
Trace Element Levels in Hashimoto Thyroiditis
Patients with Subclinical Hypothyroidism
Muhammed Erdal
&
Mustafa Sahin
&
Adnan Hasimi
&
Gökhan Uckaya
&
Mustafa Kutlu
&
Kenan Saglam
Received: 21 January 2008 / Accepted: 14 February 2008 /
Published online: 6 March 2008
# Humana Press Inc. 2008
Abstract The present study was conducted to evaluate the serum copper, zinc, magnesium,
and selenium levels in patients with subclinical hypothyroidism in the iodine-rich region of
Ankara, Turkey. The effects of hormone replacement therapy on these elements were also
studied in these patients. Basal levels of selenium and iron in patients were significantly
lower than control group (67.7±10.4 vs. 83.7±17.3
μg/dl, p=0.02; 55.7±38 vs 275.7±24,
P=0.03 μg/dl). Serum magnesium levels were significantly higher in patient group (2.16±
0.31 vs 1.95±0.13 mg/dl,
P<0.0001). There was a correlation between selenium levels with
hsCRP (
r=−0.408, p=0.007). HsCRP levels in patients with selenium levels <80 μg/l
(
n=31) was significantly higher than hsCRP levels in patients with selenium levels >80 μg/l
(
n=12; 1.99±1.0; 1.02±0.9, p=0.014). None of these biochemical risk factors and trace
elements have changed after euthyroidism in patients with SH when compared to pretreatment
levels. Selenium deficiency may contribute to cardiovascular disease risk in these patients.
Keywords Trace elements . Subclinical hypothyroidism . TSH
Introduction
Subclinical hypothyroidism (SH) is defined as serum FT4 and FT3 levels within their
respective reference ranges in the presence of abnormal serum thyrotropin-stimulating
hormone levels [
]. The prevalence of SH has been reported to be between 4% and 10%
of adult population [
]. It is most often caused by chronic lymphocytic thyroiditis, an
Biol Trace Elem Res (2008) 123:1
–7
DOI 10.1007/s12011-008-8117-8
M. Erdal
:
K. Saglam
Department of Family Medicine, Gulhane School of Medicine, Etlik, Ankara, Turkey
M. Sahin
:
G. Uckaya
:
M. Kutlu
Department of Endocrinology and Metabolism, Gulhane School of Medicine, Etlik, Ankara, Turkey
A. Hasimi
Department of Biochemistry, Gulhane School of Medicine, Etlik, Ankara, Turkey
M. Sahin (
*)
Endocrinology and Metabolism Department, Gulhane University School of Medicine,
Zulfikar sok 28/8 Buyukesat, Ankara, Turkey
e-mail: drsahinmustafa@yahoo.com

autoimmune disorder of the thyroid gland that is the most common cause of decreased
thyroid hormone production in patients with acquired mild, subclinical, or overt
hypothyroidism [
]. We do not know the prevalence of trace elements deficiency in this
patient population. We also do not know the effect of thyroid hormone replacement therapy
on serum trace elements levels.
The association between SHypo and cardiovascular disease in studies are controversial
[
]. Whether to treat SH remains also a dilemma [
,
]. Several experts concluded
that there was no sufficient evidence to recommend routine treatment for patients with TSH
between 4.5 and 10 mIU/l and suggested that patients be monitored at 6
–12-month intervals
[
]. American Association of Clinical Endocrinologist, the Endocrine Society and the
American Thyroid Association recommended routine treatment of patients with SH who
had serum TSH levels of 4.5
–10 mIU/l [
Trace elements are essential micronutrients both for humans, and they are crucial for
many physiological processes [
]. They influence the normal physiology of the thyroid
gland [
]. The concentration of these elements in the thyroid gland is higher than in any
other tissues [
]. Thyroid hormones influence the metabolism of trace elements especially
zinc and copper [
]. In one study, it is observed that basal metabolic rate and serum free
T4 levels decreased significantly during the low zinc period, and increased during
adequate-zinc period [
].
