Chemistry for Everyone
900
Journal of Chemical Education • Vol. 78 No. 7 July 2001 • JChemEd.chem.wisc.edu
Products of Chemistry
Microwave Ovens—Out of the Kitchen
Sarah L. Cresswell*
School of Applied and Molecular Sciences, University of Northumbria at Newcastle, Newcastle Upon Tyne NE1 8ST, UK;
sarah.cresswell@unn.ac.uk
Stephen J. Haswell
Department of Chemistry, Hull University, Cottingham Road, Hull HU6 7RX, UK
We are all aware of the advantages of cooking our meals
in a microwave oven, but what of the advantages of using
microwave ovens to do laboratory experiments? Since domestic
ovens first appeared in the early 1980s homes around the
world have been preparing meals in far less time than was
required by a standard convection oven and this technology
was only a small step away from similar usage in laboratories.
In 1986, papers by Richard Gedye and co-workers at
Laurentian University (
1) and George Majetich and R. J.
Giguere at the University of Georgia (
2) demonstrated that
the rate of a number of organic reactions could be increased
using a commercially available microwave oven. These papers
formed the basis of an ever-increasing range of research
applications over the next 15 years.
In the early years, the majority of published work described
digestion reactions in direct comparison to hot-plate digestion
methods (
3–7 ). The samples that are now being studied range
from environmental specimens
(
8), coal and ash materials
(
9),
and foodstuffs and oils
(
10) to metals in wine, beer, and other
alcoholic beverages
(
11). Today microwave radiation is used in
nearly all areas of chemistry. Synthetic reactions are carried
out to prepare organic (
1), organometallic (12, 13), and in-
organic materials (
14) such as catalysts. Ashing (15–17 ), ex-
traction (
18–21), and digestion (22–24) procedures can also
be routinely carried out.
Heating
Microwave radiation lies between infrared and radio fre-
quencies of the electromagnetic spectrum at wavelengths from
1 cm to 1 m (corresponding to 30 GHz to 300 MHz). Because
much of this range is dedicated to radar and telecommuni-
cations, microwave ovens are restricted to 12.2 cm or 33.3 cm
(2.45 GHz or 900 MHz) in order to prevent interferences
(
25).
Domestic microwave ovens are usually at a frequency of
2.45 GHz.
