregs, we investigated the Foxp3 mRNA
levels and protein levels in splenocytes and peripheral blood cells.
There was a significant increase of Foxp3 protein level in amygda-
lin treated groups (
C). By quantification of Foxp3 mRNA lev-
els, we found a 4.3-fold (cold) and 4.9-fold (hot) increase of
Foxp3 mRNA levels in splenocytes (
D), and 3.8-fold (cold)
and 5-fold (hot) increase of Foxp3 mRNA levels in peripheral blood
cells (
E) in amygdalin treated groups compared with un-
treated groups (p < 0.05).
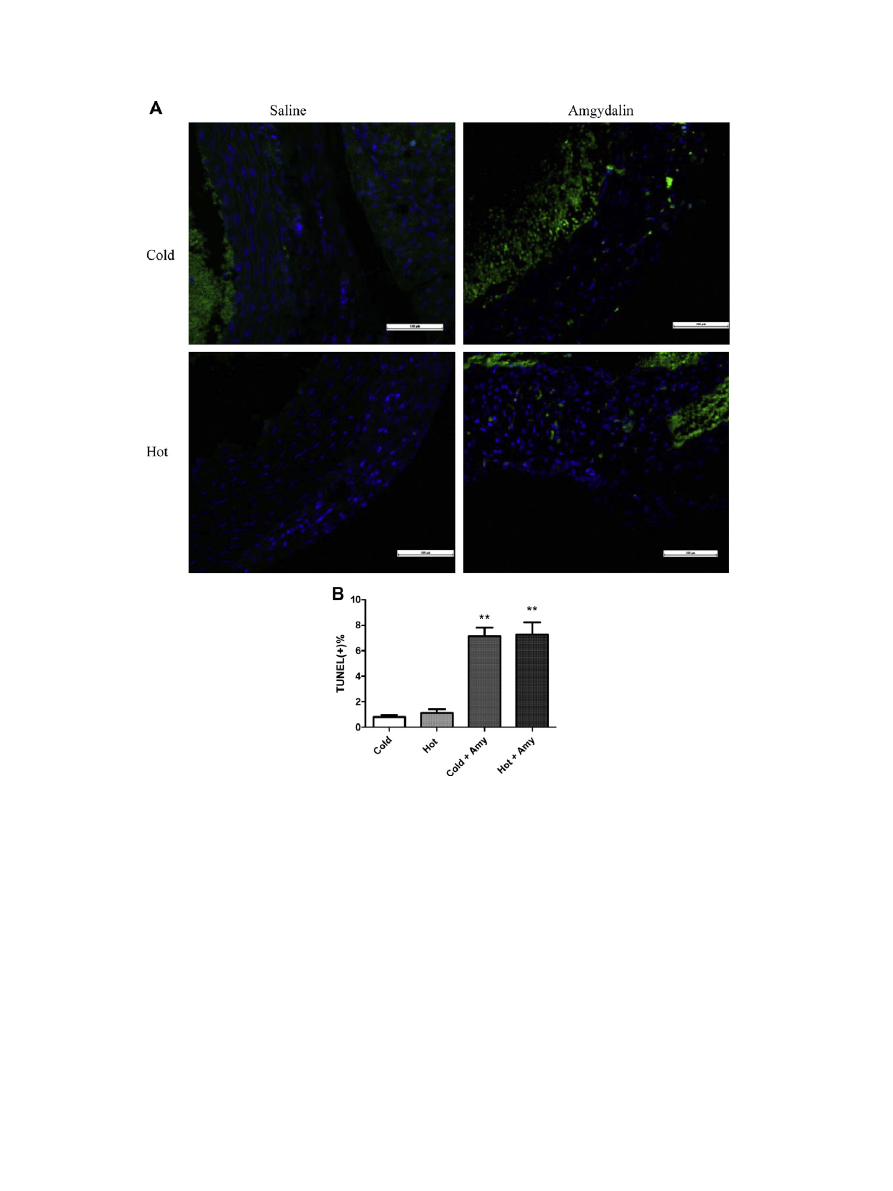
3.5. Induction of apoptosis
Aiming to establish the role of amygdalin in the progression of
atherosclerosis, we performed TUNEL staining to measure the apop-
totic percentage within atherosclerotic lesions. Recently published
data provided evidence for a functional involvement of granzyme
B and apoptosis in the immunosuppressor function of Tregs
To assess whether rates of apoptosis contribute to the anti-
atherosclerotic effect observed with amygdalin treatment, we
analyzed the percentage of DNA fragmentation within lesions of
treated and untreated mice. We observed a significantly increased
percentages of apoptotic cells within atherosclerotic lesions of
amygdalin-treated mice compared with control mice (
,
p < 0.01). Thus we hypothesized that the anti-atherosclerosis func-
tion of amygdalin was contributed to Treg-induced apoptosis.
