
* Corresponding author.
Biomaterials 21 (2000) 469}473
Ion-beam-assisted deposition (IBAD) of hydroxyapatite coating layer
on Ti-based metal substrate
Jae-Man Choi
!, Hyoun-Ee Kim!,*, In-Seop Lee"
!School of Materials Science and Engineering, Seoul National University, Seoul 151-742, South Korea
"Research Center of Orthopaedic & Rehabilitation Engineering, Inchon 403-120, South Korea
Received 12 February 1999; accepted 22 August 1999
Abstract
A hydroxyapatite layer was formed on the surface of a Ti-based alloy by ion-beam-assisted deposition. The deposition methodo-
logy comprised of an electron beam vaporizing a pure hydroxyapatite target, while an Ar ion beam was focused on the metal substrate
to assist deposition. All deposited layers were amorphous, regardless of the current level of the ion beam. The bond strength between
the layer and the substrate increased steadily with increasing current, while the dissolution rate in a physiological saline solution
decreased remarkably. These improvements were attributed to an increase in the Ca/P ratio of the layer. Without ion beam assistance,
the Ca/P ratio was much lower than the stoichiometric HAp (Ca/P"1.67). With ion-beam assistance, the Ca/P ratio of the layer
increased presumably due to the high sputtering rate of P compared to that of Ca from the layer being coated.
( 2000 Elsevier
Science Ltd. All rights reserved.
Keywords: Hydroxyapatite; Coating; Metal implant; IBAD; Ca/P ratio
1. Introduction
To improve bioactivity of metal implants, hydroxy-
apatite (HAp: Ca10(PO4)6(OH)2) or other calcium
phosphates are generally applied as a coating [1]. Be-
cause of the chemical and crystallographic similarities
with the inorganic components of human bones, HAp or
calcium phosphate layers lead to direct bonding or
earlier stabilization of implants with the surrounding
bones or tissues [2}4].
In addition to bioactivity, the bond strength of a coat-
ing layer with the metal substrate is a very important
factor. If the layer is separated from the implant during
actual applications in human body, the detached frag-
ments have very adverse e!ects on the implant or the
tissue surrounding it [5]. Another important property
that the coating layer should possess is a low dissolution
rate in aqueous solutions. If the dissolution rate is faster
than bone growth or implants stabilization, the coating is
useless. The dissolution rate of crystalline HAp has been
observed to be very low, while that of the amorphous
phase was considerably high [1].
Various techniques, such as sputtering [6}9], electron
beam deposition [7], laser deposition [11,12], and
plasma spraying [13}19] have been employed to deposit
HAp or other calcium phosphate layers on various metal
substrates. Among these, plasma spraying is most widely
used because of its simplicity and versatility. Regardless
of the coating methodology, amorphous layers are gener-
ally formed on metal substrates, which have a high
dissolution rate in aqueous solutions. Therefore, the
layers are subsequently heat-treated at approximately
6003C in order to convert the amorphous phase into
a crystalline phase [16}20]. However, the heat treatment
causes cracks in the layer due to a thermal expansion
mismatch between the coated layer and the metal sub-
strate. This leads to a severe reduction in bond strength
[16}19].
In this study, thin hydroxyapatite layers were depos-
ited on a Ti}6Al}4V alloy by an ion-beam-assisted de-
position (IBAD) method. The e!ect of ion beam intensity
on the layer-substrate bond strength in addition to the
layer dissolution rate in a physiological saline solution
was investigated.
0142-9612/00/$ - see front matter
( 2000 Elsevier Science Ltd. All rights reserved.