To understand how reactions can occur faster under micro-
wave irradiation, we must first consider the heating process
itself. When a sample is heated on a hot-plate, a convection
process takes place. The heat is transferred from the hot-plate
to the vessel and in turn from the vessel to the liquid inside
it—an inefficient method of heating. Microwave dielectric
heating uses the ability of some liquids to transform electro-
magnetic energy into heat and propagate chemical reactions
(
26 ), removing the need to heat the container.
If a liquid is exposed to microwave radiation, the micro-
waves induce rotation of the dipoles within the liquid, causing
polar molecules to align and relax in the field of oscillating
electromagnetic radiation. Energy is dissipated from these
dipole rotations, which causes the liquid to become hot. In
such a way, the heat is produced within the liquid and not
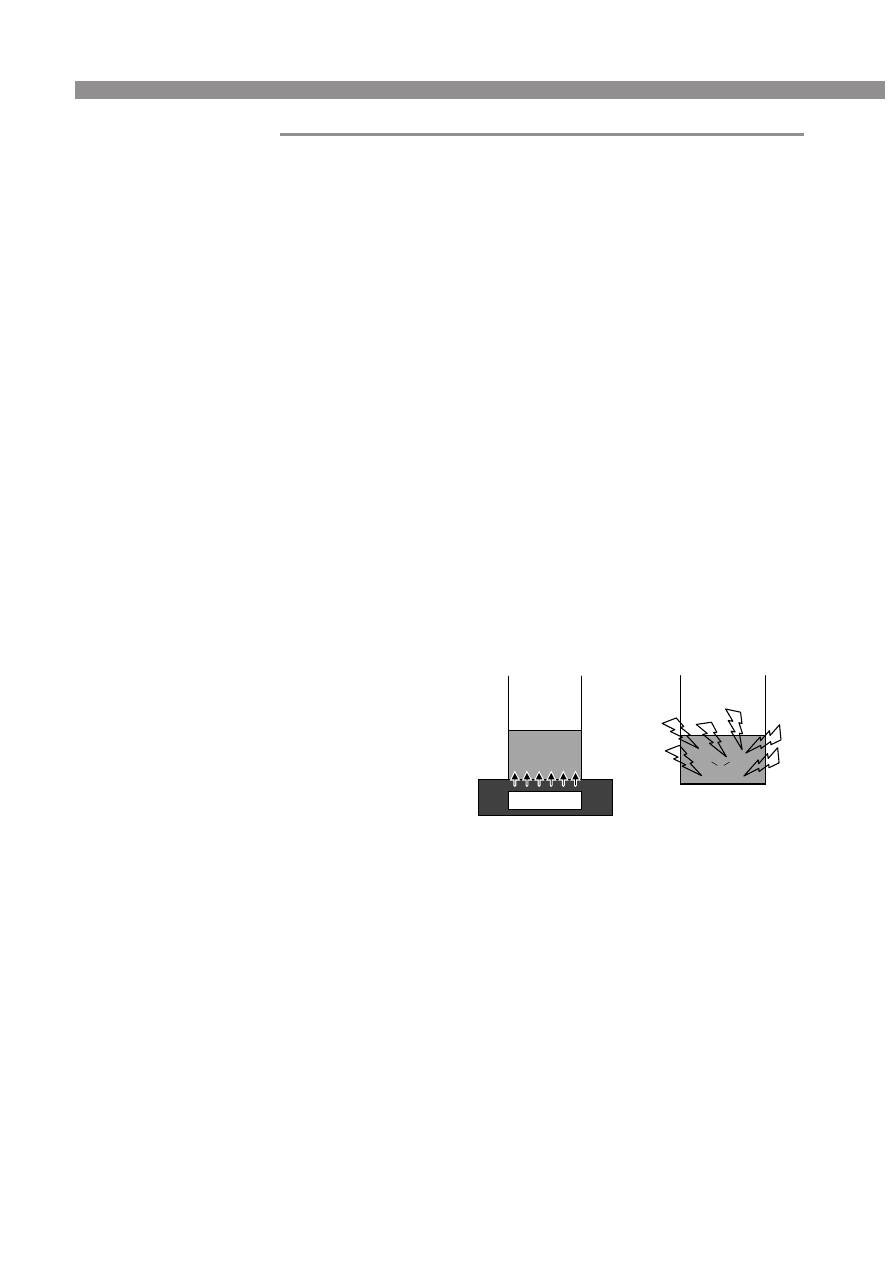
transferred from the vessel as in the hot-plate system (Fig. 1). In
a microwave oven, the liquid is therefore often at a higher tem-
Figure 1. Comparison of methods of heating. (a) Heat from the hot-
plate heats the glass base of the beaker and is transferred to the
solution by convection. The solution heats slowly. (b) Microwaves pass
through the vessel walls directly into the polar liquid. Microwaves
interact with dipolar molecules, causing rotation and vibration of
the molecular bonds, which results in heating of the mixture.
HOT PLATE
Hot-Plate Heating
δ+
δ+
δ−
O
H
H
Microwave Heating
a
b
Chemistry for Everyone
JChemEd.chem.wisc.edu • Vol. 78 No. 7 July 2001 • Journal of Chemical Education
901
perature than the vessel in which it is held. This efficient heat-
ing has been reported to lead to the increases in reaction rates,
increases in yields, and improved extraction efficiencies (
27).
Microwave Ovens
Most early research work was carried out using com-
mercially available domestic microwave ovens, although
considerations for safety and the need to have controllable and
reproducible heating has led to the development of specially
designed equipment. The new microwave ovens were fitted
with temperature and/or pressure measurement devices,
which made it possible to monitor a reaction while it was
taking place. This, coupled with improved safety, allowed
advances in the field to continue.