In addition, thyroid hormone seems to have some effect on selenium metabolism as
significantly low levels of selenium were found in patients suffering from hyperthyroidism
[
]. Thyroxine (T4) is important for iron homeostasis since its administration restores iron
levels in tissues [
]. The trace element selenium (Se) plays an important role in the thyroid
gland under normal physiological conditions and in disease. Se is effective in reducing
TPOAb titers in patients affected by thyroid autoimmune diseases, probably due to its
modification of the inflammatory and immune responses [
]. The reduction in TPOAb
titers seems to be correlated with the amount of Se administered [
]. Selenium-dependent
enzymes, such as glutathione peroxidase, maintain nitric oxide in its reduced form and
protect against oxidative stress. Via this mechanism, selenium deficiency might predispose
to cardiovascular disease also [
].
Thyroid hormone metabolism may also be affected by other dietary components,
including iodine [
], and copper [
]. Limited or inadequate supply of
both trace elements, iodine and selenium, leads to complex rearrangements of thyroid
hormone metabolism enabling adaptation to unfavorable conditions.
The present study was undertaken to investigate the serum levels of trace elements (Zn,
Cu, Mg, Fe, and Se) in subclinical hypothyroid patients in iodine-replete area before and
after thyroid replacement therapy. Also, we aimed to investigate any correlation of trace
elements (Zn, Cu, Mn, Mg, Fe, and Se) in serum and other laboratory parameters. We also
evaluated the effect of thyroid hormone replacement therapy on other laboratory parameters
in subclinical hypothyroid patients.
Material and Method
Study Subjects
Forty-three autoimmune thyroiditis patients (4 male/39 premenopausal women, mean 48.5±
4.7 years, body mass index, 25.8±4.1 kg/m
2
) were examined and followed up in the
outpatient clinic of Department of Endocrinology and Metabolism, Gulhane School of
2
Erdal et al.

Medicine, Ankara. After an overnight fast, all patients underwent full medical assessment
and laboratory examinations to rule out nonthyroidal illnesses. Ankara is an iodine-replete
area in Turkey. The exclusion criteria were as follows: coronary heart disease, pituitary/
hypothalamic disorders or other nonthyroidal diseases. None were receiving vitamins, lipid-
lowering drugs, or other medications known to interfere with homocysteine metabolism,
lipid profile, or thyroid function. Healthy age to age matched 49 controls (48.4±5.7 years,
BMI 26 kg/m
2
) were voluntarily enrolled in the study; physical examination and venous
blood samplings were performed for same parameters as patients. All the participants were
informed, and written consents were obtained. SH was defined as an elevated TSH
concentration (>5 mIU/l) in the presence of normal thyroxine levels in two determinations [
Study Design
Venous blood samples were withdrawn from brachial vein after 12 h overnight fasting,
between 08:00 and 09:00
A.M
. All the patients were treated with L-T4 (Levothyroxine, Abdi
Ibrahim, Istanbul, Turkey) starting from dose of 50
μg/day. TSH was measured every 4–
6 weeks to adjust L-T4 dose. Mean L-T4 dose required to restore euthyroidism was 65±
20
μg/day. Reevaluation was performed with venous blood sampling at least 4 months after
restoration of euthyroidism.
Methods
Fasting serum samples were immediately put on ice and kept frozen at
−70°C until analyses
were performed. Serum TSH, f-T4, free triiodothyronine (f-T3) levels (Immulite, 2000
autoanalyzer by BIO-DPC, CA, USA) and total cholesterol, triglyceride, and high-density
lipoprotein cholesterol levels (Olympus AU 2700 auto analyzer, Germany) were determined
using commercially available methods. LDL cholesterol was calculated using Friedewald
’s
formula. t-Hyc levels were determined using high pressure liquid chromatography; normal
range was 5
–12 mmol/l with a 0.4–5% intraassay coefficient of variation (CV). Vitamin
B12 and folate levels were assayed in serum by using a commercially available kit and
Immulite 2000 auto analyzer (BIO-DPC, CA, USA), average ranges were 193
–982 pmol/l
and 3
–17 nmol/l, with a 3.2–5.5% and 4.4–7% intraassay CV, respectively.
