
Stability and Change in Temperament During Adolescence
Jody M. Ganiban
George Washington University
Kimberly J. Saudino
Boston University
Jennifer Ulbricht
George Washington University
Jenae M. Neiderhiser and David Reiss
George Washington University Medical School
This study assessed genetic and environmental contributions to temperament during adolescence within
the Nonshared Environment and Adolescent Development project (NEAD; D. Reiss, J. M. Neiderhiser,
E. M. Hetherington, & R. Plomin, 2000). NEAD is a national study that includes twins and other sibling
types who vary in regard to genetic relatedness. Seven hundred twenty sibling pairs (aged 12.1–13.5
years) participated at Time 1, and 395 sibling pairs (aged 14.7–16.2 years) participated again at Time 2.
At both Times, mothers and fathers rated their children’s temperament (emotionality, activity, sociability,
and shyness). At Times 1 and 2, genetic and nonshared environmental factors accounted for variance in
temperament, whereas shared environmental contributions were negligible. However, at Time 1, genetic
contributions were inflated, and shared environmental contributions were masked if sibling contrast
effects were not taken into account. At Time 2, sibling interaction effects had little impact on estimates
of genetic and environmental contributions to temperament. Last, temperament stability was primarily
explained by genetic factors, whereas both genetic and nonshared environmental factors accounted for
change.
Keywords: temperament, adolescence, genes, twins, stepfamilies
Temperament has long been identified as a factor that affects
adjustment during adolescence. Specific temperament characteris-
tics have been linked to alcohol use and substance abuse (e.g.,
Colder & Chassin, 1997; Cloninger, Sigvardsson, & Bohman,
1988) and interact with a wide range of risk and protective factors
during adolescence (e.g., Davies & Windle, 2001; Lynam et al.,
2000). Temperament characteristics that emerge during early
childhood are also predictive of behavioral outcomes during ado-
lescence and young adulthood (Newman, Caspi, Moffitt, & Silva,
1997). Because temperament plays an important role in adolescent
development, factors that contribute to individual differences in
temperament as well as to stability and change in temperament
during this critical time require attention. Numerous studies have
examined temperament stability; however, most studies have been
confined to children younger than 12 years of age (Roberts &
DelVecchio, 2000). Moreover, few studies have focused upon
understanding sources of stability and change in temperament
during adolescence. Such information is crucial to understanding
the malleability of temperament and, in turn, whether temperament
itself can be targeted as a source of intervention. The current article
examines the degree to which genetic and environmental factors
contribute to temperament during early and late adolescence and to
stability and change throughout adolescence.
Temperament is defined as biologically based individual differ-
ences in emotional and physiological reactivity and regulation that
are expressed through children’s negativity and positive emotion-
ality, activity level, sociability, and shyness (Buss & Plomin, 1984;
Rothbart & Bates, 1998). Studies conducted with young children
generally find that individual differences in temperament appear
early in life and demonstrate increasing stability from infancy to
the preschool years (Rothbart & Bates, 1998). For example, Le-
mery, Goldsmith, Klinnert, and Mrazek (1999) reported that spe-
cific temperament characteristics undergo progressive change dur-
ing infancy, but from the toddler to preschool periods, they are
moderately stable. By middle to late childhood, temperament
characteristics also demonstrate moderate stability, with estimates
ranging from .35 to .41 for most characteristics (Roberts &
DelVecchio, 2000). During late childhood and adolescence, re-
search has focused primarily upon personality, rather than upon
temperament. Similar to temperament characteristics, personality
characteristics exhibit moderate stability between the ages of 12
and 30 years, with average cross-age correlations between .47 and
.57 (Roberts & DelVecchio, 2000). Furthermore, Roberts and
DelVecchio (2000) noted that stability continues to increase after
age 30 years and peaks after 50 years, leading to population
correlation estimates as high as .75. These findings are consistent
with more recent studies that included large community samples
(Roberts, Caspi, & Moffitt, 2001; Shiner, Masten, & Tellegen,
2002).
Jody M. Ganiban and Jennifer Ulbricht, Department of Psychology,
George Washington University; Kimberly J. Saudino, Department of Psy-
chology, Boston University; Jenae M. Neiderhiser and David Reiss, Center
for Family Research, George Washington University Medical School.
Jenae M. Neiderhiser is now at the Department of Psychology, Penn-
sylvania State University, and David Reiss is now at the Child Study
Center, Yale University.
Correspondence concerning this article should be addressed to Jody M.
Ganiban, Department of Psychology, George Washington University, 2125
G Street, NW, Washington, DC 20052. E-mail: ganiban@gwu.edu
Journal of Personality and Social Psychology
Copyright 2008 by the American Psychological Association
2008, Vol. 95, No. 1, 222–236
0022-3514/08/$12.00
DOI: 10.1037/0022-3514.95.1.222
222

These studies suggest that temperament and personality become
increasingly fixed and, thus, less malleable. When characteristics
appear highly stable, it is frequently assumed that such stability
reflects the expression of endogenous characteristics, including
those that are genetically influenced (McCrae et al., 2000). How-
ever, environmental factors can also explain continuity. It is plau-
sible that stable temperament or personality characteristics are
produced by the cumulative effects of experiences or environmen-
tal pressures, such as the internalization of emotion display rules,
the acquisition of new coping strategies, or becoming embedded
within a stable social environment. Likewise, both genetic and
environmental mechanisms can be used to account for changes in
temperament and personality. For example, new genes may be
activated with puberty, causing changes in reactions and behavior.
Changes in one’s environment may also stimulate outward
changes in temperament or personality (Rothbart, Ahadi, & Evans,
2000).
Findings from twin studies are consistent with a genetic expla-
nation for continuity. Cross-sectional studies indicate that even in
infancy, temperament is genetically influenced (e.g., Silberg et al.,
2005) and that heritability estimates tend to increase from infancy
to early childhood (Nigg & Goldsmith, 1994). In one study,
Goldsmith, Buss, and Lemery (1997) detected moderate to large
genetic contributions for two indices of negative emotionality
(social fearfulness, anger proneness), activity level, and interest/
persistence during the toddler period. However, during the pre-
school years, moderate genetic contributions (heritability estimates
ranged from .41 to .58) were found more consistently for all
temperament dimensions, including positivity (i.e., surgency),
negative affectivity, and effortful control. Fewer studies have
focused upon adolescents. One exception is the Nonshared Envi-
ronment and Adolescent Development project (NEAD; Reiss, Nei-
derhiser, Hetherington, & Plomin, 2000), which includes twins as
well as full-, half-, and unrelated siblings. Analyses that included
just the monozygotic (MZ) and dizygotic (DZ) twins consistently
detected moderate to large genetic contributions to variance in
negative emotionality, activity, sociability, and shyness during
early adolescence (Saudino, McGuire, Reiss, Hetherington, & Plo-
min, 1995). In a more recent analysis of the NEAD data, Loehlin,
Neiderhiser, and Reiss (2003) examined genetic contributions to
personality and adjustment dimensions. Again, moderate to large
heritability estimates were found for each dimension when only
twins were included in the analyses. Similarly, adult twin studies
on personality have reported high heritability estimates for extra-
version (i.e., .41–.58), neuroticism (i.e., .41–.58), and conscien-
tiousness (i.e., .38 –.53; Bouchard & McGue, 2003).
Few longitudinal analyses have examined genetic and environ-
mental contributions to continuity in temperament or personality.
In one twin study, Gillespie, Evans, Wright, and Martin (2004)
obtained self-reports of personality at ages 12, 14, and 16 years.