In parallel with the development of improved microwave
ovens, the vessels used in microwave experiments have evolved
too. In many cases the vessels are sealed (closed vessels) and
pressure therefore plays a part in the reaction. Vessels need
to be transparent to microwaves for efficient heating to take
place and are usually made of polytetrafluoroethylene (PTFE)
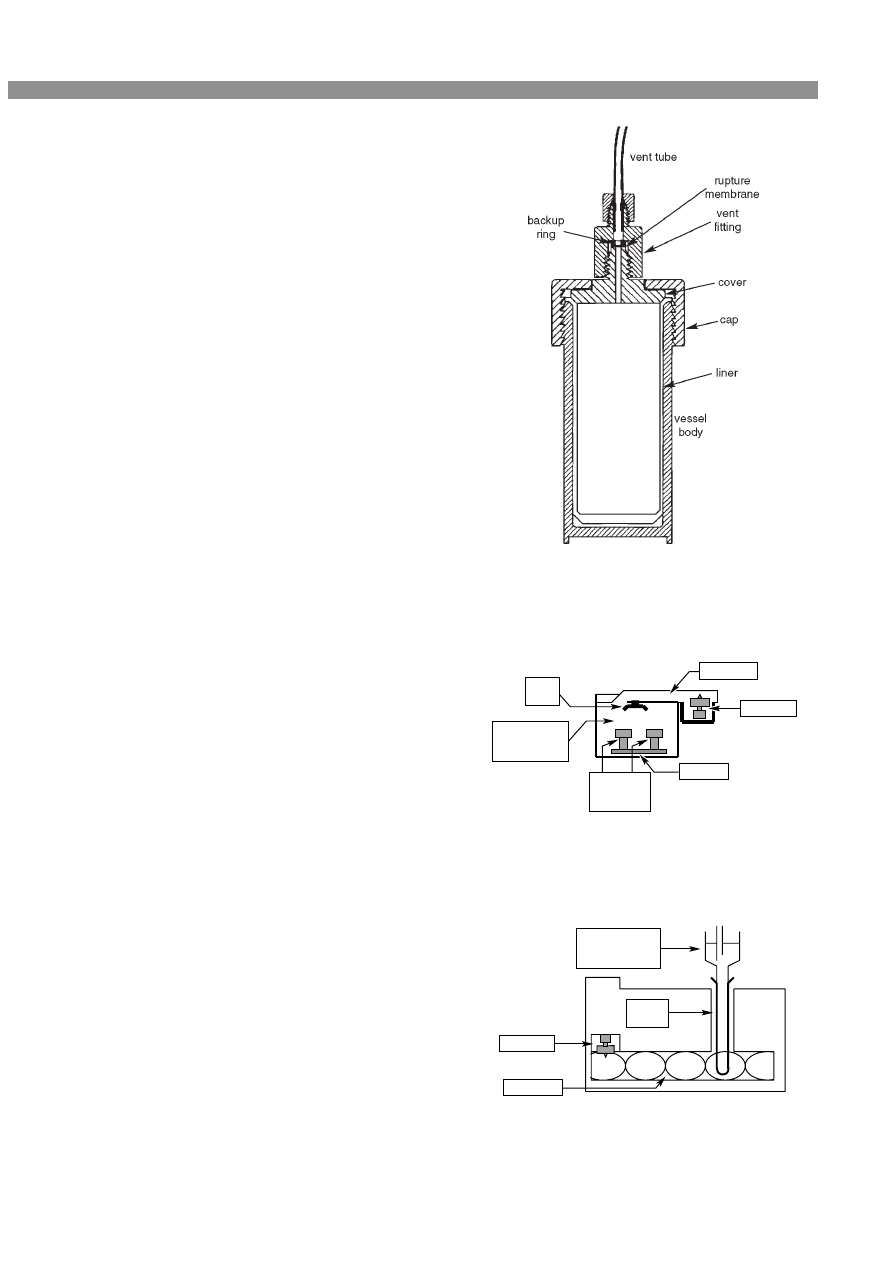
or a similar material. They have been designed with a pres-
sure outlet valve or rupture membrane, which will vent if
safe pressure is exceeded (see Fig. 2). In line with the wide
range of reactions for which microwaves can be used, there
is a wide range of vessel types. Recent advances in the manu-
facture of microwave ovens and reaction vessels has led to a
system in which each vessel can withstand up to 1500 psi
and can be independently monitored for temperature
throughout the heating process.
Components of a Microwave Oven
A schematic diagram of a microwave oven is shown in
Figure 3. Some components and features of a microwave are
described below.
Magnetron – where microwaves are generated.
Waveguide – rectangular channel of metal with reflec-
tive walls to allow transmission of microwaves from the
magnetron to the microwave cavity.