PII: S 0 1 4 2 - 9 6 1 2 ( 9 9 ) 0 0 1 8 6 - 6
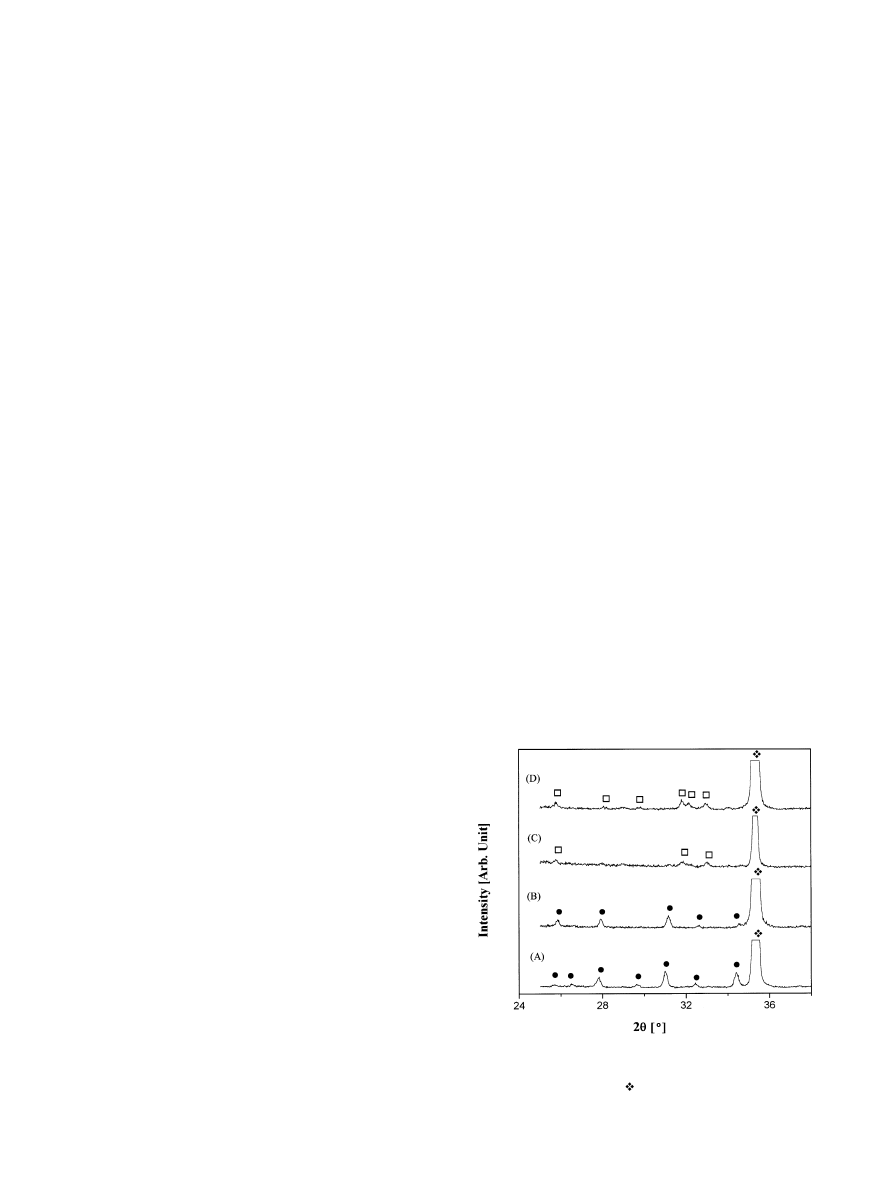
Fig. 1. XRD patterns of coating layers after heat treatment in vacuum
at 6303C. Ion beam currents are (A) 0 A, (B) 0.6 A, (C) 0.8 A, and (D)
1.0A. (
v) TCP, (h) HAp, and ( ) Ti.
2. Experimental procedure
The deposition target was synthesized by sintering a
commercial Ca10(PO4)6(OH)2 powder (Alfa Aesar Co.,
Ward Hill, MA, USA) in air at 12003C for 2 h. A com-
mercial Ti}6Al}4V alloy (Supra Alloys Inc., Camarillo,
CA, USA) was used as a substrate after machining into
disk of 25 mm in diameter and 2 mm in thickness. The
substrate surface was ground with SiC and subsequently
polished with diamond slurries down to 1
lm.
Thin hydroxyapatite layers were deposited on the
metal substrate by utilizing an ion-beam assisted depos-
ition (IBAD) technique. Using a cryopump (OB-10, Helix
Technology, Mans"eld, MA, USA), the chamber was
evacuated to a pressure of 10
~7 Torr. Subsequently, Ar
gas (P"10
~4 Torr) was introduced to the chamber.
Whilst an electron beam (Telemark, Fremont, CA, USA)
at 8.5 kV and about 0.1 A was evaporating the target, the
end-hall type ion gun (Mark II, Commonwealth Scient-
i"c, Alexandria, VA, USA) was applied to the metal
substrate surface to assist the deposition. The voltage was
"xed at 130 V and the current level was gradually increased
up to 1.0 A. The substrate was rotated at 8 rpm during
deposition in order to improve coating layer uniformity.