Their analyses indicated that continuity in most personality char-
acteristics is explained by additive genetic factors. They also noted
that genetic factors contributed to changes in temperament, per-
haps reflecting the activation of new genes during puberty. How-
ever, most changes were explained by environmental factors. In a
second study with young adults, McGue, Bacon, and Lykken
(1993) estimated genetic and environmental contributions to per-
sonality within adult twins between the ages of 20 to 30 years and
also found that genetic factors accounted for most of the observed
stability. In a subsequent, larger study that used the Finnish Twin
Registry, Viken, Rose, Kaprio, and Koskenvuo (1994) similarly
reported that genetic factors primarily explained personality sta-
bility, whereas nonshared environmental factors accounted for
changes in temperament across adulthood. In addition, they found
little evidence of new genetic effects upon personality character-
istics after age 40 years. These studies all indicate that a common
set of genetic factors account for stability in temperament from
adolescence through adulthood. However, although change in per-
sonality is explained by environmental factors during adulthood,
both genetic and environmental factors may play a role in changes
during adolescence. Therefore, the relative influences of both
factors on changes in temperament may wax and wane over time.
More longitudinal studies during transition points such as adoles-
cence are needed to determine whether this is true and to contrib-
ute to a more comprehensive developmental theory of tempera-
ment.
Although twin studies point to significant genetic contributions
to temperament stability, a different picture emerges when nontwin
samples are assessed. For example, within the first wave of NEAD,
estimates of genetic and environmental contributions to activity
and shyness significantly differed for the twin and nontwin sibling
groups. In both cases, nonshared environmental factors explained
most variance within the nontwin sibling groups, whereas genetic
contributions were negligible (Saudino et al., 1995). Within
NEAD, genetic contributions to personality characteristics also
dropped significantly when twins were excluded from analyses
pertaining to personality characteristics (Loehlin et al., 2003).
Nontwin adoption studies also routinely yield lower heritability
estimates for temperament and personality characteristics than do
twin studies (Caspi, 1998; Goldsmith et al., 1997). For example,
the Colorado Adoption Project examined the temperament of
adopted children from ages 9 to 16 years through self-, parent, and
teacher reports. There was little evidence of genetic contributions
between the ages of 9 and 12 years when parents reported on their
children’s emotionality, activity, sociability, and attention (Gagne,
Saudino, & Cherny, 2003). Self-reports obtained when the adopt-
ees were 16 years old also yielded low heritability estimates
(Plomin, Corley, Caspi, Fulker, & DeFries, 1998). However,
teacher reports of the adoptees’ negative emotionality did yield
heritability estimates that ranged from .19 to .49 (M
⫽ .36) from
9 to 12 years of age (Gagne et al., 2003).
In summary, studies that do not include twins tend to generate
lower heritability estimates for temperament than do studies that
include twins. These discrepancies have been attributed to nonad-
ditive genetic influences on temperament (Plomin et al., 1998) or
to sibling-interaction effects (Saudino et al., 1995). Nonadditive
genetic effects are caused by interactions between alleles at the
same locus (dominance) or across different loci (epistasis). Such
effects are unpredictable, and their detection relies upon the inclu-
sion of MZ twins within the study sample. Because MZ twins are
genetically identical, their phenotypes are also identical for both
genetic dominance and epistasis. However, DZ twins and full
siblings, on average, have only a 25% chance of inheriting the
same set of alleles at a locus, whereas half siblings have little
chance of inheriting the same alleles and, thus, dominant genetic
influences. The potential of inheriting the same epistatic effects is
even less predictable and harder to detect given that siblings have
a low chance of inheriting the same alleles at the same loci, let
223
ADOLESCENCE AND TEMPERAMENT

alone across loci. Consequently, only studies that include MZ
twins along with other sibling groups or relatives have sufficient
power to detect nonadditive genetic effects. There is increasing
evidence from twin and extended twin kin studies (i.e., twins and
their siblings, parents) that nonadditive and additive genetic factors
contribute to personality characteristics (e.g., Eaves et al., 1999;
Finkel & McGue, 1997; Keller, Coventry, Heath, & Martin, 2005;
Lake, Eaves, Maes, Heath, & Martin, 2000). Eaves, Heath, Neale,
Hewitt, and Martin (1998) further demonstrated that nonadditive
genetic contributions to personality may be best explained by
epistasis, rather than dominance. Thus, nontwin studies may un-
derestimate genetic influences on temperament because they do
not have the power to identify nonadditive genetic contributions.
Sibling-interaction effects may also be an important source of
discrepancies between twin and nontwin studies. These effects
were initially conceptualized to describe and measure the social-
izing influences siblings have on each others’ phenotypes (Carey,
1986; Eaves, 1976). Specifically, some siblings may imitate each
other or encourage each other to become more similar over time
(i.e., sibling assimilation). Other siblings may strive to distinguish
themselves by behaving differently and actually become less sim-
ilar to each other over time (i.e., sibling contrast or competition).
When this occurs, sibling interaction becomes an environmental
factor that affects the phenotype of each sibling and, thus, sibling
similarity. However, the degree to which siblings become more
similar to or different from each other may be influenced by their
genetic relatedness (Eaves, 1976). Because MZ twins share the
same genotype, any influence MZ twins have on each other will be
correlated with their genetic makeup (i.e., an MZ twin is socialized
by a cotwin who has the same genetic makeup). However, for
stepsiblings who are not genetically related, their influences upon
each other are not correlated with their genetic makeups. As a
result, sibling interactions will have a greater influence on sibling
similarity for sibling pairs who demonstrate the lowest levels of
genetic relatedness than for sibling pairs who demonstrate highest
levels of genetic relatedness. Because sibling-interaction effects on
phenotypes can depend on sibling relatedness, they can also mimic
nonadditive genetic effects when sibling-contrast effects are
present or mimic shared environmental effects when sibling as-
similation is present.
More pertinent to the current investigation, however, is the
application of the sibling-interaction model to account for and
assess rater bias in parents’ reports of their children’s tempera-
ments (e.g., Goldsmith et al., 1997; Neale & Stevensen, 1989;
Saudino, Cherny, & Plomin, 2000). For example, some parents
may be predisposed to focus on sibling differences and to ignore
sibling similarities (i.e., sibling contrast). Other parents may focus
on sibling similarities and ignore sibling differences (i.e., sibling
assimilation). Either tendency can lead to inaccurate assessments
of true sibling similarities or differences and affect estimates of
genetic and environmental contributions to behavior. Again, it is
expected that such report biases influence perceptions of similar-
ities or differences more for siblings who demonstrate lower levels
of genetic relatedness than for siblings who demonstrate higher
levels of relatedness. Thus, sibling contrast could mimic nonaddi-
tive effects, whereas sibling assimilation could mimic shared en-
vironmental effects.
Sibling-interaction effects are difficult to detect in twin-only
samples. Rietveld, Posthuma, Dolan, and Boomsma (2003) esti-
mated that a sample of 500 MZ and 1,000 DZ twins would be
required to detect a sibling-interaction effect with an absolute
magnitude of .15. Therefore, it is possible that the higher genetic
estimates found in twin-only studies may, in part, reflect the
impact of nonmeasured sibling-interaction effects. However, stud-
ies that include twins along with other sibling types, such as
NEAD, have greater power to detect sibling-interaction effects.
Saudino et al. (1995) examined sibling-contrast versus assimilation
effects for mother and father reports of adolescent temperament
during early adolescence within NEAD. They did not find assim-
ilation effects for MZ twins but detected significant contrast ef-
fects for parents’ reports of their adolescents’ emotionality, activ-
ity level, shyness, and sociability. Their results suggest that
sibling-interaction effects partially accounted for nonadditive ge-
netic contributions to the ratings and that they also masked the
contributions of family-wide experiences to temperament. Other
studies have detected significant contrast effects within infant and
toddler populations for difficult temperament (Silberg et al., 2005)
and for activity and shyness (Saudino et al., 2000; Saudino, Wertz,
Gagne, & Chawla, 2004).