Microwave Cavity – internal space of the oven where
samples can be placed for heating. Usually contains a
turntable to ensure that each sample experiences the same
average heating. The cavity has reflective walls to prevent
leakage of microwaves and to increase the efficiency of
the oven.
Mode Stirrer – a reflective fan-shaped paddle, which
ensures that incoming energy is distributed evenly
throughout the cavity.
Door Interlocks – safety devices to prevent the door from
being opened while the microwave energy is on.
Safety
As for all laboratory equipment, safety is an important
issue. Since many reactions are performed in closed vessels
and involve the heating of solvents beyond their boiling point,
there is a risk of explosion. Decomposition reactions, which
involve the use of acids, often produce gaseous by-products
that can result in an increase in the pressure within a closed
vessel. For example, if one heats a sample having a large
Figure 2. Closed vessel for microwave extraction and digestion.
Courtesy of CEM (Microwave Technology) Ltd, UK.
Figure 3. Schematic diagram of a microwave oven.
magnetron
wave guide
microwave
transparent
vessels
mode
stirrer
microwave
cavity with
reflective walls
turntable
Figure 4. An open-vessel microwave system.
magnetron
wave guide
sample
tube
refluxing and
reagent addition
adapter

Chemistry for Everyone
902
Journal of Chemical Education • Vol. 78 No. 7 July 2001 • JChemEd.chem.wisc.edu
number of similar bonds (a polymer), when the bonds are
degraded there is a rapid release of carbon dioxide.
This type of problem led researchers to look at the pos-
sibility of developing microwave-assisted systems that were
open to the atmosphere and therefore unable to reach elevated
pressures. These types of microwaves are often referred to as
“open-vessel systems”. Since the formation of gaseous by-
products is no longer an issue with this type of system, larger
sample sizes are possible—up to 1–2 g. An example of such
a open-vessel-type microwave system can be seen in Figure
4. With this apparatus it is possible to perform a number of
reagent addition steps, with controllable microwave heating,
in six individual vessels.
Microwaves in Synthetic Chemistry
Even with all these advances in technology the question
still remains, can we use the microwave heating effect to our
advantage? In the field of combinatorial chemistry microwave
ovens have found a niche. Combinatorial chemistry relies on
the fast preparation of a large number of similar compounds
for screening. Ian Cotterill and colleagues at EnzyMed in Iowa
City have developed a highly efficient microwave-assisted
combinatorial synthesis (MICROCOS) technology
(
28). Using
microwave energy as the power source, the system has enabled
a rapid increase in their high-throughput, automated, one-
step parallel synthesis of diverse substituted pyridines based
on the Hantzsch reaction. They have shown that there are
many advantages to using this technique, including improved
product recovery, shorter reaction times, and increased yields.
Some excellent reviews are available on this area of microwave-
enhanced chemistry (
14, 29).
Although a great deal of research is carried out on organic
reactions, this is not exclusive. Inorganic-chemistry-based
research into the use of microwaves in the synthesis and
modification of zeolite catalysts has been carried out by Colin
Cundy at UMIST
(
14). He found that the use of this technique
results in rapid reactions, which give pure products with good
crystal structure. He also found that in some cases microwave
energy can enhance the selectivity of a reaction.
Microwaves in Sample Preparation
The trend toward processing larger numbers of samples
for analysis has promoted the desire to speed up this process
considerably (
30). When looking at the extraction of organic
molecules from a sample matrix it is usual to attempt to extract
the analyte molecules into an organic solvent, usually under
reflux conditions, as with Soxhlet extraction methodology.
Soxhlet extraction is both time consuming and solvent
hungry. In addition it usually requires extraction times in
excess of 6 hours and is not an easy process to automate.
Therefore there is an obvious advantage to introducing micro-
wave extraction prior to chromatographic or spectroscopic
analysis in order to greatly reduce the total analysis time. In
1994, Jocelyn Pare and co-workers described the fundamental
physical phenomena of this process and showed how micro-
waves could be used in extractions from plant and animal
tissues, water, soil, consumer products, and cosmetics
(
31).
Looking at the preparation of solid samples for compo-
sitional analysis, Joanna Szpunar and colleagues found that
the lengthy sample-preparation step rather than the speed of
the chromatography limited the total analysis time
(
32). They
used low-power focused microwaves to reduce the time
needed for quantitative isolation of analytes from 24 hours
to between 3 and 5 minutes. They were also able to sharply
reduce the volumes of solvents they used.