The deposited layers were analyzed by X-ray di!rac-
tometer (XRD, M18XHF, Mac Science, Yokohama,
Japan), energy dispersive spectroscopy (EDS, Oxford
Instruments, Bucks, England), and Fourier transform
infrared spectroscopy (FTIR, Model Equinox 55, Bruker,
Karlsruhe, Germany). The thickness of the layer was
measured by a surface pro"ler (Model P-10, Tencor,
Santa Clara, CA, USA) after making a step through an
etching process. The morphology of the layer was ob-
served by scanning electron microscopy (SEM, JSM-
5310, JEOL, Tokyo, Japan). The e!ect of heat treatment
in a vacuum (3
]10~3 Torr) at 630
3C for 1 h on the layer
phase and morphology was also investigated.
The dissolution rate of the deposited layer in a physio-
logical saline solution was measured with the surface
pro"ler. After half of the coating layer on the specimen
was covered with a water-resistant tape, it was then
immersed in the solution. The dissolution rate was esti-
mated from the "lm thickness dissolved during the dis-
solution period.
The bond strength of the coating layer was measured
using an adhesion testing apparatus (Sebastian V, Quad
Group, Spokane, WA, USA). A stud pre-coated by the
manufacturer using an epoxy of a proprietary composi-
tion was adhered to the coating layer by curing the epoxy
at 1503C for 1 h. The stud with a diameter of 3.6 mm was
pulled with a loading rate of 4.5 mm/min until the failure
of the coating layer, and the bond strength was deter-
mined from the maximum load recorded. Care was taken
to minimize the e!ect of epoxy penetration and non-
uniform failure of the coating layer. At least 10 measure-
ments were made for each experimental condition.
3. Results and discussion
The current level of the ion beam had signi"cant e!ects
on the deposited layer composition. Before heat treat-
ment, all the coating layers in this experiment were
amorphous with a thickness of approximately 700 nm.
To estimate the composition, the layers were heat-treated
in a vacuum (3
]10~3 Torr) at 630
3C for 1 h and ana-
lyzed by XRD. The XRD patterns of the layers after the
heat treatment are shown in Fig. 1. When the ion beam
was not used to assist deposition, tricalcium phosphate
(TCP: Ca3(PO4)2) was formed after heat treatment,
Fig. 1(A). When an ion beam with the current of 0.6 A
was applied to the substrate during deposition, the com-
position of the layer was unchanged (Fig. 1(B)). However,
when the current of the ion beam was increased to 0.8 A,
weak HAp peaks were detected instead of TCP peaks as
shown in Fig. 1(C), which became stronger as the ion
beam current was increased to 1.0 A (Fig. 1(D)). These
XRD patterns indicated that the composition of the
coating layer was strongly in#uenced by the ion beam
assistance.
The e!ect of ion-beam assistance was also observed by
EDS analyses. Without the ion-beam assistance, the
coating had a low Ca/P ratio (Ca/P"1.1) as seen in
Fig. 2. At an ion beam current of 0.6 A, the Ca/P ratio
was not signi"cantly changed. However, the Ca/P ratio
increased remarkably when the current was further in-
creased to 0.8 or 1.0 A. The coating layer Ca/P ratios
observed were still smaller than those for stoichiometric
TCP (Ca/P"1.5) or HAp (Ca/P"1.67). Therefore, the
layers deposited, even after heat treatments, were not
100% crystalline TCP or HAp, but a mixture of crystal-
line and amorphous phases.