Venous blood samples were collected from the fasting subjects in the morning. Appropriate
trace-element tubes (Becton-Dickinson, Vacutainer, NJ, USA) were used for drawing blood
Table 1 Clinical Characteristics of Controls and Patients
Variable
Control (
n=49)
Patient (
n=43)
p value*
Age
48.4±5.7
48.5±4.7
NS
Sex
6/43
4/39
NS
AntiTPO
12±4
423.09±20
<0.001
f-T4 (pg/ml)
1.3
0.98±0.22
<0.001
f-T3 (ng/dl)
3.1
2.83±0.62
>0.05
TSH (mIU/l)
1.1±0.01
9.93±0.4
<0.001
Selenium
83.7±17.3
67.7±10.4
0.02
Zinc
101.5±10.7
109.3±34.3
NS
Fe
75.7±24
55.7±38
0.03
Magnesium(ng/ml)
1.95±0.13
2.16±0.31
<0.0001
Cupper(mmol/l)
106.9±14.9
108.1±53.08
NS
Trace Element Levels in Hashimoto Thyroiditis Patients
3
samples for determination of copper (Cu), zinc (Zn), magnesium (Mg), selenium (Se), and
iron (Fe) levels in blood. Serum levels of Cu, Zn, Se, were determined by use of atomic mass
spectrometry (ZEEnit 700; Analytical Jena, Germany), serum iron and magnesium levels were
determined by an autoanalyzer (Olympus AU 2700; Japan) using its original photometric kits
utilizing xylidyl blue and TPTZ [2,4,6-Tri-(2-pyridyl)-5-triazine] methods, respectively. All
tests were run with the quality control samples recommended by the manufacturer in a
laboratory following an external quality assurance program (Bio-Rad EQAS, UK).
The data that were analyzed with the help of SPSS 11.0 program with the Wilcoxon signed-
rank test because the data was not fitting to normal distribution. Values were given as medians.
Results
Main findings of control and patient groups were given in Table
. No patients had vitamin
B12 and folate deficiencies. There were significant difference between basal selenium,
Basal serum selenium level (
µ
g/L)
125,00
100,00
75,00
50,00
25,00
0,00
Basal hs-CRP (mg/L)
4,00
3,00
2,00
1,00
0,00
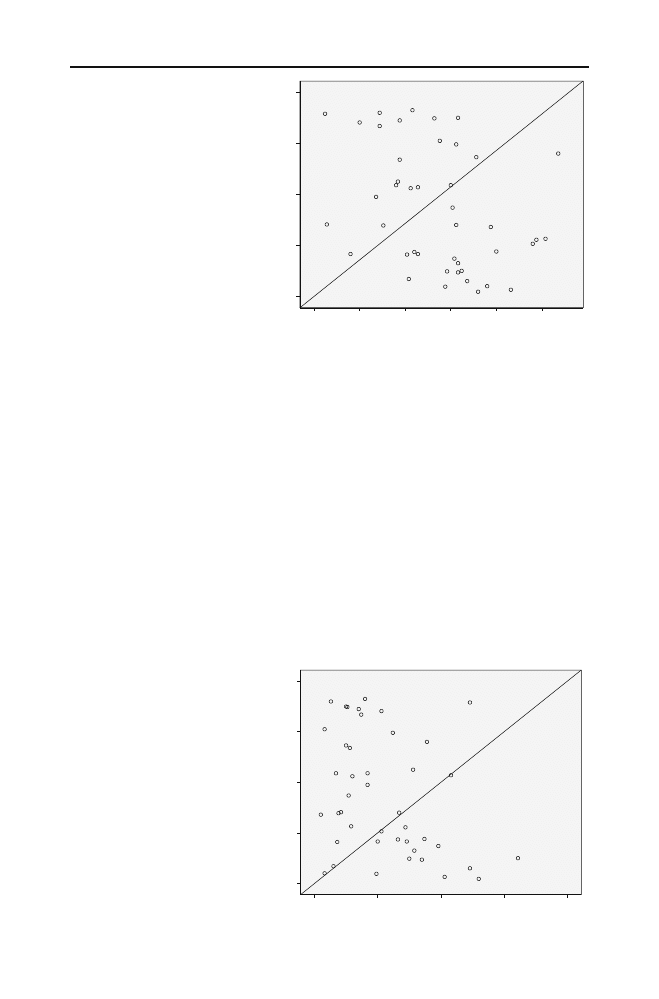
Fig. 1 Correlation between
hsCRP levels and selenium levels
Basal Fe level
200,00
150,00
100,00
50,00
0,00
Basal hs-CRP (mg/L)
4,00
3,00
2,00
1,00
0,00
Fig. 2 Correlation between
hsCRP levels and iron levels
4
Erdal et al.
magnesium, and iron levels in patients according to control subjects. Basal levels of
selenium and iron in patients were significantly lower than control group (67.7±10.4 vs.