Over the past few years a number of papers have looked at
the use of microwave energy in extraction procedures. In all cases,
the researchers concluded that faster reactions and higher
extraction efficiencies are possible with microwave-assisted
techniques than with conventional methodology.
It is not only in the field of extraction that microwaves
can be used for sample preparation; microwave digestion of
materials for inorganic elemental analysis is also an important
process. Sample digestion is usually performed by wet or dry
decomposition, which releases and stabilizes the analytes of
interest as a liquid sample.
In microwave digestion, closed vessels are again employed
in a batch method, although online “continuous-flow” systems
have been developed for routine analysis of multiple samples
(
33). Like microwave extraction procedures, microwave diges-
tion decreases time requirements and increases controllability
and reaction yields. However, the use of microwaves as a di-
gestion energy source depends upon the correct selection of
the acid or acid mixture and method employed.
Metal speciation studies using microwave energy sources
have also been published. Paul Worsfold and colleagues of
Plymouth University described an online microwave system
for determining the oxidation states of selenium in biological
and environmental samples (
34). They first analyzed for
selenium(IV) and then with the assistance of rapid micro-
wave heating chemically reduced the selenium(VI) to its IV
form. This allowed them to measure the total selenium
present and, by difference, to determine the selenium(VI)
present in samples.
Chun-mao Tseng and co-workers in Pau studied the
leaching of mercury species from sediments and biomaterials
(
35). Using a two-step procedure in open microwave vessels
they were able to reduce the sample preparation time from
1–2 hours to just 2–4 minutes.
Continuous-Flow Systems
Most of the applications described here are batch processes.
Each microwave vessel holds a discrete sample. In some cases
the efficiency of microwave processes is limited by the re-
stricted number of samples that can be digested or extracted
in any one run. Then these vessels must be left to cool
before analysis. If this process could be made continuous, so
that samples could be extracted or digested in an online system
and directly analyzed, the throughout of samples would be
greatly increased.
Since the first step toward this goal was made by Strauss, at
CISRO in Australia (
36 ), continuous-flow microwave systems
have been manufactured by CEM Corporation in North
Carolina and more recently by Milestone. Using this instru-
mentation, we developed a method for the online extraction
of polycyclic aromatic hydrocarbons (PAHs) from sediment
(
37 ). Our results show that our extraction is as effective as
and in some cases more effective than the U.S. Environmental
Protection Agency (EPA) batch microwave method (
38).

Chemistry for Everyone
JChemEd.chem.wisc.edu • Vol. 78 No. 7 July 2001 • Journal of Chemical Education
903
Ultimately the aim is to produce a system that can be run
without constant supervision. Complete automation of the
sample extraction and analysis steps would lead to a substan-
tial increase in sample throughput and accuracy and would
significantly reduce the chance of sample contamination.
The disadvantage of making a continuous-flow system
at the moment is that most magnetrons work on a duty cycle.
This means that the duration of microwave production is
varied rather than the power of the microwaves. For example,
to obtain 50% power, the magnetron would be on full power
for 10 seconds and then off for 10 seconds—on average, 50%
power. This is a problem for continuous-flow samples because
if a sample happens to be passing through the microwave cavity
during the power-off section of the duty cycle, it will not be
irradiated.
Another advantage of continuous-flow systems is a reduc-
tion in the volumes of solvent required. During the extraction
of PAHs we used 40% less solvent than is required for the
EPA batch method. This may be significant if a large number
of samples are analyzed or if regulations governing the disposal
of organic solvents are an issue.
Process Applications
Microwave instruments are starting to find uses in other,
less obvious areas—for example, process control. Theisen
(
39)
has produced a low-power microwave sensor capable of making
density and concentration measurements, which is currently
in use as a process-control mechanism in the sugar industry.
Another example is the use of high-power microwave
energy to cure resins. Boey’s group at Nanyang Technological
University in Singapore (
40) compared microwave curing
with conventional curing of a thermosetting resin and found
that the microwave procedure was faster and easier to control.
Like Theisen’s sensor, its main advantage, however, was that
it could be operated within a process environment.
