
292
*Corresponding author: Mailing address: Department of
Molecular Microbiology and Immunology, Nagasaki Uni-
versity Graduate School of Biomedical Sciences, 1-7-1
Sakamoto, Nagasaki 852-8501, Japan. Tel: +81-95-819-
7276, Fax: +81-95-819-7285, E-mail: kakeya@nagasaki-u.
ac.jp
292
Jpn. J. Infect. Dis., 64, 292-296, 2011
Original Article
Synergistic Antifungal Effect of Lactoferrin with Azole Antifungals
against Candida albicans and a Proposal for
a New Treatment Method for Invasive Candidiasis
Tsutomu Kobayashi, Hiroshi Kakeya*, Taiga Miyazaki, Koichi Izumikawa, Katsunori Yanagihara
1
,
Hideaki Ohno
3
, Yoshihiro Yamamoto, Takayoshi Tashiro
2
, and Shigeru Kohno
Department of Molecular Microbiology and Immunology,
1
Department of Laboratory Medicine, and
2
Department of Health Sciences,
Nagasaki University Graduate School of Biomedical Sciences, Nagasaki 852-8501; and
3
Department of Chemotherapy and Mycoses, National Institute of Infectious Diseases,
Tokyo 162-8640, Japan
(Received March 14, 2011. Accepted April 27, 2011)
SUMMARY: The combination of lactoferrin with fluconazole has been reported to synergistically en-
hance the antifungal activity of fluconazole against
Candida spp. and inhibit the hyphal formation in
fluconazole-resistant strains of
Candida albicans. In this study, we investigated the association between
the therapeutic effects of this combination and the pharmacological characteristics of fluconazole and
itraconazole and the variation in these effects with differences among the strains in terms of the suscep-
tibility and resistance mechanisms. Lactoferrin enhanced the growth-inhibitory activity of fluconazole
against two different ergosterol mutants but not againt pump mutants or an azole-susceptible strain; but
increased the activity of itraconazole against all the strains tested in this study. Exogenous iron cancelled
the synergistic effect, which suggests that the iron-chelating function of lactoferrin may contribute to
the synergism. Besides, radiolabeled fluconazole assays revealed that lactoferrin did not affect the in-
tracellular concentrations of fluconazole, thereby indicating that these synergistic effects were not due
to the alteration of the intracellular uptake of the drug. The development of new clinical treatments and
therapeutic method against resistant
Candida will depend on our understanding of the resistance
mechanisms and methods to overcome them by the application of suitable drug combinations with syn-
ergistic effects. The results of this study might contribute to the improvement of our understand of the
mechanisms underlying the resistance of
Candida strains.
INTRODUCTION
Candidiasis is the commonest invasive mycosis en-
countered in clinical settings, even in the current
``echinocandin era,'' and it remains refractory to treat-
ment and has a high mortality of 30z or higher (1).
Although fluconazole (FLCZ) and echinocandins are
the primary choices for therapy against invasive can-
didiasis, more effective treatments or prophylactic
methods need to be developed.
Lactoferrin (LF) is a broad-spectrum antimicrobial
peptide against bacteria, fungi, viruses, and protozoa
and shows potent synergism with FLCZ in azole-suscep-
tible isolates of
Candida albicans obtained from ne-
onates with sepsis (2); this synergism might also be ap-
plicable against refractory candidiasis. Wakabayashi et
al. also showed that a combination of FLCZ with LF-
related compounds exerted synergistic effects on cell
growth, even in the case of azole-resistant
C. albicans
(3), but it has not yet been elucidated exactly how com-
bination with LF will influence effectiveness of different
drugs against various resistance mechanisms.
Candida
spp. are known to acquire azole resistance by at least
three different mechanisms: altered sterol synthetic
pathway from native ergosterol due to
ERG3 mutation,
resulting in the production of non-toxic alternative
sterol in the presence of azoles (4); decreased substrate
affinity due to mutations in the target molecule, 14-
alpha-demetlylase, which is encoded by
ERG11 (5);
and decreased intracellular concentrations of drugs due
to overexpression of genes encoding efflux pumps, such
as
CDR1 and CaMDR (6). Here, we investigated the ef-
fects of LF combinations with FLCZ and itraconazole
(ITCZ) on strains that exhibit one or more of the
abovementioned resistance mechanisms.
MATERIALS AND METHODS
Fungal strains: The following
C. albicans strains were
tested: SC5314, an azole-susceptible strain; CAE3DU3,
the erg3 disrupted mutant (7); Darlington strain, a clini-
cal strain carrying mutations in both erg3 and erg11
(4,8,9) (kindly provided by Dr. John E. Bennett,
NIAID, NIH, Md., USA); C26, a
CDR1 overexpressing
mutant (10) (provided by Dr. S. Maesaki, Saitama Med-
ical School, Saitama, Japan); and C40, a
CaMDR over-
expressing mutant (10) (see Table 1).