In summary, current studies indicate that nonadditive genetic
factors contribute to temperament and personality and that sibling-
interaction effects exist. Both factors could explain discrepancies
between the findings of twin and nontwin studies. Specifically,
many twin studies that focus on personality do not include contrast
effects in their analytical model or lack sufficient power to detect
sibling-interaction effects. Conversely, nonadditive genetic effects
may not be detectable within studies that do not include MZ twins.
Therefore, studies that include both twins and nontwin sibling
groups are particularly suited to estimate the relative importance of
both genetic and contrast effects on temperament ratings. NEAD
affords this opportunity because it includes twin and nontwin
sibling pairs. In the current study, we revisit and extend the
findings of Saudino et al. (1995), who examined genetic contribu-
tions and sibling-interaction effects within NEAD’s first wave of
data collection. In the current report, we include a second set of
temperament ratings made 3 years after the initial assessment and
examine factors that contribute to stability and change in temper-
ament at this later time point.
Method
Participants
NEAD represents a nationwide sample of two-parent families
that included never-divorced families and stepfamilies. Several
inclusion criteria were used to select families: (a) family had two
adolescent same-sex siblings no more that 4 years apart in age
(M
⫽ 1.61 ⫾ 1.29 years apart); and (2) family was in existence for
at least 5 years prior to the first Time 1 (M
⫽ 8.9 ⫾ 3.7 years of
marriage). At Time 1, 720 families participated. At Time 1, the
adolescent children (N
⫽ 1,420) ranged in age from 13.5 ⫾ 2.0
years (Child 1) to 12.1
⫾ 1.3 years (Child 2). Participating families
were grouped into one of the following six sibling categories, in
one of the two family types (i.e., never divorced or stepfamily):
MZ twin pairs (n
⫽ 93), dizygotic DZ twin pairs (n ⫽ 99), and full
sibling (FI) pairs (n
⫽ 95) from never divorced families and full
sibling (FS) pairs (n
⫽ 182), half sibling (HS) pairs (n ⫽ 109), and
genetically unrelated sibling (US) pairs (n
⫽ 130) residing in
224
GANIBAN, SAUDINO, ULBRICHT, NEIDERHISER, AND REISS

stepfamilies. For the three sibling pair types within the stepfami-
lies, we matched the age of the oldest child and age spacing
between siblings across families to maximize their comparability.
At Time 2, 395 families from Time 1 participated. This subset
of Time 1 families included 63 MZ twin pairs, 75 DZ twin pairs,
and 58 FI pairs from nondivorced families and 95 FS pairs, 60 HS
pairs, and 44 US pairs residing in stepfamilies. In all, 790 children
participated at Time 2 and ranged in age from 16.2
⫾ 2.1 years
(Child 1) to 14.7
⫾ 1.9 years (Child 2). The decrease in the number
of participating families from Time 1 to Time 2 was not due to
attrition. Rather, only families with both adolescents still residing
at home for at least half of the time with both parents were eligible
to participate at Time 2. Of the ineligible families, 15% experi-
enced a divorce; in 79% cases one or both of the adolescents had
moved out of the home, and the remaining 6% were unable to be
classified. At Time 2, 91% of eligible families participated. There
were no mean differences in the demographic characteristics (par-
ents’ education, family income, gender of the siblings, and age
difference between siblings) for families who participated at both
Times 1 and 2 versus those who only participated at Time 1. In
regard to temperament, children who participated in the study at
Times 1 and 2 were rated by their mothers as slightly more
sociable than were children who only participated at Time 1.
However, no differences were found between the fathers’ temper-
ament ratings of each group. For the 27 eligible families who
refused to participate at Time 2, analyses indicated significant
main effects for age and variables related to age (i.e., the
adolescents were older and received less parental monitoring)
when compared with eligible families who chose to participate
at Time 2.
Twin Zygosity
Twins were rated for physical similarity (e.g., eye and hair
color) by the interviewer, by the parents, and with self-reports
using a questionnaire designed for adolescents (Nichols & Bilbro,
1966). If any differences in physical characteristics were reported
(e.g., eye color, hair color) or if respondents reported that people
never were confused about the identity of the twins, the twin pair
was classified as dizygotic. Ten of the twin pairs could not be
classified as either monozygotic or dizygotic and were excluded
from these analyses (7% of the twin pairs). Questionnaire methods
of assigning zygosity have been found to be at least 90% accurate
when compared with tests of single-gene markers in blood pheno-
types (Nichols & Bilbro, 1966; Spitz et al., 1996).
Procedures
At Time 1, each family participated in two 3-hour home visits
during which family members were interviewed, completed ques-
tionnaires, and were observed during interactions. The home visits
were scheduled 2 weeks apart. At Time 2, families were visited
once by one interviewer. Both parents and the two adolescents
completed questionnaires and were videotaped during each visit.
Additional questionnaire data were obtained from take-home ques-
tionnaires, which were mailed ahead and collected by the inter-
viewer. At Times 1 and 2, data were gathered from the children
and both parents regarding the children’s temperament character-
istics; relationship with siblings, parents, and peers; and psycho-
logical adjustment, as well as parental mental health, marital
quality, and stability.
Measures
The EAS Temperament Survey–Parent Form (Buss & Plomin,
1984) includes 20 descriptive statements that assess children’s
negative emotionality, activity level, sociability, and shyness.
Mothers and fathers completed the EAS for each adolescent sib-
ling in the study. For each statement, parents were asked to rate the
degree to which it described their children over the past 2 weeks,
using a 5-point Likert scale. At Time 1, the alphas for the EAS
ratings averaged .73 (range .60 –.81) for mothers and .72 (range
.61–.81) for fathers. The average alphas for ratings at Time 2 were
.75 (range .64 –.87) for mothers, and .70 (range .56 –.81) for
fathers.
Analyses
Preliminary analyses.
The following potential confounding
variables were regressed from the temperament ratings: maternal
age, child age, age differences between nontwin siblings, child
gender, and Child Age
⫻ Child Gender interaction (McGue &
Bouchard, 1984). Next, the means and standard deviations were
computed for the study variables, and the distribution of each
variable was examined for normality. The temperament subscales
demonstrated significant skew. Therefore, we ranked the residu-
alized temperament ratings and normalized them across the entire
sample, using procedures described by Blom (1958). This strategy
for dealing with skewed data has been used in previous behavioral
genetic analyses (e.g., Eaves et al., 1997). The raw transformed
data were used in the model-fitting analyses. These data-analytic
methods differ from those of Saudino et al. (1995). In this earlier
report of the Time 1 NEAD data, Saudino et al. (1995) used
double-entered, unranked data and variance/covariance matrices in
the model-fitting analyses. The current analyses also included 41
additional sibling pairs that were not available at the time of the
original report.
Twin/sibling intraclass correlations.
We computed intraclass
twin/sibling correlations to explore whether additive genetic (A),
dominant genetic (D), shared environmental (C), and nonshared
environmental (E) factors contribute to children’s temperament
characteristics and to assess potential sibling-interaction effects.
For each sibling group, temperament ratings for Sibling 1 were
correlated with those for Sibling 2. We double entered data for
these analyses to guard against the possibility that the original
designations of siblings as 1 or 2 were not random.
Genetic contributions are inferred if the magnitude of intraclass
correlations closely parallels the genetic relatedness of the sibling
pairs. If this was the case, then correlations would be highest for
sibling pairs that are the most genetically similar (e.g., MZ twins,
who share 100% of their genes) and lowest for sibling pairs that
are least genetically similar (US siblings, who share 0% of their
genes). If the MZ twin correlation is approximately twice as large
as the DZ twin and full sibling correlations, then additive genetic
contributions are inferred. If this difference is larger, nonadditive
genetic contributions may be present as well. The presence of
environmental factors that cause sibling similarity (i.e., shared
environment) are inferred if the intraclass twin/sibling correlations
225
ADOLESCENCE AND TEMPERAMENT
are greater than would be predicted by genetic relatedness. Shared
experiences, such as the same household environment or being
raised by the same parents, may account for sibling similarities that
are independent of genetic relatedness. Nonshared environment
encompasses the unique experiences of siblings that make them
different from each other (e.g., having different peers, being in
different classrooms, and even differential parental treatment), as
well as measurement error. Because MZ twins share 100% of their
genes and are reared in the same family, any deviation from a
correlation of 1.0 for this sibling group indicates nonshared envi-
ronmental influences.