Since microwaves are able to selectively heat materials
on the basis of their structure, Robert Osiander and co-workers
are attempting to develop nondestructive thermographic
evaluation techniques for the analysis of materials
(
41). This will
work because the defects in a material and the rest of the
sample matrix will heat at different rates, allowing identifi-
cation of the presence, quantity, and distribution of defects.
Nonthermal Effects
No review on microwave chemistry would be complete
without mention of nonthermal effects. As anyone who owns
a mobile phone knows, there are numerous articles in the
press about the possibility of microwaves causing damage to
brain tissue. Evidence for the existence of nonthermal effects
has been mounting over the past 5 years or so.
Work at Ohio State University by Sheryl Barringer and
co-workers (
42) showed that the rate of heating of oil–water
mixtures and emulsions depends upon the dispersion of the
two liquids. This was attributed to the increased power ab-
sorption at the large number of interfaces present, and while
interesting, it may or may not be a real nonthermal effect.
A search for specific local microwave interaction in a
single amphiphilic bilayer assembled on silicon was carried out
by Rirka Moaz and co-workers at the Weizmann Institute
in Israel
(
43). When irradiated with microwaves the bilayer
became depleted, and this effect could not be repeated using
a conventional thermal heat source.
It is also possible to influence the retention time of
components on a silica–diol column held within a microwave
field (
44), an effect that was attributed to nonthermal micro-
wave properties. Although these findings are not conclusive,
there does appear to be evidence to support the existence of
nonthermal microwave effects. More research is obviously
needed.
Microwave Ovens in Undergraduate Laboratories
Laboratory classes are an important part of any under-
graduate degree course and should provide students with the
chance to undertake experiments that mirror industrial reac-
tions. In microwave chemistry there are a number of reactions
that lend themselves to undergraduate experiments (
45–48).
For example, Ng et al. (
49) used a domestic microwave oven to
demonstrate the advantages of microwave curing of polymers.
They used Fourier-transform infrared spectroscopy (FTIR)
to compare the percentage curing of methyl methacrylate by
thermal and microwave oven methods. They concluded micro-
wave curing offers faster curing times, improved efficiency,
and enhanced properties of the polymer.
The use of microwaves to enhance the rate of organic
reactions is well documented by Bose (
50) and co-workers, who
have devised a number of undergraduate laboratories that
demonstrate the advantages of microwave heating. Students are
able to carry out synthetic organic reactions in open vessels
in domestic microwave ovens. Promising results have been
achieved with a number of reactions, including the Bischler–
Napieralski reaction, the Wolff–Kishner reduction, and free-
radical dehalogenation reactions. These reactions come un-
der their heading of MORE (Microwave-Induced Organic-
Reaction Enhancement) chemistry techniques. Using MORE,
Bose et al. developed and tested a number of meaningful,
safe, and inexpensive synthetic experiments for undergraduate
students.
Conclusions
Microwave ovens have successfully made the transition
from our kitchens to laboratories and have been used to en-
hance many reactions. But the story does not end here; re-
search in the many fields of microwave chemistry will con-
tinue. Already this year some 45 or so papers have been pub-
lished in this field. These include “Automated Microwave
Digestion of Certifiable Color Additives for Determination of
Mercury by Cold Vapor Atomic Absorption Spectrometry” (
51)
and “Microwave Assisted Solid Support Synthesis of Novel
1,2,4-Triazolo[3,4-b]-1,3,4-thiadiazepines As Potent Anti-
microbial Agents” (
52). The relationship between microwave
power applications and spectroscopic measurements in the
microwave region seems also to be an exciting area for future
development.
Acknowledgment
Figure 2 was produced with kind permission of CEM
(Microwave Technology) Ltd, UK.

Chemistry for Everyone
904
Journal of Chemical Education • Vol. 78 No. 7 July 2001 • JChemEd.chem.wisc.edu
Literature Cited
1. Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L.;
Laberge, L.
Tetrahedron Lett. 1986, 27, 279–282.
2. Giguere, R. J.; Bray, T. L.; Duncan, S. N.; Majetich, G.
Tetra-
hedron Lett. 1986, 28, 4945–4948.
3. Abu-Samra, A.; Morris, J. S.; Koirtyohann, S. R.
Anal. Chem.
1995, 47, 1475.
4. Abu-Samra, A.; Morris, J. S.; Koirtyohann, S. R.
Trace Subst.