470
J.-M. Choi et al. / Biomaterials 21 (2000) 469 }473
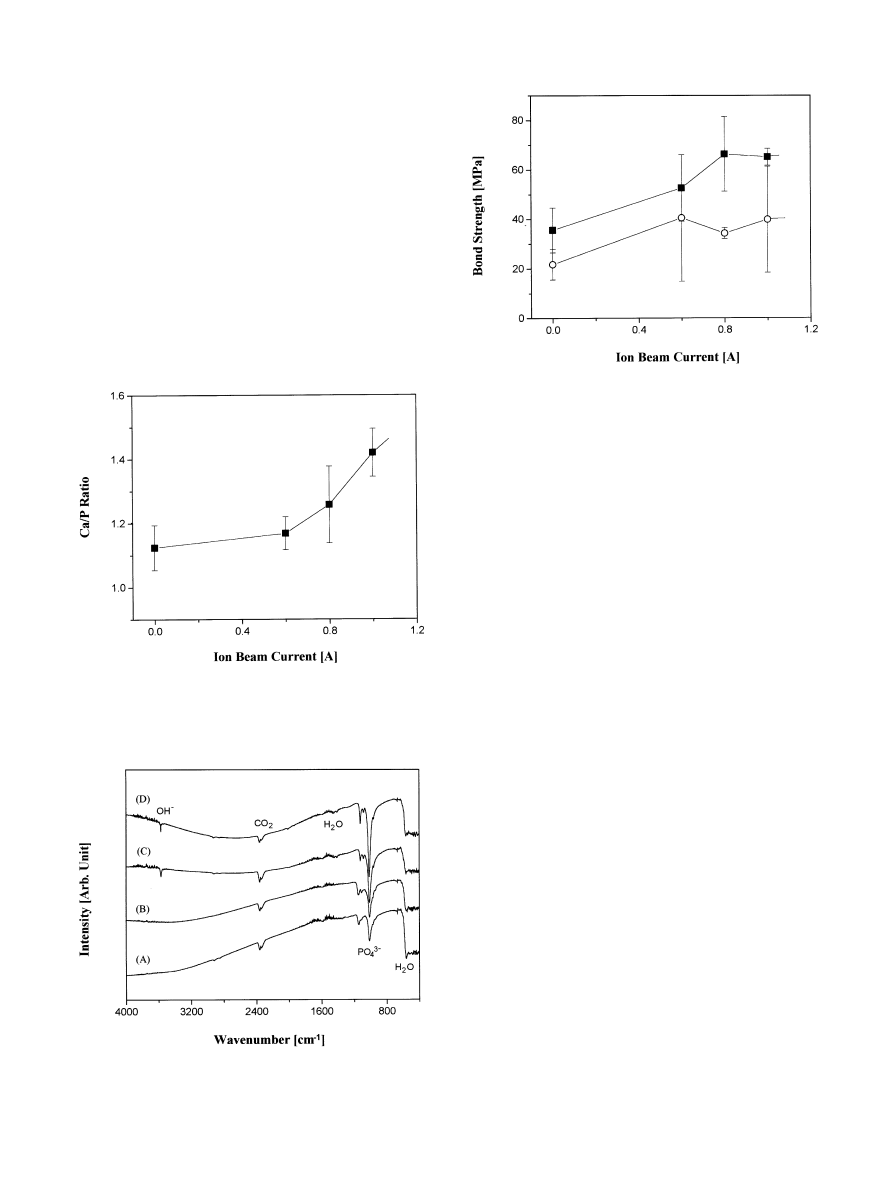
Fig. 3. FTIR spectra of the coating layers after the heat treatment. Ion
beam currents are (A) 0 A, (B) 0.6 A, (C) 0.8 A, and (D) 1.0 A.
Fig. 2. Ca/P ratio of the coating layer depending on the current level
for the ion beam.
Fig. 4. Layer}metal substrate bond strengths, before and after heat
treatment, as a function of ion beam current.
The e!ect of ion beam current on the coating layer
structure was also observed by FTIR spectroscopy
(Fig. 3). When the ion beam was o! or the current level
was low (0.6 A), no OH
~ stretch was detected after heat
treatment. However, when the ion beam current was
increased to 0.8 or 1.0 A, an OH
~ stretch was clearly
observed by FTIR spectroscopy [14,20].
These variations in composition have an e!ect on the
properties of the deposited layers. The bond strengths are
shown in Fig. 4 as a function of the ion beam current,
before and after the heat treatment. Before the heat
treatment, the bond strength increased steadily with in-
creasing current. Ion bombardment during deposition is
known to broaden the atomic intermixed zone, thereby
increasing the adhesion strength between the coating
layer and the substrate [21].
The bond strength decreased as a result of heat treat-
ment as has been frequently observed by other investiga-
tors [16}19]. SEM micrographs of the coating layer
before and after the heat treatment are shown in Fig. 5.