83.7±17.3,
p=0.02; 55.7±38 vs 275.7±24, p=0.03). Serum magnesium levels were
significantly higher in patient group (2.16±0.31 vs 1.95±0.13,
p<0.0001). There were no
significant differences in ferritin levels between patient and control group.
There was a correlation between selenium levels with hsCRP (
r=−0.408, p=0.007;
Fig.
). There was also a significant correlation between iron levels and hsCRP levels
(
r=−0.318, p=0.038; Fig.
). hsCRP levels in patients with selenium levels <80
μg/l (n=
31) was significantly higher than hsCRP levels in patients with selenium levels >80
μg/l
(
n=12; 1.99±1.0; 1.02±0.9, p=0.014; Fig.
Pre- and posttreatment values of patients are given in Table
. While TSH levels reduced
significantly, f-T4 levels also increased significantly as compared pre- to posttreatment
levels (both
p<0.001). While the pretreatment median for TSH was 9.93±0.5 mIU/l, its
posttreatment level became 2.25±1.3 mIU/l. While the pre-treatment median was 0.98±
Basal selenium level < 80
µ
g/L
1,00
,00
Basal s-CRP levels
4,00
3,00
2,00
1,00
0,00
12
Fig. 3 hsCRP levels in patients
according to selenium levels
Table 2 Labaratory and Clinical Parameters Before Treatment and After Treatment
Variable
Pretreatment (
n=43)
Posttreatment (
n=43)
p value
BMI (kg/m
2
)
25.8±4.1
25.8±4.1
>0.05
f-T4 (pg/ml)
0.98±0.22
1.3±0.22
<0.001
f-T3 (ng/dl)
2.83±0.62
3.03±0.64
>0.05
TSH (mIU/l)
9.93±0.5
2.25±1.3
<0.001
Total Cholesterol (mg/dl)
202.4±43.06
197.7±30.04
>0.05
Triglycerides (mg/dl)
163.8±123
135.2±77
>0.05
LDL Cholesterol (mg/dl)
124.9±34.5
124.7±29.4
>0.05
HDL Cholesterol (mg/dl)
44.9±12.3
46.06±12.4
>0.05
CRP
1.78±1.04
1.72±1.19
>0.05
Homocysteine (mmol/l)
9.77±1.7
9.23±1.9
>0.05
Selenium
67.7±10.4
66.2±15.7
>0.05
Zinc
109.3±34.3
103.6±35.3
>0.05
Fe
55.7±38
73.2±54.7
>0.05
Magnesium(ng/ml)
2.16±0.31
2.23±0.39
>0.05
Cupper(mmol/l)
108.1±53.08
118.6±46.5
>0.05
Trace Element Levels in Hashimoto Thyroiditis Patients
5

0.22 pg/ml, its posttreatment level became 1.3±0.22 pg/ml. No significant changes were
observed in BMI, cholesterol levels, homocysteine, hsCRP, vitamin B12, and folate levels
by the end of study (Table
Discussion
There is no consensus on the thyroid hormone, and opinions differ regarding tissue effects,
symptoms and signs, and the cardiovascular risk. There are many studies about the role of
thyroid replacement therapy on health status, cardiovascular risk in patients with subclinical
hypothyroidism [
]. It has been shown that the thyroid hormones do influence the
metabolism of trace elements [
]. According to our knowledge, our study is the first
study evaluating thyroid hormone replacement therapy on serum levels of trace elements.
We did not find significant change in trace elements after thyroid hormone replacement
therapy.