Environ. Health 1975, 9, 297.
5. Abu-Samra, A.; Koirtyohann, S. R.; Morris, J. S.
Anal. Chem.
1975, 47, 1475–1477.
6. Kingston, H. M.; Jassie, L. B.
Anal. Chem. 1986, 58, 2534–
2541.
7. Kingston, H.
M.; Jassie, L.
B. In
Introduction to Microwave
Sample Preparation:
Theory
and
Practice;
Jassie,
L.
B.;
Kingston,
H.
M., Eds.; American Chemical Society: Washington DC,
1988; p 93.
8. Martinez-Garcia, M. L.; Zubieta, A. C.; Lorenzo, S. M.;
Lopez-Mahia, P.; Rodriguez, D. P.
Anal. Lett. 1999, 32, 139–
157.
9. Lachas, H.
Analyst 1999, 124, 177–184.
10. Pare, J. R. J.; Matini, G.; Belanger, J. M. R.; Li, K.; Rule, C.;
Thibert, B.; Yaylayan, V.; Liu, Z.; Mathe, D.; Jacquault, P.
J.
AOAC Int. 1997, 80, 928–933.
11. Lopez, F. F.; Cabrera, C.; Lorenzo, M. L.; Lopez, M. C.
Sci.
Tot. Environ. 1998, 220, 1–9.
12. Ali,
M.;
Bond,
S.
P.;
Mbogo,
S.
A.;
McWhinnie,
W.
R.;
Watts,
P.
M.
J. Organomet. Chem. 1989, 371, 11–13.
13. Dabirmanesh, Q.; Roberts, R. M. G.
J. Organomet. Chem.
1993, 460, C28–C29.
14. Cundy, C. S.
Collect. Czech. Chem. Commun. 1998, 63, 1699–
1723.
15. Zhang, H.; Dotson, P.
Commun. Soil Sci. Plant Anal. 1994,
25, 1321–1327.
16. Gillain, G.
Talanta 1982, 29, 651–654.
17. Dunemann, L.; Meinerling, M.
Fresenius J. Anal. Chem. 1992,
342, 714–718.
18. Sarawati,
R.;
Vetter,
T.
W.;
Watters,
R.
L.
Analyst
1995,
120,
95–99.
19. Llompart,
M.
P.;
Lorenzo,
R.
A.;
Cela,
R.
F.;
Li,
K.;
Belanger,
J.
M.
R.; Pare, J. M. J.
J. Chromatogr. A 1997, 774, 243–251.
20. Tomaniova, M.; Hajslova, J.; Pavelka, J.; Kocourek, V.;
Holadova, K.; Klimova, I.
J. Chromatogr. A 1998, 827, 21–29.
21. Xiong, G. H.; Tang, B. Y.; He, Z. Q.; Zhao, M. Q.; Zhang,
Z. P.; Zhang, Z. X.
Talanta 1999, 48, 333–339.
22. Zischka, M.; Kettisch, P.; Kairnrath, P.
Atom. Spectrosc. 1998,
19, 223–227.
23. Perez-Cid, B.; Lavilla, I.; Bendicho, C.
Anal. Chim. Acta 1999,
378, 201–210.
24. Sahuquillo, A.; Rubio, R.; Rauret, G.
Analyst 1999, 124, 1–4.
25. Kappe, C. O.
Microwave Enhanced Chemistry; http://www-ang.
kfunigraz.ac.at/~kappeco/microwave.htm (accessed Mar 2001).
26. Mingos, D. M. P.; Baghurst, D. R.
Chem. Soc. Rev. 1991, 20,
1–47.
27. Whittaker, G.
Microwave Chemistry Pages; http://www. ed.ac.uk/
~ah05/microwave.html (accessed Apr 2001).
28. Cotterill, I. C.; Usyatinsky, A. Y.; Arnold, J. M.; Clark, D. S.;
Dordick, J. S.; Michels, P. C.; Khmelnitsky, Y. L.
Tetrahedron
Lett. 1998, 39,
1117–1120.
29. Caddick, S.
Tetrahedron 1995, 51, 10403–10432.
30. Kingston, H. M.
SamplePrep Web; http://www.sampleprep.
duq.edu/sampleprep/ (accessed Mar 2001).
31. Pare, J. R. J.; Belanger, J. M. R.; Stafford, S. S.
Trends Anal.