The morphologies were more or less similar regardless of
the current level. Before the heat treatment, the layer was
rather featureless as shown in Fig. 5(A). The lines at the
interface are Wallner lines frequently observed when
hard coating layers are detached from a metal substrate
[10]. After heat treatment, however, the layer became
severely cracked (Fig. 5(B)) apparently due to a thermal
expansion mismatch between the substrate and the layer
[22]. The reduction in bond strength as a result of heat
treatment is clearly due to these cracks. In addition to
cracks, the micrograph also indicates that the metal sur-
face was slightly oxidized, presumably by OH in the
coating layer [22]. The epoxy used to attach the coating
layer to the stud may have penetrated through the cracks
formed on the heat-treated specimen. Therefore, the
bond strengths after heat treatment might be even lower
than observed [14].
Despite the reduction in bond strength, heat treatment
has been carried out in order to crystallize the coating
layer. Otherwise, the dissolution rate of the layer in
a physiological solution is too high for actual applica-
tions [16}20]. In this study, when an ion beam was used
to assist deposition, the dissolution rate was reduced
remarkably even in the absence of heat treatment as
shown in Fig. 6. With ion-beam assistance, the dissolu-
tion rate decreased by more than a factor of 10. There-
fore, by employing ion-beam assistance technique, a HAp
coating layer that has a high bond strength and at the
same time a low dissolution rate can be deposited on
a metal substrate.
J.-M. Choi et al. / Biomaterials 21 (2000) 469 }473
471
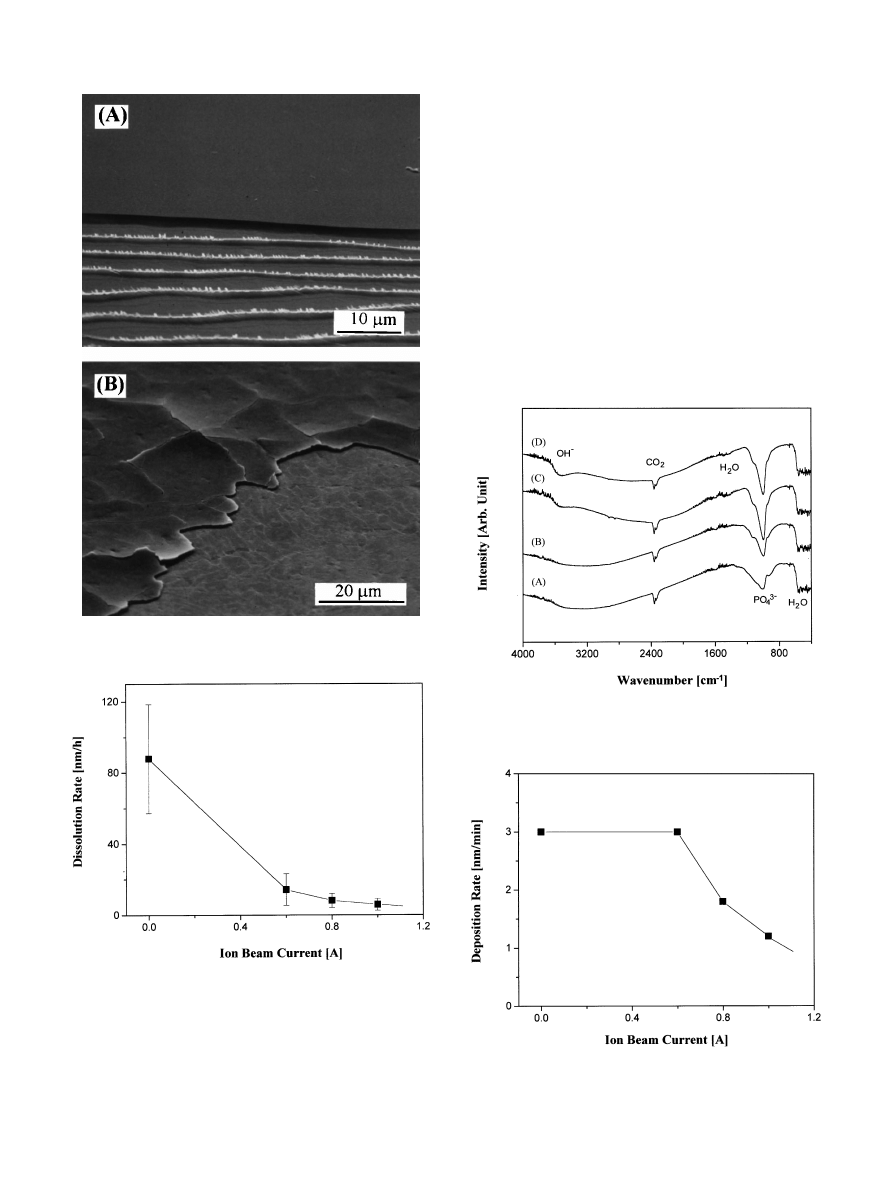
Fig. 5. SEM micrographs of the coating layer (A) before and (B) after
the heat treatment.