4. Discussion
It is widely acknowledged that Tregs play an important role in
the development of atherosclerosis, proved by adoptive transfer
studies of lymphocytes
and Th2 cytokines produced by
Tregs. In this paper, we showed the anti-atherosclerotic function
of amygdalin by its successful induction of Tregs in vivo. Although
amygdalin has controversial function in cancer therapy, we first
examined its therapeutic effect in atherosclerosis. Compared by
the blood cholesterol levels, blood lipids were significantly
Table 1
Blood lipids (TG, TC, HDL-c, LDL-c) of different groups of mice (n = 4 in each group).
Groups
TG (mmol/L)
TC (mmol/L)
HDL-c (mmol/L)
LDL-c (mmol/L)
Normal control (C57BL/6J)
1.32 ± 0.13
1.73 ± 0.42
0.75 ± 0.05
0.80 ± 0.28
ApoE
/
control
6.39 ± 0.72
7.93 ± 0.38
1.02 ± 0.13
8.71 ± 0.33
Cold factor group
8.53 ± 0.82
9.77 ± 1.40
1.05 ± 0.21
10.03 ± 0.47
Hot factor group
9.75 ± 1.21
11.61 ± 3.27
1.10 ± 0.09
10.46 ± 0.36
Amygdalin group (cold)
5.06 ± 0.51
6.51 ± 0.38
1.16 ± 0.12
6.58 ± 0.35
Amygdalin group (hot)
4.12 ± 0.74
8.02 ± 0.61
1.06 ± 0.07
6.65 ± 0.25
#
p < 0.05.
*
p < 0.05 versus same factor control group.
**
p < 0.01.
D. Jiagang et al. / Biochemical and Biophysical Research Communications 411 (2011) 523–529
525
decreased after amygdalin treatment. Then questions are raised
about what caused the blood lipid changes in amygdalin treated
mice. We moved forward to investigate on the atherosclerotic
lesions. Statistics of aortic sinus, lumen areas, plaque areas and
aortic plaque coverage percentages further proved the pathological
changes in aortas of atherosclerotic mice.
However, the main purpose of this study was not limited on the
pharmacological function of amygdalin in atherosclerosis. Explor-
ing its mechanism of how it relived the symptom of atherosclerosis
was our goal. Atherosclerosis is caused by an imbalance between
pathogenic T cells and Tregs, which suppress the overactive
immune response to self antigen. Because recent studies high-
Fig. 1. Amygdalin (Amy) could relief the symptom of atherosclerosis. Aortic plaque was formed in both cold and hot model (A), while after the administration of amygdalin
for 4 weeks, there is significantly increased vessel area (B) and lumen area (C), decreased plaque area (D). Aortic plaque coverage percentage was increased (E).
⁄
p < 0.05 and
⁄⁄
p < 0.01 vs. untreated groups of control.
526
D. Jiagang et al. / Biochemical and Biophysical Research Communications 411 (2011) 523–529
lighted the role of Tregs in the progression of atherosclerosis
strategies to induce CD4
+
CD25
+
Foxp3
+
Treg cells in vivo have
received
more
attention.