There was no association between thyroid function and trace element levels. In
Hashimoto thyroiditis with subclinical hypothyroidism, several trace elements may be
different from healthy subjects. Our patients live in iodine-replete area in Turkey. Patients
who live in iodine-deficient areas may have different profile. We did not evaluate urinary
iodine levels, which may show individual differences in iodine ingestion.
Selenium deficiency might be a risk factor cardiovascular disease in patients with
subclinical hypothyroidism. Our study should be interpreted within the context of its
possible limitations, such that we cannot exclude with certainty that the protective effect of
selenium on the development of cardiovascular risk might, owing to other factors, strongly
associate with selenium, such as dietary protein intake. If confirmed, our findings may have
important implication for public health.
In conclusion, deficiency of the antioxidant selenium, which is prevalent in autoimmune
thyroiditis patients with subclinical hypothyroidism, might be an underestimated risk factor
for the development of high cardiovascular risk.
References
1. Col NF, Surks MI, Daniels GH (2004) Subclinical thyroid disease: clinical applications. JAMA 291
(2):239
–243
2. Fatourechi V (2001) Subclinical thyroid disease. Mayo Clin Proc 76(4):413
–416
3. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE ,
Serum TSH (2002) T(4), and thyroid antibodies in the United States population (1988 to 1994): National
Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489
–499
4. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC (2000) The Colorado thyroid disease prevalence
study. Arch Intern Med 160:526
–534
5. Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, Evans JG, Young E, Bird T, Smith
PA (1977) The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol
(Oxf) 7:481
–493
6. Ross DS (2005) Subclinical hypothyroidism. In: Braverman LE, Utiger RD (eds) Werner & Ingbar
’s The
Thyroid: a fundamental and clinical text, 8th edn. Williams & Wilkins, Philadelphia Lippincott, pp
1070
–10785
7. Perez A, Cubero JM, Sucunza N, Ortega E, Arcelus R, Rodriguez-Espinosa J, Ordonez-Llanos J,
Blanco-Vaca F (2004) Emerging cardiovascular risk factors in subclinical hypothyroidism: lack of
change after restoration of euthyroidism. Metabolism 53:1512
–1515
8. Tieche M, Lupi GA, Gutzwiller F, Grob PJ, Studer H, Burgi H (1981) Borderline low thyroid function
and thyroid autoimmunity. Risk factors for coronary heart disease? Br Heart J 46:202
–206
6
Erdal et al.

9. Dean JW, Fowler PB (1985) Exaggerated responsiveness to thyrotrophin releasing hormone: a risk factor
in women with coronary artery disease. Br Med J 290:1555
–1561
10. Mya MM, Aronow WS (2002) Subclinical hypothyroidism is associated with coronary artery disease in
older persons. J Gerontol A Biol Sci Med Sci 57:658
–659
11. Ringel MD, Mazzaferri EL (2005) Subclinical thyroid dysfunction
–can there be a consensus about the
consensus? J Clin Endocrinol Metab 90:588
–590
12. Cooper DS (2004) Subclinical thyroid disease: consensus or conundrum? Clin Endocrinol (Oxf) 60:410
–
412
13. Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, Franklyn JA, Hershman JM, Burman
KD, Denke MA, Gorman C, Cooper RS, Weissman NJ (2004) Subclinical thyroid disease: scientific
review and guidelines for diagnosis and management. JAMA 291:228
–238
14. Gharib H, Tuttle RM, Baskin HJ, Fish LH, Singer PA, McDermott MT (2005) American Association of
Clinical Endocrinologists; American Thyroid Association; The Endocrine Society Consensus Statement
#1: Subclinical thyroid dysfunction: a joint statement on management from the American Association of
Clinical Endocrinologists, the American Thyroid Association, and The Endocrine Society. J Clin