Fig. 6. Dissolution rate of the coating layer in physiological saline
solution.
Fig. 8. Deposition rate of the coating layer as a function of ion beam
current.
Fig. 7. FTIR spectra of the coating layer before heat treatment. Ion
beam currents are (A) 0 A, (B) 0.6 A, (C) 0.8 A, and (D) 1.0 A.
The low dissolution rate observed is believed to be
related to the structure of the layer. As manifested by
XRD, EDS and FTIR analyses, the Ca/P ratio of the
layer increased remarkably by applying the ion beam. By
increasing the Ca/P ratio, there is a high chance for the
layer to be similar to crystalline HAp, which is supported
by FTIR spectroscopy. As seen in Fig. 7, when the cur-
rent level of the ion beam was high (0.8 and 1.0 A), broad
OH
~ peaks were observed even before heat treatment.
Therefore, with ion-beam assistance, the composition
and structure of the coating layer appears to become
similar to crystalline HAp, which has a low dissolution
rate.
Application of the ion beam to the deposited layer not
only consolidated it but also resulted in sputtering by
impacting ions. The deposition rate, therefore, decreased
with increasing current level as shown in Fig. 8. This
sputtering e!ect is deemed to have a!ected the layer
Ca/P ratio. When a HAp layer was deposited on a metal
substrate by a r.f. sputtering method, the Ca/P ratio of
472
J.-M. Choi et al. / Biomaterials 21 (2000) 469 }473

the layer was much higher than that of the target [23].
This discrepancy suggests that Ca has higher sputtering
yield compared to P. Therefore, to obtain a stoichiomet-
ric HAp layer by the r.f. sputtering method, the Ca/P
ratio of the target should be lower than 1.67 [21]. How-
ever, in this experiment, the sputtering e!ect by the ion
beam decreased the relative concentration of P, resulting
in the observed increases in the Ca/P ratio in the layer.
4. Conclusions
The bond strength of a HAp coating layer with a metal
substrate and the dissolution rate of such layer in
a physiological saline solution was improved signi"-
cantly by applying an Ar ion beam during deposition.
Even though amorphous, the composition and structure
of the coating layer became similar to that of crystalline
HAp as a result of ion beam assistance. The higher
sputtering e$ciency of Ca compared to that of P was
attributed to the observed increase in the Ca/P ratio of
the coating layer.
Acknowledgements
We thank Dr. Mark C. Barnes for his helpful com-
ments when reviewing the manuscript.
References
[1] Lace"eld WR. Hydroxyapatite coating. Ann NY Acad Sci 1988;
523:72}80.
[2] Hench LL. Bioceramics. J Am Ceram Soc 1998;81:1705}28.
[3] Suchanek W, Yoshimura M. Processing and properties of hy-
droxyapatite-bases biomaterials for use as hard tissue replace-
ment implants. J Mater Res 1998;13:94}117.
[4] LeGeros RZ. Biodegradation and bioresorption of calcium phos-
phate ceramics. Clin Mater 1993;14:65}88.
[5] Wang S, Lace"eld WR, Lemons JE. Interfacial shear strength and
histology of plasma sprayed and sintered hydroxyapatite im-
plants in vivo. Biomaterials 1996;17:1965}70.
[6] van Dijk K, Schaeken HG, Wolke JGC, Jansen JA. In#uence of
annealing temperature on r.f. magnetron sputtered calcium phos-
phate coatings. Biomaterials 1996;17:405}10.
[7] Ong JL, Lucas LC, Lace"eld WR, Rigney ED. Structure, solubil-
ity and bond strength of thin calcium phosphate coatings produ-
ced by ion beam sputter deposition. Biomaterials 1992;13:249}54.
[8] Chen TS, Lace"eld WR. Crystallization of ion beam deposited
calcium phosphate. J Mater Res 1994;9:1284}90.