293
Table 1. Antifungal susceptibilities and FIC indexes
C. albicans
strain
Characteristic
Origin or
reference
IC
50
FIC index of
FLCZ + LF
1)
IC
50
FIC index of
ITCZ + LF
1)
IC
50
of
LF alone
FLCZ
alone
FLCZ/LF
in combination
ITCZ
alone
ITCZ/LF
in combination
SC5314
ERG3/ERG3
,
URA3/URA3
Wild type
0.25
0.25/À6,400
2 (I)
0.125
0.031/200
0.28(S)
À
6,400
CAE3DU3
Derg3/Derg3
,
ura3/ura3
URA3
(+)
(7)
À
64
0.5/50
º
0.016 (S)
À
16
0.063/200
º
0.035 (S)
À
6,400
Darlington
erg3/erg3
,
URA3/URA3
,
erg11/erg11
(9)
À
64
16/25
º
0.25 (S)
À
16
2/50
º
0.13(S)
À
6,400
C26
CDR1
overexpressing
(10)
À
64
À
64/À6,400
2 (I)
À
16
0.016/200
º
0.03 (S)
À
6,400
C40
CaMDR
overexpressing
(10)
À
64
À
64/À6,400
2 (I)
À
16
0.016/200
º
0.03 (S)
À
6,400
1)
: The FIC indexes are calculated as described in the text, and the interpretation is indicated in the parenthesis; S, synergy; I, indifference.
293
Antifungal susceptibility test: Antifungal susceptibili-
ties were determined using a slightly modified version of
the CLSI M27A method (11). FLCZ (Pfizer Japan,
Tokyo, Japan) and ITCZ (Janssen Pharmaceuticals,
Cork, Ireland) were dissolved in dimethylsulfoxide
(DMSO; Wako, Osaka, Japan) and diluted with RPMI
1640 medium (with l-glutamine, without NaHCO
3
, and
supplemented with 2z glucose; pH 7.0 from Gibco
BRL, Paisley, Scotland) with 0.165 mol/l 3-mor-
pholinopropanesulfonic acid (MOPS); the latter was
prepared according to the manufacturer's instructions.
All solutions were sterilized by passing them through
filters (pore size, 0.22
mm; Millipore, Bedford, Mass.,
US, Millex
}
-GV Syringe Driven Filter Unit) and added
to the microtiter plates. Tested ranges of antifungal sus-
ceptibilities were 0.0625–64
mg/ml and 0.0156–16 mg/ml
for FLCZ and ITCZ, respectively. The 50z inhibitory
concentrations (IC
50
s) were defined as the minimal con-
centrations required to inhibit 50z of the growth com-
pared to control (without treatment).
Preparation of LF and iron: Bovine LF (Wako) was
dissolved in RPMI1640 media, and the tested LF con-
centration in combination with azoles was 200
mg/ml,
which represents the LF concentration in peripheral
blood (12), while 2-fold serially diluted concentrations
of LF, from 0 to 6,400
mg/ml, were tested to evaluate
the antifungal effect of LF alone. Iron sulfate II and
iron chloride III were added at 10, 50, or 100
mM.
Cell preparation: Each
C. albicans strain was grown
on a Sabouraud dextrose agar (SDA) plate for 24 h at
359
C. Five colonies of each strain were scraped out
from the plate and suspended in 5 ml of phosphate
buffered saline (PBS). Cell densities were counted on a
hemocytometer and adjusted to 5 × 10
3
cells/
ml. One
hundred microliter of this cell suspension was diluted
with 9.9 ml PBS and vortexed well; 0.5 ml of the diluted
cell suspension was then further diluted in 4.5 ml of
RPMI1640 to achieve a final concentration of 5 × 10
3
cells/ml.
Growth-inhibition assay: One hundred microliter of
the final cell suspension was applied to each well of a
sterile 96-well plastic flat-bottom plate with a cover
(Microtest
TM
Tissue Culture Plate, 96 well, flat bottom
with low evaporation lid; Becton Dickson, Sparks, Md.,
USA), and 100
ml of the prepared antifungal solutions
were added to each well at series of concentrations. The
plates were incubated for 48 h at 359
C without agita-
tion, and the turbidity was measured by absorption at
620 nm, using an automated microplate reader (Mul-
tiskan
Ascent; Labsystems, Helsinki, Finland). All ex-
periments were performed in triplicate.