We also examined the pattern of intraclass twin/sibling corre-
lations for evidence of sibling-contrast effects. If parents exagger-
ated true differences between siblings, sibling contrast would be
greatest for the least genetically related sibling pairs. This would
result in greater increases in variance and deflation of intraclass
sibling correlations for the least genetically related sibling pairs
(Carey, 1986; Eaves, 1976). Therefore, MZ twin correlations that
are more than two times those of DZ twin or full sibling correla-
tions could be caused by sibling-contrast effects. The presence of
significant negative twin/sibling correlations would also provide
strong evidence of sibling-contrast effects, as they would indicate
that parents’ ratings of one sibling are in opposition to the ratings
of the other sibling.
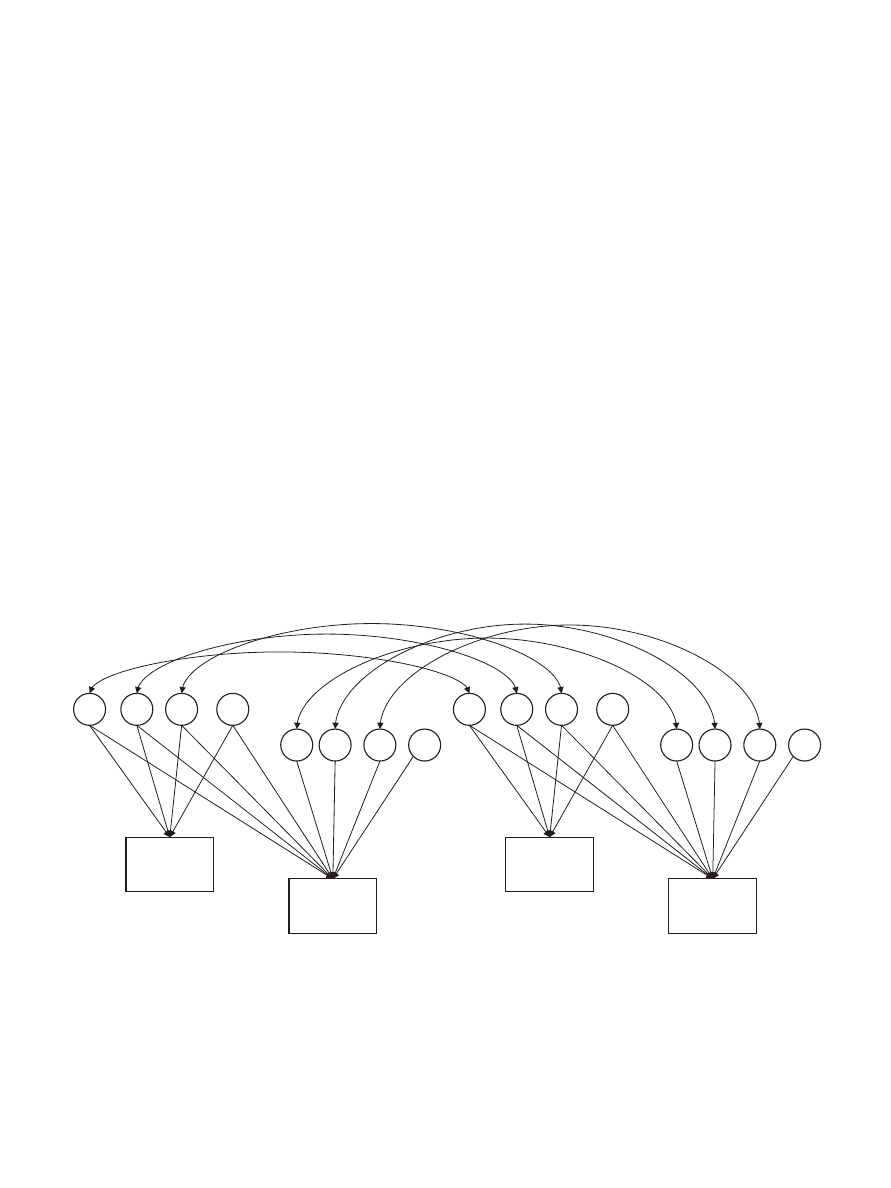
Biometric model fitting.
We used model fitting to estimate
genetic and environmental contributions to each temperament di-
mension (Neale & Cardon, 1992). We used a Cholesky model to
assess genetic and environmental contributions to temperament
ratings at Times 1 and 2 and covariance between both time points
(see Figure 1). This model includes latent additive genetic (A1,
A2), nonadditive genetic (D1, D2), shared environmental (C1, C2),
and nonshared environmental (E1, E2) factors. Variance in Time 1
ratings is explained by latent factors A1, D1, C1, and E1. Squaring
paths a
11
, d
11
, c
11
, and e
11
can be used to estimate genetic and
environment contributions to these ratings. Variance in Time 2
ratings, however, is explained by factors unique to the Time 2
ratings (i.e., A2, D2, C2, E2) and by latent factors associated with
Time 1 ratings (i.e., A1, D1, C1, E1). Summing the squares of
paths from these latent factors to the Time 2 ratings can be used to
estimate genetic (a
21
2
⫹ a
22
2
⫹ d
21
2
⫹ d
22
2
), shared environmen-
tal (c
21
2
⫹ c
22
2
), and nonshared environmental (e
21
2
⫹ e
22
2
)
contributions to variance in the Time 2 ratings. This variance can
be further decomposed into variance that is shared with the Time
1 ratings and variance that is unique to Time 2. Shared variance
between Times 1 and 2 provides an estimate of temperament
stability, whereas variance unique to Time 2 provides an estimate
of change.
Some paths in Figure 1 were fixed. Specifically, the paths
between additive genetic factors (A1, A2) for Siblings 1 and 2
were set to 1.0 for MZ twin pairs, .50 for DZ twin pairs and full
sibling pairs, .25 for half siblings, and 0 for unrelated siblings. The
paths between nonadditive genetic factors (D1, D2) for the siblings
were also fixed to 1.0 for MZ twin pairs, .25 for DZ and full sibling
pairs, and 0 for half sibling and unrelated sibling pairs. For all
sibling groups, the path between shared environmental factors (C1,
C2) for the siblings was set to 1.0.
The Cholesky model assumes that nonadditive genetic effects
reflect dominance, rather than epistasis. Eaves et al. (1998) re-
Temperament
Time 1
Sibling 1
Temperament
Time 2
Sibling 1
A1
D1
C1
E1
a
11
d
11
c
11
e
11
a
21
d
21
c
21
e
21
A2
D2
C2
E2
e
22
a
22
d
22
c
22
Temperam
Time 1
Sibling 2
A1
D1
C1
E1
ent
Temperament
Time 2
Sibling 2
a
11
d
11
a
21
d
21
c
11
e
11
c
21
e
21
A2
D2
C2
E2
e
22
a
22
d
22
c
22
Figure 1.
Cholesky model for estimating additive genetic (A1, A2), nonadditive genetic (D1, D2), shared
environmental (C1, C2), and nonshared environmental (E1, E2) contributions to temperament ratings at Times
1 and 2. Genetic and environmental correlations between siblings are represented by double-headed arrows. The
paths between A1 for Siblings 1 and 2 and between A2 for Siblings 1 and 2 were set to 1.0 for monozygotic
twins, .50 for dizygotic twins and full siblings, .25 for half siblings, and .00 for unrelated siblings. The paths
between D1 for Siblings 1 and 2 and between D2 for Siblings 1 and 2 were set to 1.0 for monozygotic twins,
.25 for dizygotic twins and full siblings, and .00 for half siblings and unrelated siblings. Paths between C1 for
Siblings 1 and 2 and between C2 for Siblings 1 and 2 were set to 1.0 for all sibling groups. All paths were
constrained to be equal for Siblings 1 and 2.