[9] Yoshinari M, Ohtsuka Y, Derand T. Thin hydroxyapatite coating
produced by the ion beam dynamic mixing method. Biomaterials
1994;15:529}35.
[10] Choi JM, Kong YM, Kin S, Kim HE, Hwang CS, Lee IS. Forma-
tion and characterization of hydroxyapatite coating layer on
Ti-based metal implant by electron beam deposition. J Mater Res
Soc 1999;14:2980}5.
[11] Singh RK, Qian F, Nagabushnam V, Damodaran R, Moudgil
BM. Excimer laser deposition of hydroxyapatite thin "lms. Bio-
materials 1994;15:522}8.
[12] Cotell CM, Chrisey DB, Grabowski KS, Spregue JA. Pulsed laser
deposition of hydroxyapatite thin "lms on Ti}6Al}4V. J Appl
Biomater 1992;8:87}93.
[13] Ducheyne P, Raemdonck WV, Heughebaert JC, Heughebaert M.
Sturctural analysis of hydroxyapatite coating on titanium. Bio-
materials 1986;7:97}103.
[14] Tsui YC, Doyle C, Clyne TW. Plasma sprayed hydroxyapatite
coating on titanium substrates. Part 1: mechanical properties and
residual stress levels. Biomaterials 1998;17:2015}29.
[15] Zyman Z, Weng J, Liu X, Zhang X, Ma Z. Amorphous phase and
morphological structure of hydroxyapatite plasma coatings. Bio-
materials 1993;14:225}8.
[16] Ji H, Marquis PM. E!ect of heat treatment on the microstructure
of plasma-sprayed hydroxyapatite coating. Biomaterials 1933;
14:64}8.
[17] Chen J, Wolke JGC, de Groot K. Microstructure and crystallinity
in hydroxyapatite coatings. Biomaterials 1994;15:396}9.
[18] Brossa F, Cigada A, Chiesa R, Paracchini L, Consonni C. Post-
deposition treatment e!ects on hydroxyapatite vacuum plasma
spray coatings. J Mater Sci Mater Med 1994;5:855}7.
[19] Chen J, Tong W, Coa Y, Feng J, Zhang X. E!ect of atmosphere
on phase transformation in plasma sprayed hydroxyapatite coat-
ings during heat treatment. J Biomed Mater Res 1997;34:15}20.
[20] Gross KA, Gross V, Berndt CC. Thermal analysis of amorphous
phases in hydroxyapatite coatings. J Am Ceram Soc 1998;
81:106}12.
[21] Cui FZ, Luo ZS, Feng QL. Highly adhesive hydroxyapatite
coatings on titanium alloy formed by ion beam assisted depos-
ition. J Mater Sci Mater Med 1997;8:403}5.
[22] Choi JW, Kong YM, Kim HE, Lee IS. Reinforcement of hy-
droxyapatite bioceramic by addition of Ni3Al and Al2O3. J Am
Ceram Soc 1998;81:1743}8.
[23] Hamagami JI, Kokubu D, Umegaki T, Yamashita K. Target-
composition e!ect on the hydroxyapatite thin "lms coated on
titanium by r.f. sputtering. Presented on the Third International
Meeting of Paci"c Rim Ceramic Societies 1998; paper
d 13-O-12,
Kyongju, Korea.
J.-M. Choi et al. / Biomaterials 21 (2000) 469 }473
473
Wyszukiwarka
Podobne podstrony:
14 2002 Mayer Ion beam analysis roughness
Floor beam ver 1 Student id 178 Nieznany
Platon z Aten Ion
02 Projekt BLUE BEAM dokladna informacja
68 979 990 Increasing of Lifetime of Aluminium and Magnesium Pressure Die Casting Moulds by Arc Ion
ion sputtering
Butterworth Finite element analysis of Structural Steelwork Beam to Column Bolted Connections (2)
blue beam
7 Modal Analysis of a Cantilever Beam
Beam Finishing PL
15m beam
8 Harmonic Analysis of a Cantilever Beam
Źródła prawa, zrodla 2, Mutuum & Depositum Irregulare
1 Effect of Self Weight on a Cantilever Beam
5 lect6 beam students
Projekt BLUE BEAM
9 Transient Analysis of a Cantilever Beam
więcej podobnych podstron