Checkerboard analysis: The synergistic growth-
inhibitory effects of antifungal-LF combinations were
verified by the checkerboard method reported by
Eliopoulos and Moellering with slight modification
(13). Briefly, the fractional inhibitory concentration
(FIC) indexes were calculated as a summation of the
IC
50
for drug A in the combination/IC
50
for drug A
alone and the IC
50
for drug B in the combination/IC
50
for drug B alone. When the IC
50
s were greater than the
highest concentrations tested, the highest concentra-
tions were substituted for the IC
50
s. The effects of the
drugs were interpreted to be indicative of synergy, in-
difference, or antagonism when the FIC indexes were
º1, 1 to 4, or 4º, respectively (14).
Evaluation of intracellular FLCZ concentrations: In-
tracellular concentrations of FLCZ were measured by a
protocol as previously reported (15). Briefly, each tested
C. albicans strain was inoculated in yeast nitrogen base
broth (Difco Labs., Detroit, Mich., USA) with 2z glu-
cose (pH 6.0) and incubated with or without LF.
3
H-
thymidine-labeled FLCZ (
3
H-FLCZ), which was kindly
provided by Pfizer Japan Inc., was added to the over-
night culture to achieve a final specific radioactivity of
8.35 kBq/ml (0.225
mCi/ml). Immediately or 1 h after
the addition, excessive
3
H-FLCZ was removed by filtra-
tion and a brief wash, and the radioactivity of the incor-
porated
3
H-FLCZ was measured by LSC-5001 liquid