Steffens
et
al.
reported
that
anti-CD3 antibody treatment could induce markedly decreased
plaque lesion, and this was associated with the induction of TGF-
b
in lymph node and Foxp3 expression in splenocytes and periph-
eral blood cells
. van Puijvelde et al. demonstrated that oral
administration of oxLDL could induce attenuated atherosclerosis
in ldlr
/
mice
. CD4
+
CD25
+
Foxp3
+
Treg cells were also found
in spleens and mesenteric lymph nodes of ldlr
/
mice. However,
as anti-CD3 antibody is an immunosuppressive agent used in clin-
ical for inducing tolerance after transplantation, patients adminis-
trated this drug are in high risk of infectious disease. So in the
present study, we reported that amygdalin therapy successfully
induced CD4
+
CD25
+
Foxp3
+
Tregs, which was testified by the cyto-
kine secre tion levels and Foxp3 mRNA levels. We found that mice
treated with amygdalin have comparatively higher levels of TGF-b.
TGF-b is a cytokine required for the peripheral maintenance of
natural Treg and the in vivo immune suppressive function of Treg
relies on TGF-b
. So we further testified our hypothesis that
the anti-atherosclerosis function of amygdalin could be related
with Treg. IL-10, secreted from effect CD4
+
CD25 T cells, is induced
by Treg and mediates natural Tregs function by turning effect T
cells into Tregs. Chronic antigenic stimulation or mucosal adminis-
tration of antigen can also generate the IL-10 producing Tregs
Our study proved that TGF-b and IL-10 were increased after amyg
dalin therapy, which is mainly due to the increased CD4
+
CD25
+
Foxp3
+
Treg cells. By assaying the number of
CD4
+
CD25
+
and
Foxp3
+
cells in spleen and peripheral blood cells, we found that
mice without amygdalin therapy had a significantly reduced num-
ber of splenic Tregs and blood Tregs. Thus, we hypothesized that
amygdalin could ameliorate atherosclerosis and retard lesion pro-
gression by inducing Tregs.
Foxp3 has been shown to govern the development and function
of Tregs
. Mutations in FOXP3 will eliminate CD25
+
Tregs and
cause autoimmune disorders
. Thus a study on Foxp3 mRNA
level and Foxp3 expression level was performed. We explored
whether the Tregs induction function of amygdalin was associated
Fig. 2. Treg-cell-associated cell factors were increased in amygdalin administrated groups. IL-10 (A) which mediates Treg function was increased after the drug delivery. TGF-
b
(B) which is required for the peripheral maintenance of Treg cells and mediates their suppressive function in vivo was also significantly increased in drug groups.
⁄
p < 0.05
vs. untreated controls.
Fig. 3. Analyze Tregs in different groups. Flow cytometry analysis of CD4
+
CD25
+
Foxp3
+
cells on lymph node and spleen-derived cells (A and B), Western blotting (C), and real-
time PCR analysis of FoxP3 mRNA level in spleen (D) and in blood (E).
⁄
p < 0.05 vs. untreated controls.
D. Jiagang et al. / Biochemical and Biophysical Research Communications 411 (2011) 523–529
527
with Foxp3. By quantification of Foxp3 mRNA and protein levels,
we found that amygdalin could induce FOXP3 gene tran-
scription and translation, thus induce the Treg development. These
findings suggested that amygdalin could be causally associated
with the increase of Tregs. However the exact mechanisms of
how Tregs mediate immune suppressive function, and whether
costimulatory signals are also involved in this process have not
yet been elucidated.
Besides the mechanism study of amygdalin from Treg perspec-
tive, and the pharmacological study on atherosclerosis, we found
that cold factor and hot factor were both conducive to the progres-
sion of atherosclerosis. This result suggested that these two factors
could be considered as one of the causal factors in the development
of atherosclerosis. However, mechanisms of how these factors con-
tribute to the progression of atherosclerosis need to be further
studied.