226
GANIBAN, SAUDINO, ULBRICHT, NEIDERHISER, AND REISS
ported that nonadditive genetic influences may be best explained
by epistasis, rather than dominance. However, because epistatic
effects are unpredictable, the influence of such effects can only be
estimated reliably in very large samples. Consequently, the model
used in this study may actually underestimate overall genetic
effects if epistasis is a key genetic mechanism.
The Cholesky model was fitted by maximum likelihood estima-
tion with raw transformed data, using the Mx statistical package
(Neale, Baker, Xie, & Maes, 2003). Power analyses indicated that
the full ADCE model could not be tested with the current sample
because the relative contributions of A and D could not be distin-
guished with accuracy. Power analyses also indicated that only
moderate shared environmental influences could be detected when
nonadditive genetic effects were also present, suggesting that
shared environmental influences may be underestimated in the
ADCE model. Consequently, we tested and compared the relative
fits of three models: (a) a model that included additive and non-
additive genetic and nonshared environmental factors (ADE mod-
el); (b) a model that included additive genetic, shared environmen-
tal, and nonshared environmental factors (ACE model); and (c) an
environmental factors model (CE model). Because the ADE and
ACE models were not nested, their relative fits were judged by the
⫺2 log likelihood (–2LL) values for each model. Generally, mod-
els with lower –2LL values are considered to represent better fits
to the data than are models with higher values (Price et al., 2005).
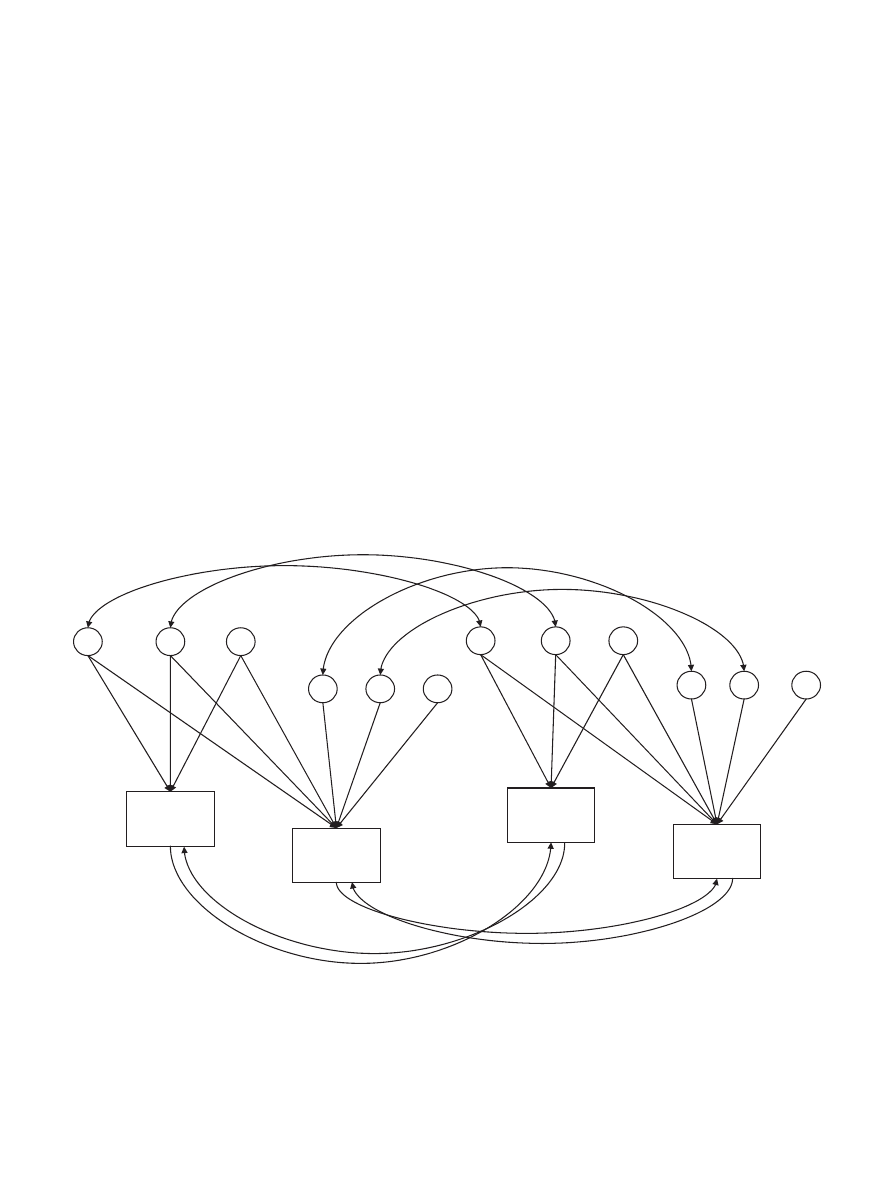
A second set of analyses examined whether sibling-interaction
effects were present and significant. As depicted in Figure 2,
sibling-interaction paths were included at Time 1 (B
1
) and Time 2
(B
2
). Because of power constraints, the sibling-interaction model
included only one genetic parameter (either A or D), and this
parameter is denoted as “G” in the sibling-interaction model
(GCE-B
1
B
2
). The sibling-interaction model was compared with a
baseline model that included the same latent genetic factor (GCE).
The relative fits of the ACE versus DCE models were compared,
and the better fitting model was used as the baseline GCE model.
We also examined the relative fits of two additional sibling-
interaction models. In one model, all genetic effects were elimi-
nated (CE-B
1
B
2
model). The second model constrained estimates
of G, C, and E to be the same for all sibling groups but permitted
sibling-interaction effects to vary for twin versus nontwin sibling
groups (GCE-B
1
B
2
B
3
B
4
model). In this latter model, B
1
and B
2
are estimates of Time 1 and Time 2 sibling-interaction effects for
the twin groups, respectively, whereas B
3
and B
4
are estimates of
Time 1 and Time 2 sibling-interaction effects for nontwin siblings,
respectively. To determine if the addition of sibling-interaction
paths affected model fit, we compared the fit of the GCE model
with each sibling-interaction model. Because the various models
were nested, we used the likelihood-ratio chi-square test to assess
the relative fits of the models. The difference between the –2LL of
the baseline GCE model and each sibling-interaction model was
Temperament
Time 1
Sibling 1
Temperament
Time 2
Sibling 1
G1
C1
E1
G2
C2
E2
Temperament
Time 1
Sibling 2
Temperament
Time 2
Sibling 2
G1
C1
E1
G2
C2
E2
g
11
g
21
c
21
c
11
e
11
e
21
g
22
c
22
e
22
g
22
c
22
e
22
g
11
g
21
c
21
c
11
e
11
e
21
B
1
B
2
Figure 2.
Longitudinal sibling-interaction model, with latent genetic (G1, G2), shared environmental (C1, C2),
and nonshared environmental (E1, E2) factors and sibling-interaction effects at Time 1 (B
1
) and Time 2 (B
2
).
Genetic and environmental correlations between siblings are represented by double-headed arrows. Because the
best-fitting model included nonadditive genetic contributions, paths between G1 for Siblings 1 and 2 and
between G2 for Siblings 1 and 2 were set to 1.0 for monozygotic twins, .25 for dizygotic twins and full siblings,
and 0 for half siblings and unrelated siblings. Paths between C1 for Siblings 1 and 2 and between C2 for Siblings
1 and 2 were set to 1.0 for all sibling groups. All estimates were constrained to be equal for Siblings 1 and 2.