scintillation counter (Aloka, Tokyo, Japan). The
radioactivity was corrected for the control background
level using heat-killed cells. Viable cell numbers were
also counted by plating, and the radioactivity per cell
was calculated. Results were expressed as counts per
minute (cpm) per 10
6
cells. All experiments were per-
formed in triplicate.
Statistical analysis: The data were analyzed using
analysis of variance. Unless otherwise indicated, the
data are presented as the mean ± standard error (SE) of
triplicates. The error bars represent the SE, and the data
are representative of 2 or more individual experiments.
RESULTS
LF synergistically enhances the growth-inhibitory ef-
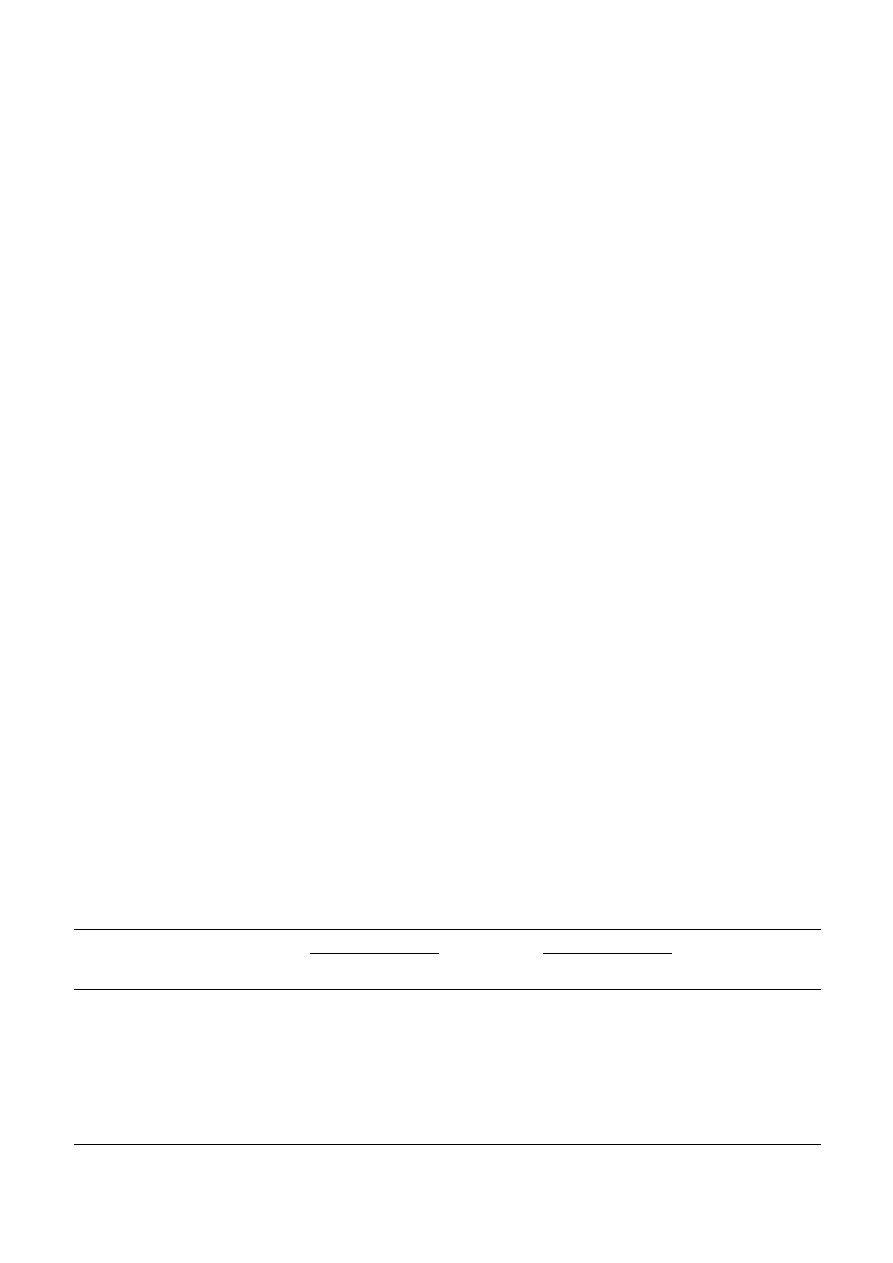
294
Fig. 1. Inhibition of C. albicans growth by fluconazole (FLCZ) or itraconazole (ITCZ) in combination with lac-
toferrin (LF). The growth of each C. albicans strain measured by optical density at 620 nm (OD
620
) is shown. Data
are presented as means of triplicates. Strains are SC5314 for A and E, CAE3DU3 for B and F, Darlington for C
and G, and C26 for D and H. Closed squares () are data for FLCZ or ITCZ alone, open squares () are for
FLCZ or ITCZ with LF. LF significantly enhanced the growth inhibitory effects of FLCZ only against CAE3DU3
and Darlington strain while LF significantly enhanced the growth inhibitory activity of ITCZ against all strains
tested.
294
295
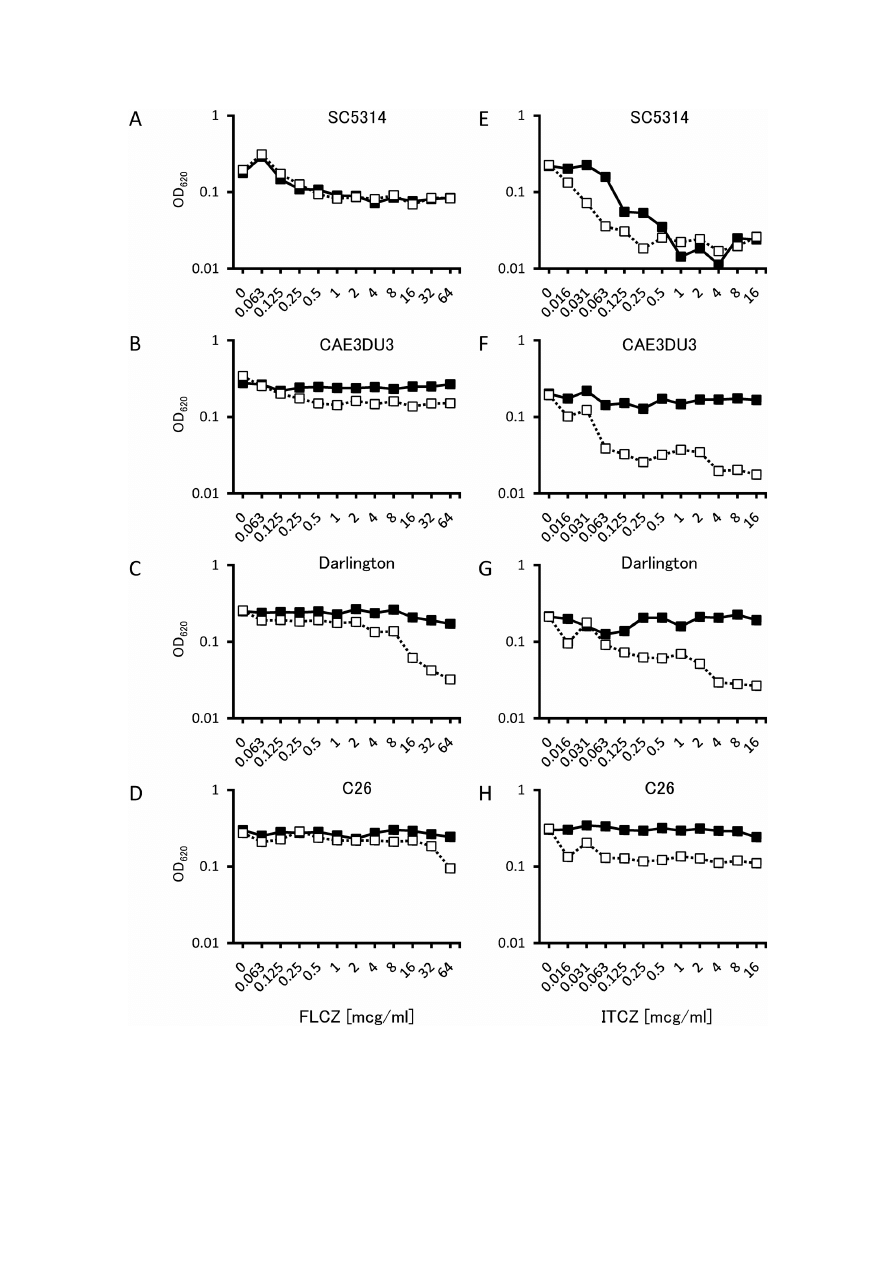
Fig. 2.