In summary, out study showed that in the development of ath-
erosclerosis in ApoE
/
mice both hot and cold factors almost
simultaneously exerted some influences, and the development of
atherosclerosis was significantly retarded by amygdalin. The inhib-
itory function of amygdalin was closely associated with Tregs,
which suggested that delivery of amygdalin to atherosclerotic mice
could be seen as beneficial. Further studies are also required to
determine its long-term toxicity and other possible mechanisms
in atherosclerotic treatment of amygdalin. In any case, our results
clearly indicated that amygdalin could be novel potential thera-
peutic drug in atherosclerotic treatment.
Acknowledgments
This study was supported in part by the National Basic Research
Program of China (No. 2007CB512602) and the project sup-
ported by Guangxi Science Foundation (No. 09320005).
References
[1] C.J. Binder, M.K. Chang, P.X. Shaw, Y.I. Miller, K. Hartvigsen, A. Dewan, et al.,
Innate and acquired immunity in atherogenesis, Nat. Med. 8 (11) (2002) 1218–
1226.
Fig. 4. Increased apoptosis percentage in atherosclerotic lesion of amygdalin treated groups of mice. Quantification of TUNEL(+)% of treated and untreated groups of mice (A).
Representative pictures of immunofluorescent staining of TUNEL of different groups of mice (B).
⁄⁄
p < 0.01 vs. untreated controls.
528
D. Jiagang et al. / Biochemical and Biophysical Research Communications 411 (2011) 523–529

[2] G.K. Hansson, Inflammation, atherosclerosis, and coronary artery disease, N.
Engl. J. Med. 352 (16) (2005) 1685–1695.
[3] P. Davenport, P.G. Tipping, The role of interleukin-4 and interleukin-12 in the
progression of atherosclerosis in apolipoprotein E-deficient mice, Am. J. Pathol.
163 (3) (2003) 1117–1125.
[4] L.J. Pinderski, M.P. Fischbein, G. Subbanagounder, M.C. Fishbein, N. Kubo, H.
Cheroutre, et al., Overexpression of interleukin-10 by activated T lymphocytes
inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte
and macrophage phenotypes, Circ. Res. 90 (10) (2002) 1064–1071.
[5] S. Potteaux, B. Esposito, O. van Oostrom, V. Brun, P. Ardouin, H. Groux, et al.,
Leukocyte-derived
interleukin
10
is
required
for
protection
against
atherosclerosis
in
low-density
lipoprotein
receptor
knockout
mice,
Arterioscler. Thromb. Vasc. Biol. 24 (8) (2004) 1474–1478.
[6] A. Gojova, V. Brun, B. Esposito, F. Cottrez, P. Gourdy, P. Ardouin, et al., Specific
abrogation of transforming growth factor-beta signaling in T cells alters
atherosclerotic lesion size and composition in mice, Blood 102 (12) (2003)
4052–4058.
[7] Z. Mallat, A. Gojova, C. Marchiol-Fournigault, B. Esposito, C. Kamate, R. Merval,
et al., Inhibition of transforming growth factor-beta signaling accelerates
atherosclerosis and induces an unstable plaque phenotype in mice, Circ. Res.
89 (10) (2001) 930–934.
[8] Z. Mallat, H. Ait-Oufella, A. Tedgui, Regulatory T-cell immunity in
atherosclerosis, Trends Cardiovasc. Med. 17 (4) (2007) 113–118.
[9] H. Ait-Oufella, B.L. Salomon, S. Potteaux, A.K. Robertson, P. Gourdy, J. Zoll, et al.,
Natural Tregs control the development of atherosclerosis in mice, Nat. Med. 12
(2) (2006) 178–180.
[10] A. Mor, D. Planer, G. Luboshits, A. Afek, S. Metzger, T. Chajek-Shaul, et al., Role
of naturally occurring CD4+CD25+ Tregs in experimental atherosclerosis,
Arterioscler. Thromb. Vasc. Biol. 27 (4) (2007) 893–900.