Endocrinol Metab 90:581
–585
15. Margalioth EJ, Schenker JG, Chevion M (1983) Copper and Zinc levels in normal and malignant tissues.
Cancer 52:868
–872
16. Zaichick VY, Tsyh AF, Vtyurini BM (1995) Trace elements and thyroid cancers. Analyst 120:817
–821
17. Henkin RI (1976) Trace metals in endocrinology. Med Clin North Am 60:779
–797
18. Kralik A, Eder K, Kirchgessner M (1996) Influence of zinc and selenium deficiency on parameters
relating to thyroid hormone metabolism. Horm Metab Res 28:223
–226
19. Aihara K, Nishi Y, Hatano S, Kihara M, Yoshimitsu K, Takeichi N, Ho T, Ezaki H, Usui T (1984) Zinc,
copper manganese and selenium metabolism in thyroid disease. Am J Clin Nutr 40:26
–35
20. Leblondel G, Allain P (1989) Effects of thyroparathyroidectomy and of thyroxine and calcitonin on
tissue distribution of twelve elements in the rat. Biol Trace Element Res 19:171
–183
21. Gartner R, Gasnier BCH, Dietrich JW, Krebs B, Angstwurm MWA (2002) Selenium supplementation in
patients with autoimmune thyroiditis decrease thyroid peroxidase antibodies concentrations. J Clin
Endocrinol Metab 87:1687
–1691
22. Duntas LH, Mantzou E, Koutras DA (2003) Effects of a six month treatment with selenomethionine in
patients with autoimmune thyroiditis. Eur J Endocrinol 148:389
–393
23. Turker O, Kumanlioglu K, Karapolat I, Dogan I (2006) Selenium treatment in autoimmune thyroiditis:
9-month follow-up with variable doses. J Clin Endocrinol Metab 190:151
–156
24. Salonen JT, Alfthan G, Huttunen JK, Pikkarainen J, Puska P (1982) Association between cardiovascular
death and myocardial-infarction and serum selenium in a matched-pair longitudinal study. Lancet 2:175
–
179
25. Clugston GA, Hetzel BS (1994) Iodine. In: Shils ME, Olson JA, Shike M (eds) Modern nutrition in
health and disease,. 8thth edn. Lea & Febiger, Philadelphia PA
26. Brigham D, Beard J (1996) Iron and thermoregulation: a review. Crit Rev Food Sci Nutr 36:747
–763
27. Kralik A, Kirchgessner M, Eder K (1996) Concentrations of thyroid hormones in serum and activity of
hepatic 5- monodeiodinase in copper deficient rats. Z Ernaehrswiss 35:288
–291
28. Helfand M (2004) Screening for subclinical thyroid dysfunction in non-pregnant adults: a summary of
the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 140:128
–141
Trace Element Levels in Hashimoto Thyroiditis Patients
7
Document Outline
Wyszukiwarka
Podobne podstrony:
High Choline Concentrations in the Caudate Nucleus in Antipsychotic Naive Patients With Schizophreni
A Proton MRSI Study of Brain N Acetylaspartate Level After 12 Weeks of Citalopram Treatment in Drug
Glutamate and Glutamine Measured With 4 0 T Proton MRS in Never Treated Patients With Schizophrenia
Serum cytokine levels in patients with chronic low back pain due to herniated disc
Effects of Clopidogrel?ded to Aspirin in Patients with Recent Lacunar Stroke
Difficult airway management in a patient with traumatic asphyxia
Impaired Sexual Function in Patients with BPD is Determined by History of Sexual Abuse
Breast and other cancers in 1445 blood relatives of 75 Nordic patients with ataxia telangiectasia
Konstatinos A Land versus water exercise in patients with coronary
A Ser49Cys Variant in the Ataxia Telangiectasia, Mutated, Gene that Is More Common in Patients with
Muscle Mass Gain Observed in Patients with Short Bowel Syndrome
Difficult airway management in a patient with traumatic asphyxia
Continuous mechanical chest compression during in hospital cardiopulmonary resuscitation of patients
Proton Magnetic Resonance Spectroscopy of the Medial Prefrontal Cortex in Patients With Deficit Schi
(IV)Intertester reliability of the McKenzie evaluation in assessing patients with mechanical low bac
The Effects of Probiotic Supplementation on Markers of Blood Lipids, and Blood Pressure in Patients
Bacteremia in adult patients with acquired immunodeficiency syndrome
Personality Constellations in Patients With a History of Childhood Sexual Abuse
Effect of high dose intravenous ascorbic acid on the level of inflammation in patients with rheumato
więcej podobnych podstron