Decreased synergistic effect of LF with FLCZ by addi-
tion of iron in Darlington strain. Solid lines represent FLCZ
alone (), FLCZ with iron sulfate ($), or iron chloride ().
Dotted lines represent FLCZ with LF (), and further addition
of iron sulfate (#) or iron chloride ().
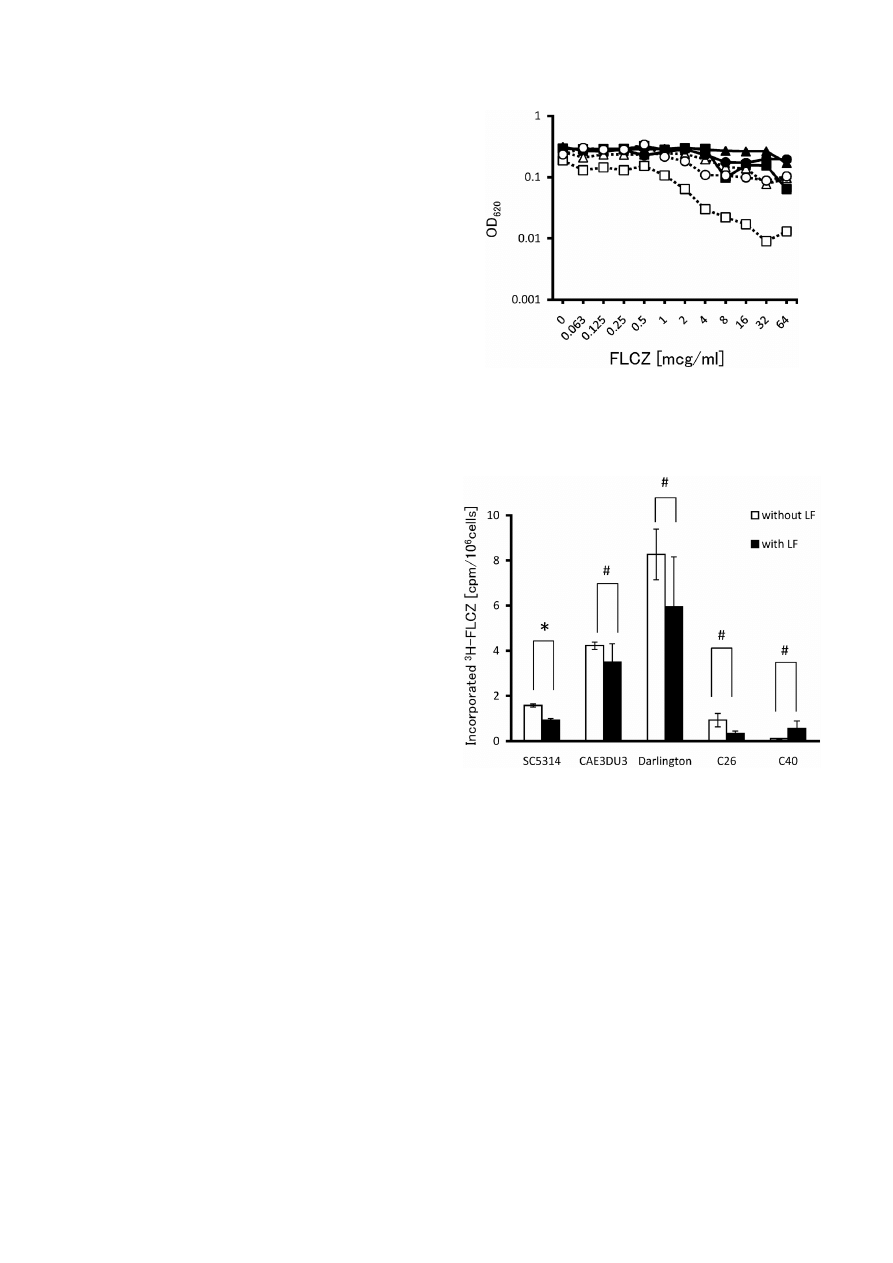
Fig. 3. Intracellular concentrations of FLCZ in C. albicans
strains in the absence and presence of LF. Intracellular concen-
trations of FLCZ are presented as the radioactive counts of in-
corporated
3
H-FLCZ in the absence and in the presence of LF,
and are expressed as cpm per 10
6
cells. Open bars and closed
bars indicate the counts for the absence of LF and for the
presence of LF, respectively. LF does not significantly promote
incorporation of
3
H-FLCZ for any strains tested though sig-
nificant difference of
3
H-FLCZ counts is observed between ab-
sence and presence of LF in SC5314.