[11] J.D. Fontenot, M.A. Gavin, A.Y. Rudensky, Foxp3 programs the development
and function of CD4+CD25+ Tregs, Nat. Immunol. 4 (4) (2003) 330–336.
[12] S. Hori, T. Nomura, S. Sakaguchi, Control of regulatory T cell development by
the transcription factor Foxp3, Science (New York, NY) 299 (5609) (2003)
1057–1061.
[13] Z. Mallat, A. Gojova, V. Brun, B. Esposito, N. Fournier, F. Cottrez, et al., Induction
of a regulatory T cell type 1 response reduces the development of
atherosclerosis in apolipoprotein E-knockout mice, Circulation 108 (10)
(2003) 1232–1237.
[14] H.K. Chang, M.S. Shin, H.Y. Yang, J.W. Lee, Y.S. Kim, M.H. Lee, et al., Amygdalin
induces apoptosis through regulation of Bax and Bcl-2 expressions in human
DU145 and LNCaP prostate cancer cells, Biol. Pharm. Bull. 29 (8) (2006) 1597–
1602.
[15] W.R. Laster Jr., F.M. Schabel Jr., Experimental studies of the antitumor activity
of amygdalin MF (NSC-15780) alone and in combination with beta-
glucosidase (NSC-128056), Cancer Chemother. Rep. 59 (5) (1975) 951–965.
[16] S. Milazzo, S. Lejeune, E. Ernst, Laetrile for cancer: a systematic review of the
clinical evidence, Support. Care Cancer 15 (6) (2007) 583–595.
[17] R.E. Heikkila, F.S. Cabbat, The prevention of alloxan-induced diabetes by
amygdalin, Life Sci. 27 (8) (1980) 659–662.
[18] A. Baroni, I. Paoletti, R. Greco, R.A. Satriano, E. Ruocco, M.A. Tufano, et al.,
Immunomodulatory effects of a set of amygdalin analogues on human
keratinocyte cells, Exp. Dermatol. 14 (11) (2005) 854–859.
[19] H.J. Hwang, H.J. Lee, C.J. Kim, I. Shim, D.H. Hahm, Inhibitory effect of amygdalin
on lipopolysaccharide-inducible TNF-alpha and IL-1beta mRNA expression
and carrageenan-induced rat arthritis, J. Microbiol. Biotechnol. 18 (10) (2008)
1641–1647.
[20] E.G. Nabel, P. Ganz, J.B. Gordon, R.W. Alexander, A.P. Selwyn, Dilation of
normal and constriction of atherosclerotic coronary arteries caused by the cold
pressor test, Circulation 77 (1) (1988) 43–52.
[21] I. Antony, E. Aptecar, G. Lerebours, A. Nitenberg, Coronary artery constriction
caused by the cold pressor test in human hypertension, Hypertension 24 (2)
(1994) 212–219.
[22] I. Antony, G. Lerebours, A. Nitenberg, Angiotensin-converting enzyme
inhibition restores flow-dependent and cold pressor test-induced dilations in
coronary arteries of hypertensive patients, Circulation 94 (12) (1996) 3115–
3122.
[23] A.S. Plump, J.D. Smith, T. Hayek, K. Aalto-Setala, A. Walsh, J.G. Verstuyft, et al.,
Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient
mice created by homologous recombination in ES cells, Cell 71 (2) (1992) 343–
353.
[24] J. George, D. Harats, B. Gilburd, A. Afek, A. Shaish, J. Kopolovic, et al., Adoptive
transfer of beta(2)-glycoprotein I-reactive lymphocytes enhances early
atherosclerosis in LDL receptor-deficient mice, Circulation 102 (15) (2000)
1822–1827.
[25] J. George, A. Afek, B. Gilburd, Y. Shoenfeld, D. Harats, Cellular and humoral
immune responses to heat shock protein 65 are both involved in promoting
fatty-streak formation in LDL-receptor deficient mice, J. Am. Coll. Cardiol. 38
(3) (2001) 900–905.