227
ADOLESCENCE AND TEMPERAMENT
computed for each temperament dimension. The difference in
–2LL values between models are distributed as chi-square values
for each change in degree of freedom. If the change in –2LL value
was not significant at p
⬍ .05, then the sibling-interaction paths
were judged to be nonsignificant.
Results
Phenotypic Analyses
The means and standard deviations for each temperament sub-
scale at each Time are presented in Table 1. We used repeated-
measures ANOVAs to assess the degree to which ratings changed
from Time 1 to Time 2. Adolescents’ shyness seemed to de-
crease—for mothers’ reports, F(1, 781)
⫽ 6861, p ⬍ .01; for
fathers’ reports, F(1, 768)
⫽ 4.45, p ⬍ .05—and emotionality
increased, according to fathers’ reports, F(1, 763)
⫽ 5.50, p ⬍ .05,
over time. Stability was apparent in individuals’ rank ordering of
temperament characteristics. The cross-age correlations ranged
from .32 to .56 for mothers’ ratings and from .42 to .53 for the
fathers’ ratings.
Sibling intraclass correlations.
Sibling intraclass correlations
again indicated that the MZ twins were more similar to each other
at each time than to any of the remaining sibling groups (Table 1).
With the exception of mothers’ and fathers’ ratings of emotionality
at Time 1, the MZ correlations were more than double those of DZ
twins and full siblings from intact and remarried families, consis-
tent with the presence of nonadditive genetic or sibling-interaction
effects. In addition, the MZ cross-sibling correlations were less
than 1.0, indicating that nonshared environmental factors or mea-
surement error also play a role in explaining stability. Last, for 10
out of 16 variables, some of the cross-sibling correlations for the
nontwin siblings were significantly negative. The presence of
negative correlations and the magnitude of the MZ sibling corre-
lations relative to other sibling pairs are suggestive of sibling-
contrast effects.
Biometric Model Fitting
Mother ratings.
As summarized in Table 2, the ADE model
yielded a better fit to the data than did the CE or ACE models for
each of the temperament dimensions at Times 1 and 2, suggesting
that only genetic and nonshared environmental factors account for
significant variance in temperament at each age. The path esti-
mates for the best-fitting models for each variable are included in
Table 2. For all variables except emotionality, nonadditive genetic
factors accounted for genetic variance, whereas the path estimates
for additive genetic factors were essentially zero.
The next set of analyses evaluated the fit of the sibling-
interaction model (see Figure 2). Because of power constraints, the
baseline and sibling-interaction models included only one genetic
parameter. In each case, latent factor D was selected because the
DCE model yielded a better fit to the data than did the ACE model.
The fit statistics for the baseline GCE model and sibling-
interaction models are included in Table 3. The addition of sibling-
interaction paths B
1
and B
2
led to improved model fits for activity,
shyness, and sociability. For activity and shyness, the fit of the
model was improved further by permitting the sibling-interaction
Table 1
Means, Standard Deviations, and Sibling Intraclass Correlations for Mothers’ and Fathers’ Reports of Temperament
Variable
Overall sample
Sibling intraclass correlations by sibling type
M
SD
MZ
DZ
FI
FS
HS
US
Mothers’ reports
Time 1
Activity
18.69
3.64
.71
ⴱ
.14
⫺.11
⫺.06
.00
⫺.22
ⴱ
Emotionality
11.51
3.77
.56
ⴱ
.37
ⴱ
.35
ⴱ
.04
.09
.20
ⴱ
Shyness
11.82
3.81
.64
ⴱ
.09
⫺.20
ⴱ
⫺.22
ⴱ
⫺.03
⫺.27
ⴱ
Sociability
17.51
2.93
.52
ⴱ
.06
⫺.02
.03
.07
⫺.31
ⴱ
Time 2
Activity
18.74
3.90
.70
ⴱ
.04
⫺.10
.10
⫺.28
ⴱ
⫺.38
ⴱ
Emotionality
11.90
4.49
.58
ⴱ
.20
ⴱ
.31
ⴱ
.19
ⴱ
.02
.03
Shyness
11.56
a
3.97
.61
ⴱ
.12
⫺.20
ⴱ
⫺.11
⫺.27
ⴱ
⫺.30
ⴱ
Sociability
17.63
3.29
.50
ⴱ
⫺.03
⫺.06
⫺.09
⫺.11
⫺.26
ⴱ
Fathers’ reports
Time 1
Activity
18.34
3.12
.77
ⴱ
.24
ⴱ
⫺.10
⫺.02
⫺.10
⫺.09
Emotionality
11.85
3.40
.61
ⴱ
.35
ⴱ
.34
ⴱ
.25
ⴱ
.19
ⴱ
⫺.10
Shyness
11.98
3.24
.65
ⴱ
.15
.02
.17
.04
⫺.20
ⴱ
Sociability
17.58
2.36
.42
ⴱ
.00
.10
.17
ⴱ
.03
⫺.09
Time 2
Activity
18.03
3.16
.79
ⴱ
.06
⫺.03
.05
⫺.26
ⴱ
.01
Emotionality
13.02
a
3.10
.64
ⴱ
.12
.11
.10
.25
ⴱ
.14
Shyness
12.33
a
3.67
.70
ⴱ
.04
.15
ⴱ
.01
⫺.12
⫺.41
ⴱ
Sociability
16.81
2.49
.69
ⴱ
.18
ⴱ
⫺.04
.31
ⴱ
.09
⫺.29
ⴱ
Note.
MZ
⫽ monozygotic twins; DZ ⫽ dizygotic twins; FI ⫽ full siblings from never-divorced families; FS ⫽ full siblings from stepfamilies; HS ⫽ half
siblings; US
⫽ unrelated siblings.
a
Time 2 ratings significantly differ from Time 1 ratings of the same temperament dimension ( p
⬍ .05).
ⴱ
p
⬍ .05.
228
GANIBAN, SAUDINO, ULBRICHT, NEIDERHISER, AND REISS

path estimates to vary for the twin and nontwin sibling groups. In
each case, estimates for the sibling-interaction paths were smaller
or nonsignificant for the twins than for the nontwin siblings. The
only variable to demonstrate nonsignficant sibling-interaction
paths for all sibling groups was emotionality. In this case, the
addition of sibling-interaction paths led to a marginal improvement
in model fit ( p
⬍ .07).
The parameter estimates for the best-fitting sibling-interaction
models are summarized in Table 4. We also included the parameter
estimates for emotionality in Table 4 to illustrate the potential
effects of sibling-contrast effects on variance estimates, even
though the sibling-interaction paths were nonsignificant for this
variable. In most cases, the sibling-interaction parameters were
negative and indicative of sibling-contrast effects. Overall, the
estimates of the sibling-interaction paths were of greater magni-
tude at Time 1. In the two instances in which the sibling-
interaction estimates were positive (i.e., emotionality and shyness),
they tended to occur at Time 2 but were not statistically significant.
For each temperament characteristic, paths related to shared envi-
ronmental factors at Time 1 increased relative to estimates derived
from models that did not include sibling-interaction paths. Specif-
ically, estimates for c
11
ranged from
⫺.56 to .85 for the sibling-
interaction model. Estimates of Time 1 genetic contributions (d
11
)
were lower in the sibling-interaction models than in the
nonsibling-interaction models and ranged from .44 to .63. How-
ever, inclusion of sibling-interaction terms did not seem to affect
genetic and environmental path estimates for the Time 2 ratings.