3
H-FLCZ,
3
H-thymidine
labeled fluconazole; cpm, counts per minute. *P º 0.01;
#
No
significant difference.
295
fects of FLCZ and ITCZ: The IC
50
s of FLCZ and ITCZ
for the strains used are summarized in Table 1. Strains
other than SC5314 were extremely resistant to FLCZ
and ITCZ alone. LF alone exhibited no antifungal activ-
ity, even at a concentration of 6,400
mg/ml, although it
did increase yeast growth in the media to some extent,
contrary to our expectations. LF significantly enhanced
the growth-inhibitory effects of FLCZ in CAE3DU3 or
in the Darlington strain (Fig. 1B and C), leading to a
significant reduction of the IC
50
s (Table 1); however,
the effect of the LF-FLCZ combination remained un-
clear in the case of SC5314 and the pump mutants (Fig.
1A and D).
The effect of the LF-FLCZ combination was limited
only to ergosterol mutants; however, LF significantly
enhanced the growth-inhibitory activity of ITCZ against
all strains tested, irrespective of their azole susceptibili-
ties and resistance mechanisms (Fig. 1E–H), which led
to a significant reduction in the IC
50
s (Table 1). Thus,
LF seemed to exert different effects depending on
whether it was combined with FLCZ or ITCZ.
To verify the effects of the combinations, the FIC in-
dexes were calculated (Table 1). The FIC indexes of the
LF-FLCZ and LF-ITCZ combinations were lower than
1, except in the case of that of the LF-FLCZ combina-
tion against SC5314 and the pump mutants. Therefore,
LF was verified to exert a synergistic action with FLCZ
and ITCZ, thereby enhancing their growth-inhibitory
activity.
Exogenous iron cancels the synergism: We sought to
examine whether exogenous iron canceled the effect of
LF, since LF is known to be an iron chelator. Both iron
sulfate II and iron chloride III reversed the resistance of
the Darlington strain against FLCZ, at concentrations
of 50
mmol or higher (Fig. 2). Thus, exogenous iron
cancelled the synergistic effect of LF with FLCZ, sug-
gesting that the chelating function of LF may be related
to the synergism. It is plausible that additional iron is es-
sential for the operation of the defense mechanism of
Candida against azoles and that it may have play some
role in the promotion or suppression of ergosterol syn-
thesis in the
Candida cell membrane.
LF does not increase the intracellular concentrations
of FLCZ: LF did not promote the uptake of
3
H-FLCZ
for any of the
C. albicans strains tested (Fig. 3). The
statistically significant decrease observed for the
SC5314 strain might be attributable to experimental er-
rors, and since the decrease was not marked, it is un-
likely to contribute to the effect. These findings suggest
that the synergistic effect of LF is not due to a change in
FLCZ uptake.
DISCUSSION
Innate immunity plays an important role in protec-
tion against systemic candidiasis, and LF is an anti-in-
fective peptide secreted from human cells that acts di-
rectly on
Candida cells at the site of inflammation. LF is
believed to exert its effects via the inhibition of Fe
3+
up-
take by microorganisms, which is essential to them, but
recent studies have revealed other biochemical actions
of LF (16,17). For instance, it has been reported that
binding to bacterial polysaccharide contributes to the
reduction in the inflammation of the oral mucosa (16)
and that lactoferricin, the N-terminus of LF, has fungi-
cidal activity similar to that of defensin (17). Another
study also revealed that LF synergistically enhanced the
antifungal effects of the azole class of antifungal agents
(18), and we focused on this synergistic effect in this
study.