[26] S.P. Cobbold, R. Castejon, E. Adams, D. Zelenika, L. Graca, S. Humm, et al.,
Induction of Foxp3+ Tregs in the periphery of T cell receptor transgenic mice
tolerized to transplants, J. Immunol. 172 (10) (2004) 6003–6010.
[27] D.C. Gondek, L.F. Lu, S.A. Quezada, S. Sakaguchi, R.J. Noelle, Cutting edge:
contact-mediated suppression by CD4+CD25+ regulatory cells involves a
granzyme B-dependent, perforin-independent mechanism, J. Immunol. 174 (4)
(2005) 1783–1786.
[28] X. Zhou, A. Nicoletti, R. Elhage, G.K. Hansson, Transfer of CD4(+) T cells
aggravates atherosclerosis in immunodeficient apolipoprotein E knockout
mice, Circulation 102 (24) (2000) 2919–2922.
[29] S. Steffens, F. Burger, G. Pelli, Y. Dean, G. Elson, M. Kosco-Vilbois, et al., Short-
term treatment with anti-CD3 antibody reduces the development and
progression of atherosclerosis in mice, Circulation 114 (18) (2006) 1977–
1984.
[30] G.H. van Puijvelde, A.D. Hauer, P. de Vos, R. van den Heuvel, M.J. van
Herwijnen, R. van der Zee, et al., Induction of oral tolerance to oxidized low-
density lipoprotein ameliorates atherosclerosis, Circulation 114 (18) (2006)
1968–1976.
[31] M. Belghith, J.A. Bluestone, S. Barriot, J. Megret, J.F. Bach, L. Chatenoud, TGF-
beta-dependent mechanisms mediate restoration of self-tolerance induced by
antibodies to CD3 in overt autoimmune diabetes, Nat. Med. 9 (9) (2003) 1202–
1208.
[32] O. Akbari, R.H. DeKruyff, D.T. Umetsu, Pulmonary dendritic cells producing IL-
10 mediate tolerance induced by respiratory exposure to antigen, Nat.
Immunol. 2 (8) (2001) 725–731.
[33] H.D. Ochs, S.F. Ziegler, T.R. Torgerson, FOXP3 acts as a rheostat of the immune
response, Immunol. Rev. 203 (2005) 156–164.
[34] A. Mor, G. Luboshits, D. Planer, G. Keren, J. George, Altered status of
CD4(+)CD25(+) Tregs in patients with acute coronary syndromes, Eur. Heart
J. 27 (21) (2006) 2530–2537.
[35] M.E. Brunkow, E.W. Jeffery, K.A. Hjerrild, B. Paeper, L.B. Clark, S.A. Yasayko,
et al., Disruption of a new forkhead/winged-helix protein, scurfin, results in
the fatal lymphoproliferative disorder of the scurfy mouse, Nat. Genet. 27 (1)
(2001) 68–73.
D. Jiagang et al. / Biochemical and Biophysical Research Communications 411 (2011) 523–529
529
Document Outline
- Amygdalin mediates relieved atherosclerosis in apolipoprotein E deficient mice through the induction of regulatory T cells
Wyszukiwarka
Podobne podstrony:
1 s2 0 S0006291X05021595 main
1 s2 0 S0006291X07005785 main
1 s2 0 S0020025512001946 main
1 s2 0 S0378382002000085 main
1 s2 0 S0304397599001000 main
1 s2 0 S0040603111000104 main 2
1 s2 0 S0944501312001358 main
1 s2 0 S0166218X96000583 main
1 s2 0 S0005273614000303 main
1 s2 0 S0304397502001342 main
1 s2 0 S0377221798003622 main (1)
1 s2 0 S0022169496031423 main
1 s2 0 S1046592814002101 main
1 s2 0 0166218X93E0153P main
1 s2 0 S0022000006001474 main
1 s2 0 S000925099800520X main
1 s2 0 S0022283610008843 main
1 s2 0 S0960852409006385 main
więcej podobnych podstron