Table 4 summarizes the contributions of genetic and environ-
mental factors to variance in mothers’ temperament ratings. The
top part of the table provides estimates based upon the best-fitting
models that did not include sibling-interaction paths. The bottom
part of the table provides estimates generated from the best-fitting
sibling-interaction models. When sibling-interaction effects were
not included in the model, genetic factors explained 52% to 73%
of variance in ratings at Time 1 and 37% to 63% of variance in
temperament ratings at Time 2. Most of the genetic variance was
explained by nonadditive genetic factors. The last six columns in
Table 4 differentiate between variance that is shared between the
Time 1 and Time 2 ratings and variance that is unique to Time 2
ratings. Sixteen percent to 37% of variance in the Time 2 ratings
was explained by genetic and environmental factors associated
with the Time 1 ratings. In each case, most of the covariance
between the Time 1 and 2 ratings was accounted for by genetic
factors. As illustrated by the last three columns, change in tem-
perament over time was explained by the emergence of unique
genetic and nonshared environmental factors at Time 2.
As summarized in the last four rows of Table 4, the addition of
sibling-interaction paths to the models affected estimates of ge-
netic and environmental contributions to the Time 1 ratings. Esti-
mates of genetic contributions decreased, whereas shared environ-
mental contributions increased. Specifically, within the sibling-
interaction models, at Time 1, genetic contributions ranged from
19% to 40%, whereas shared environmental contributions ranged
from 31% to 72%. A different pattern of findings was obtained for
the Time 2 data. In most instances, the inclusion of sibling-
interaction paths did not affect heritability estimates for mothers’
temperament ratings, which ranged from 47% to 65%, and shared
environmental contributions continued to be low. In some cases,
Table 2
Model Fit Statistics and Parameter Estimates for Mothers’ Reports of Adolescent Temperament at Times 1 and 2
Model
Fit indices
Activity
Emotionality
Shyness
Sociability
df
⫺2LL
df
⫺2LL
df
⫺2LL
df
⫺2LL
ADE
2821
6666.05
2821
6633.72
2821
6772.92
2821
6840.10
ACE
2821
6717.99
2821
6636.42
2821
6807.11
2821
6858.38
CE
2824
6764.04
2824
6669.55
2824
6827.15
2824
6880.39
Parameter
Parameter estimates for best-fitting models
Activity (CI)
Emotionality (CI)
Shyness (CI)
Sociability (CI)
Time 1
a
11
.00 (
⫺.34, .34)
.52 (.06, .75)
.00 (
⫺.30, .30)
.00 (.53, .53)
d
11
.86 (.76, .90)
.60 (.20, .82)
.72 (.59, .81)
.74 (.44, .82)
c
11
—
—
—
—
e
11
.52 (.44, .62)
.61 (.53, .70)
.69 (.59, .81)
.67 (.57, .79)
Time 1
3 2
a
21
.00 (
⫺.31, .31)
.00 (
⫺.57, .37)
.00 (
⫺.29, .29)
.00 (
⫺.33, .33)
d
21
.47 (.37, .58)
.56 (.16, .78)
.44 (.29, .60)
.32 (.15, .65)
c
21
—
—
—
—
e
21
.14 (.01, .27)
.24 (.14, .36)
.20 (.06, .34)
.25 (.10, .41)
Time 2
a
22
.00 (
⫺.31, .31)
.49 (
⫺.64, .64)
.00 (
⫺.29, .29)
.00 (
⫺.33, .33)
d
22
.64 (.49, .71)
.00 (
⫺.47, .47)
.55 (.32, .65)
.52 (
⫺.63, .63)
c
22
—
—
—
—
e
22
.59 (.52, .68)
.62 (.56, .69)
.68 (.60, .78)
.75 (.66, .84)
Note.
⫺2LL ⫽ ⫺2 log likelihood; A ⫽ additive genetic factors; D ⫽ nonadditive genetic factors; C ⫽ shared environmental factors; E ⫽ nonshared
environmental factors; CI
⫽ 95% confidence interval. The fit indices for the best-fitting and most parsimonious models are italicized and in bold.
229
ADOLESCENCE AND TEMPERAMENT

genetic contributions to the temperament ratings appeared to in-
crease when sibling-interaction effects were added to the model.
However, the Time 2 path estimates generated by the sibling-
interaction models were within the confidence intervals of the
nonsibling-interaction models. Sibling-interaction effects also had
limited impact on genetic contributions to covariance between
Time 1 and Time 2 temperament ratings. Within the sibling-
interaction models, covariance between these ratings ranged from
23% to 33%.
Father ratings.
For each temperament dimension, the ACE
and CE models yielded worse model fits than did the ADE model,
indicating that genetic factors account for significant variance in
the fathers’ temperament ratings and that the contributions of
shared environmental factors are negligible. The model fits and
path estimates for the best-fitting models are included in Table 5.
In each case, significant nonadditive genetic paths were detected,
and additive genetic paths estimates tended to be low.
In the next set of analyses, sibling-interaction paths were added
to the best-fitting GCE model. Again, the DCE model was used as
the baseline GCE model because it yielded a relatively better fit to
the data than did the ACE model. For each temperament dimen-
sion, the best-fitting models included sibling-interaction paths and
genetic factors. In addition, for emotionality, the best-fitting
sibling-interaction model also permitted estimates for the sibling-
interaction paths to vary for twin and nontwin siblings. The path
estimates for the best-fitting sibling-interaction models are in-
cluded in Table 6. For each temperament dimension, the sibling-
interaction paths were negative, indicating sibling contrast. At
Time 1, inclusion of sibling-interaction paths altered genetic and
environmental path estimates for most ratings. In particular, esti-
mates of d
11
decreased for activity, emotionality, and sociability.
At the same time, estimates of c
11
increased for all temperament
dimensions. At Time 2, the addition of sibling-interaction paths,
however, seemed to influence genetic paths only for emotionality.
In this case d
21
and d
22
fell to nearly 0, whereas c
21
and c
22
rose
to .37 and .93, respectively.
Genetic and environmental contributions to variance in the
fathers’ ratings are summarized in Table 7. Variance components
based upon models that did not include sibling-interaction paths
are included at the top Table 7, and variance components derived
from models that included sibling-interaction paths are included at
the bottom of Table 7. When sibling-interaction paths were not
Table 3
Model Fit Statistics and Parameter Estimates for Mothers’ Reports of Adolescent Temperament (Sibling-Interaction Models)
Model
Activity
Emotionality
Shyness
Sociability
df
⫺2LL
df
⫺2LL
df
⫺2LL
df
⫺2LL
GCE
2821
6666.05
2821
6631.11
2821
6772.92
2821
6840.10
CE-B
1
B
2
2822
6764.04
b
2822
6669.55
b
2822
6826.57
b
2822
6822.01
a
GCE-B
1
B
2
2819
6626.23
a
2819
6625.68
2819
6730.18
a
2819
6812.64
a
GCE-B
1
B
2
B
3
B
4
2817
6613.40
ac
2817
6622.20
2817
6711.11
ac
2817
6810.53
a
Path
Path estimates for the best-fitting sibling-interaction model
Activity (CI)
Emotionality (CI)
Shyness (CI)
Sociability (CI)
Time 1
g
11
.44 (.15, .70)
.53 (.004, .77)
.63 (.38, .79)
.60 (.17, .81)
c
11
.85 (.59, .98)
.73 (.12, 1.0)
⫺.56 (⫺.84, ⫺.18)
.70 (.26, .98)
e
11
.28 (.10, .43)
.44 (.004, .71)
.54 (.37, .71)
.40 (.11, .58)
B
1
All siblings
—
⫺.22 (⫺.99, .06)
—
⫺.31 (⫺.78, ⫺.10)
B
1
Twins only
⫺.35 (⫺.73, ⫺.12)
—
⫺.11 (⫺.34, .04)
—
B
3
Nontwins only
⫺.50 (⫺.80, ⫺.27)
—
⫺.28 (⫺.50, ⫺.13)
—
Time 1
3 Time 2
g
21
.53 (.41, .64)
.49 (.34, .63)
.51 (.35, .69)
.42 (.29, .54)
c
21
.18 (.03, .31)
.15 (
⫺.16, .40)
⫺.02 (⫺.18, .23)
.08 (
⫺.16, .24)
e
21
.14 (.03, .25)
.24 (.11, .37)
.19 (.06, .33)
.20 (.07, .33)
Time 2
g
22
.61 (.45, .70)
.48 (.23, .60)
.47 (
⫺.63, .63)
.60 (.44, .69)
c
22
.00 (
⫺29, .29)
.00 (
⫺.31, .31)
.00 (
⫺.34, .34)
.00 (
⫺.40, .40)
e
22
.55 (.44, .60)
.67 (.59, .76)
.69 (.57, .83)
.65 (.56, .76)
B
2
All siblings
—
.04 (
⫺.04, .09)
—
⫺.08 (⫺.16, ⫺.04)
B
2
Twins only
⫺.04 (⫺.12, .04)
—
.04 (
⫺.05, .14)
—
B
4
Nontwins only
⫺.12 (⫺.17, ⫺.07)
—
⫺.13 (⫺.19, ⫺.08)
—
Note.