Although studies indicate that the synergistic effect of
LF is evident in
C. albicans strains that have low suscep-
tibility to azoles (18), it remains to be clarified whether
this particular synergism also depends on the resistance
mechanisms adopted by the strain. Our data suggest
that the antifungal synergism of the LF-FLCZ combina-

296
296
tion depends on the azole susceptibility of the strain and
the resistance mechanisms adopted by it, while that of
the LF-ITCZ combination is independent both these
factors. This reason behind the differences in the effects
of the LF-FLCZ and LF-ITCZ combinations could not
be fully elucidated, but can be partially explained by
previously reported findings. According to Goldberg et
al., a positively charged molecule such as lysozyme in-
tensifies the hydrophobicity of the cell surface of
microorganisms (19). LF is one such positively charged
molecule and can be speculated to have a similar effect
although it has not yet been proven. ITCZ is also
hydrophobic, and therefore, its antifungal activity
might be enhanced by hydrophobic interaction,
although the detailed mechanisms are not understood
(20).
Clearly, the difference in the synergism between
SC5314 and CAE3DU3 is due to the latter's ergosterol
mutation, although the other strains have such different
genetic backgrounds that it is still impossible to ascer-
tain what characteristic or characteristics actually con-
trol their response to synergism. Assays using radiola-
beled FLCZ also revealed that LF did not alter the in-
tracellular level of FLCZ, suggesting that the synergistic
effect of LF is not due to the modification of FLCZ up-
take. In addition, exogenous iron canceled the syner-
gism, and thus the chelating function of LF might con-
tribute to its synergistic antifungal activity.
With regard to the temperatures, 379C is the internal
temperature of healthy mammals, and 359
C is the opti-
mum temperature for the growth of
Candida; which
may explain both the effectiveness of fevers in fighting
candidiasis and why undernourished or otherwise com-
promised patients and individuals exposed to cold tend
to succumb to the infection.
Our previous study revealed that FLCZ had antifun-
gal activity in vivo against CAE3DU3, which is ex-
tremely resistant to FLCZ in vitro; this finding suggest-
ed the existence of some intrinsic factors that might
potentiate the antifungal activity of FLCZ (7). There-
fore, our results suggest that both FLCZ and ITCZ can
act in tandem with endogenous LF, thereby increasing
their clinical efficacy against
C. albicans to an extent
greater than that expected by the traditional in vitro sus-
ceptibility of the organisms. In addition, reports indi-
cate that LF concentrations decreased under certain
conditions, such as those found in AIDS patients (21),
and thus, exogenous LF can be considered a promising
therapy to potentiate the antifungal activity of FLCZ
and ITCZ when endogenous LF is insufficient.
In conclusion, the development of new clinical treat-
ments and therapeutic approaches against
Candida
resistance will depend on our understanding of the
mechanisms underlying this resistance, and the applica-
tion of various combinations exhibiting synergistic ac-
tion. The findings of this study shed some light in this
regard.
Acknowledgments
This work was partly presented in the 42th
Interscience Conference on Antimicrobial Agents and Chemotherapy,
and is supported by grants from the Ministry of Health, Labour and
Welfare of Japan.
Conflict of interest
None to declare.
REFERENCES
1. Horn, D.L., Neofytos, D., Anaissie, E.J., et al. (2009): Epidemi-
ology and outcomes of candidemia in 2019 patients: data from
the prospective antifungal therapy alliance registry. Clin. Infect.
Dis., 48, 1695–1703.
2. Venkatesh, M.P. and Rong, L. (2008): Human recombinant lac-
toferrin acts synergistically with antimicrobials commonly used in
neonatal practice against coagulase-negative staphylococci and
Candida albicans
causing neonatal sepsis. J. Med. Microbiol., 57,
1113–1121.
3. Wakabayashi, H., Abe, S., Teraguchi, S., et al. (1998): Inhibition
of hyphal growth of azole-resistant strains of Candida albicans by
triazole antifungal agents in the presence of lactoferrin-related
compounds. Antimicrob. Agents Chemother., 42, 1587–1591.
4. Miyazaki, Y., Geber, A., Miyazaki, H., et al. (1999): Cloning, se-
quencing, expression and allelic sequence diversity of ERG3 (C-5
sterol desaturase gene) in Candida albicans. Gene, 236, 43–51.
5. Vanden Bossche, H., Marichal, P., Gorrens, J., et al. (1990): Mu-
tation in cytochrome P-450-dependent 14 alpha-demethylase
results in decreased affinity for azole antifungals. Biochem. Soc.
Trans., 18, 56–59.
6. Albertson, G.D., Niimi, M., Cannon, R.D., et al. (1996): Multi-
ple efflux mechanisms are involved in Candida albicans flucona-
zole resistance. Antimicrob. Agents Chemother., 40, 2835–2841.
7. Miyazaki, T., Miyazaki, Y., Izumikawa, K., et al. (2006):
Fluconazole treatment is effective against a Candida albicans
erg3/erg3 mutant in vivo despite in vitro resistance. Antimicrob.