Chem. 1994, 13 (4), 176–184.
32. Szpunar, J.; Schmitt, V. O.; Donard, O. F. X.; Lobinski, R.
Trends Anal. Chem. 1996, 15
(4), 181–187.
33. Burguera, M.; Burguera, J. L.
Anal. Chim. Acta 1998, 366
(1–3), 63–80.
34. Pitts, L.; Worsfold, P. J.; Hill, S. J.
Analyst 1994, 119, 2785–
2788.
35. Tseng, C. M.; Garraud, H.; Amouroux, D.; Donard, O. F.
X.; de Diego, A.
J. Automat. Chem. 1998, 20(4), 99–108.
36. Cablewski, T.; Faux, A. F.; Strauss, C. R.
J. Org. Chem. 1994,
59, 3408–3412.
37. Cresswell, S. L.; Haswell, S. J.
Analyst 1999, 124, 1361–1366.
38.
Microwave-Assisted Solvent Extraction; EPA Method 3546; U.S.
Environmental Protection Agency: Washington, DC, 1996.
39. Theisen, K. H.
Zuckerindustrie 1999, 124, 157–158.
40. Chia, L. H. L.; Jacob, J.; Boey, F. Y. C.
J. Mater. Process.
Technol. 1995, 48
(1–4), 445–449.
41. Osiander, R.; Spicer, J. M. W.; Murphy, J. C.
Mater. Eval.
1995, 53, 942–948.
42. Barringer, S. A.; Ayappa, K. G.; Davis, E. A.; Davis, H. T.;
Gordon, J.
J. Food Sci. 1995, 60, 1132–1136.
43. Moaz, R.; Cohen, H.; Sagiv, J.
Langmuir 1998, 14, 5988–
5993.
44. Haswell, S. J.; Howarth, N.
Anal. Chim. Acta 1999, 387
(2),
113–120.
45. Bari, S. S; Bose, A. K.; Chaudhary, A. G.; Manhas, M. S.;
Raju, V. S.; Robb, E. W.
J. Chem. Educ. 1992, 69, 938–939.
46. Nelson,
D.
A.;
Devin,
C.;
Hoffmann,
S.;
Lau,
A.
Abstracts
of
Papers, Part 1, 213th National Meeting of the American
Chemical Society, San Francisco, Apr 13–17, 1997; Ameri-
can Chemical Society: Washington, DC, 1997; CHED 101.
47. Freeman, R. G.; McCurdy, D. L.
J. Chem. Educ. 1998, 75,
1033–1034.
48. Banik, B. K.; Barakat, K. J.; Wagle, D. R.; Manhas, M. S.;
Bose, A. K.
J. Org. Chem.
1999, 64, 5746–5753.
49. Ng, L. T.; Chia, L. H. L.
Polym. Int. 1999, 48, 952–955.
50. Bose, A. K.; Manhas, M. S.; Banik, B. K.; Robb, E. W.
Res.
Chem. Intermed. 1994, 20, 1–11.
51. Hepp, N. M.; Cargill, A. M.; Shields, W. B.
J. AOAC Int.
2001, 84, 117–122.
52. Kidwai, M.; Sapra, P.; Misra, P.; Saxena, R. K.; Singh, M.
Bioorg. Med. Chem. 2001, 9, 217–220.
Wyszukiwarka
Podobne podstrony:
Breaking out of the Balkans Ghetto Why IPA should be changed
Out of the Armchair and into the Field
Journeys Out of the Body
HP BladeSystem Adaptive Infrastructure out of the box
Breaking out of the Balkans Ghetto Why IPA should be changed
Out Of The Flames Leya, E M
lewis clive staples out of the silent planet
Out of the Wreckage CeeCee James
Eric Flint Genie Out of the Bottle
Ethel Watts Mumford Out of the Ashes
Phaedra M Weldon Zoe Martinique 01a Out of the Dark
Out of the Dark(1)
FIDE Trainers Surveys 2012 01 11 Efstratios Grivas The King Out of the Way
Jamie Lynn Miller Out of the Shadows
Charles Tart Six Studies of Out of the Body Experiences (OBE)
Hemi Sync Support for Journeys Out of the Body
EternalE Out of the Mouths of Babes
Out of the East
więcej podobnych podstron