Agents Chemother., 50, 580–586.
8. Warnock, D.W., Johnson, E.M., Richardson, M.D., et al.
(1983): Modified response to ketoconazole of Candida albicans
from a treatment failure. Lancet, 1, 642–643.
9. Kakeya, H., Miyazaki, Y., Miyazaki, H., et al. (2000): Genetic
analysis of azole resistance in the Darlington strain of Candida
albicans.
Antimicrob. Agents Chemother., 44, 2985–2990.
10. Sanglard, D., Kuchler, K., Ischer, F., et al. (1995): Mechanisms
of resistance to azole antifungal agents in Candida albicans iso-
lates from AIDS patients involve specific multidrug transporters.
Antimicrob. Agents Chemother., 39, 2378–2386.
11. Clinical Laboratory Standards Institute (2008): Reference
Method for Broth Dilution Antifungal Susceptibility Testing of
Yeasts; Approved Standard–3rd ed. M27-A3. Clinical Laborato-
ry Standards Institute, Wayne, Pa.
12. Barthe, C., Galabert, C., Guy-Crotte, O., et al. (1989): Plasma
and serum lactoferrin levels in cystic fibrosis. Relationship with
the presence of cystic fibrosis protein. Clin. Chim. Acta, 181,
183–188.
13. Eliopoulos, G.M. and Moellering, R.C. (1991): Antimicrobial
combinations. p. 432–492. In Lorian, V. (ed.), Antibiotics in
Laboratory Medicine. 3rd ed. Williams & Wilkins, Baltimore.
14. Johnson, M.D., MacDougall, C., Ostrosky-Zeichner, L. et al.
(2004): Combination antifungal therapy. Antimicrob. Agents
Chemother., 48, 693–715.
15. Bennett, J.E., Izumikawa, K. and Marr, K.A. (2004): Mechanism
of increased fluconazole resistance in Candida glabrata during
prophylaxis. Antimicrob. Agents Chemother., 48, 1773–1777.
16. Tomita, M., Yamauchi, K., Teraguchi, S., et al. (1998): Host
defensive effects of orally administered bovine lactoferrin. Adv.
Exp. Med. Biol., 443, 189–197.
17. Bellamy, W., Wakabayashi, H., Takase, M., et al. (1993): Killing
of Candida albicans by lactoferricin B, a potent antimicrobial
peptide derived from the N-terminal region of bovine lactoferrin.
Med. Microbiol. Immunol., 182, 97–105.
18. Kuipers, M.E., de Vries, H.G., Eikelboom, M.C., et al. (1999):
Synergistic fungistatic effects of lactoferrin in combination with
antifungal drugs against clinical Candida isolates. Antimicrob.
Agents Chemother., 43, 2635–2641.
19. Goldberg, S., Doyle, R.J. and Rosenberg, M. (1990): Mechanism
of enhancement of microbial cell hydrophobicity by cationic
polymers. J. Bacteriol., 172, 5650–5654.
20. Zasloff, M. (2002): Antimicrobial peptides of multicellular organ-
isms. Nature, 415, 389–395.
21. van der Strate, B.W., Harmsen, M.C., The, T.H., et al. (1999):
Plasma lactoferrin levels are decreased in end-stage AIDS
patients. Viral Immunol., 12, 197–203.
Wyszukiwarka
Podobne podstrony:
Abstract Synergistic Antifungal Effect of Lactoferrin with Azole Antifungals against Candida albican
Synergistic Fungistatic Effects of Lactoferrin in Combination with Antifungal Drugs against Clinical
Effect of long chain branching Nieznany
Effect of Kinesio taping on muscle strength in athletes
53 755 765 Effect of Microstructural Homogenity on Mechanical and Thermal Fatique
Effect of File Sharing on Record Sales March2004
31 411 423 Effect of EAF and ESR Technologies on the Yield of Alloying Elements
21 269 287 Effect of Niobium and Vanadium as an Alloying Elements in Tool Steels
(10)Bactericidal Effect of Silver Nanoparticles
Effect of?renaline on survival in out of hospital?rdiac arrest
Effects of the Great?pression on the U S and the World
4 effects of honed cylinder art Nieznany
Effects of the Atomic Bombs Dropped on Japan
Effect of aqueous extract
Effect of Active Muscle Forces Nieznany
Effects of Kinesio Tape to Reduce Hand Edema in Acute Stroke
1 Effect of Self Weight on a Cantilever Beam
effect of varying doses of caffeine on life span D melanogaster
Possible Effects of Strategy Instruction on L1 and L2 Reading
więcej podobnych podstron