⫺2LL ⫽ ⫺2 log likelihood; G ⫽ genetic factors; C ⫽ shared environmental factors; E ⫽ nonshared environmental factors; CI ⫽ 95% confidence
interval. The GCE-B
1
B
2
and CE-B
1
B
2
models constrain all estimates to be equivalent for all sibling groups. In these models, B
1
corresponds to
sibling-interaction effects at Time 1, and B
2
corresponds to sibling-interaction effects at Time 2. The GCE-B
1
B
2
B
3
B
4
model permits estimates of sibling
interaction effects to vary for twin and nontwin sibling groups. Within this model, B
1
and B
2
correspond to sibling-interaction effects at Times 1 and
2, respectively, for the twins. B
3
and B
4
correspond to sibling-interaction effects at Times 1 and 2, respectively, for the nontwin siblings. The best-fitting
and most parsimonious model for each temperament dimension italicized and in bold.
a
Model fit is significantly better than the GCE model ( p
⬍ .05).
b
Model fit is significantly worse than the GCE model ( p
⬍ .05).
c
Model fit is significantly
better than the GCE-B
1
B
2
model ( p
⬍ .05).
230
GANIBAN, SAUDINO, ULBRICHT, NEIDERHISER, AND REISS
included in the model, heritability estimates ranged from 59%
to 83% at Time 1 and 63% to 79% at Time 2. In addition, the
Time 1 ratings accounted for 20% to 69% of the variance in
Time 2 ratings. Most of the covariance between the Time 1 and
Time 2 ratings was related to genetic factors. With the excep-
tion of negative emotionality, genetic factors also primarily
explained change in temperament ratings and accounted for 4%
to 61% of unique variance in Time 2 ratings. Nonshared envi-
ronmental factors also contributed to change, explaining 19% to
35% of unique variance in Time 2 ratings.
Table 4
Genetic and Environmental Contributions to Mothers’ Reports of Adolescent Temperament at Times 1 and 2 for Models That
Excluded Sibling-Interaction Paths and Models That Included Sibling-Interaction Paths
Variable
Time 1 total variance
Time 2 total variance
Time 2
Variance related to
Time 1 ratings
Variance unique
to Time 2
h
2
c
2
e
2
h
2
c
2
e
2
h
2
c
2
e
2
h
2
c
2
e
2
Nonsibling-interaction model
Activity
.73
—
.27
.63
—
.37
.22
—
.02
.41
—
.35
Emotionality
.63
—
.37
.55
—
.45
.31
—
.06
.24
—
.39
Shyness
.52
—
.48
.49
—
.50
.19
—
.04
.30
—
.46
Sociability
.55
—
.45
.37
—
.62
.10
—
.06
.27
—
.56
Sibling-interaction model
Activity
.19
.72
.08
.65
.03
.32
.28
.03
.02
.37
.00
.30
Emotionality
.28
.53
.19
.47
.02
.52
.24
.02
.06
.23
.00
.45
Shyness
.40
.31
.29
.48
.00
.52
.26
.00
.04
.22
.00
.48
Sociability
.36
.49
.16
.54
.01
.46
.18
.01
.04
.36
.00
.42
Note.
h
2
⫽ heritability; c
2
⫽ variance explained by shared environmental factors; e
2
⫽ variance explained by nonshared environmental factors. For
emotionality, the GCE-SI model was not significantly different from the GCE model, indicating that the sibling-interaction terms were not statistically
significant.
Table 5
Model Fit Statistics and Parameter Estimates for Fathers’ Reports of Adolescent Temperament at Times 1 and 2
Model
Fit indices
Activity
Emotionality
Shyness
Sociability
df
⫺2LL
df
⫺2LL
df
⫺2LL
df
⫺2LL
ADE
2821
6666.37
2821
6730.38
2821
6758.20
2821
6868.86
ACE
2821
6757.08
2821
6745.04
2821
6796.31
2821
6899.22
CE
2824
6801.60
2824
6798.53
2824
6842.58
2824
6950.04
Parameter
Parameter estimates for best-fitting models
Activity (CI)
Emotionality (CI)
Shyness (CI)
Sociability (CI)
Time 1
a
11
.00 (
⫺.30, .30)
.77 (.53, .86)
.03 (
⫺.54, .54)
.00 (
⫺.46, .46)
d
11
.91 (.85, .93)
.32 (.03, .67)
.83 (.58, .87)
.77 (.58, .84)
c
11
—
—
—
—
e
11
.42 (.36, .51)
.55 (.47, .63)
.56 (.49, .66)
.63 (.55, .74)
Time 1
3 2
a
21
.00 (
⫺.26, .26)
.04 (
⫺.23, .30)
.00 (
⫺.28, .28)
.00 (
⫺.53, .53)
d
21
.43 (.36, .51)
.82 (.14, .89)
.44 (.32, .67)
.44 (.22, .65)
c
21
—
—
—
—
e
21
.10 (.00, .21)
.14 (.03, .26)
.13 (.004, .26)
⫺.01 (⫺.11, .10)
Time 2
a
22
.00 (
⫺.25, .25)
.00 (
⫺.48, .48)
.00 (
⫺.28, .28)
.00 (
⫺.50, .50)
d
22
.78 (.71, .82)
.19 (
⫺79, .79)
.66 (.41, .74)
.75 (.50, .81)
c
22
—
—
—
—
e
22
.44 (.38, .53)
.52 (.45, .62)
.59 (.51, .69)
.49 (.43, .57)
Note.
⫺2LL ⫽ ⫺2 log likelihood; A ⫽ additive genetic factors; D ⫽ nonadditive genetic factors; C ⫽ shared environmental factors; E ⫽ nonshared
environmental factors; CI
⫽ 95% confidence interval. The fit indices for the best-fitting and most parsimonious models are italicized and in bold.
231
ADOLESCENCE AND TEMPERAMENT

As illustrated by the last four rows of Table 7, inclusion of
sibling-interaction paths led to lower estimates of genetic variance
and higher estimates of shared environmental variance for the
Time 1 ratings. Specifically, at Time 1, genetic contributions to
father-rated temperament ranged from 0 to 66%, whereas shared
environmental contributions ranged from 32% to 100%. This pat-
tern was also present for the Time 2 ratings of emotionality:
Shared environmental factors accounted for all variance in emo-
tionality ratings once sibling-interactions effects were statistically
controlled. For the remaining variables, the Time 2 estimates of
genetic and environmental contributions to activity, shyness, and
sociability were not dramatically influenced by inclusion of the
sibling-interaction terms, and genetic contributions to these tem-
perament dimensions ranged from 72% to 84%. Last, within the
sibling-interaction model, the Time 1 ratings explained 14% to
24% of the variance in Time 2 ratings. For activity, shyness, and
sociability, this covariance was primarily explained by genetic
factors. For emotionality, covariance was related to shared envi-
ronmental factors. Regarding change in temperament ratings
across time, genetic factors explained change in activity, shyness,
and sociability ratings and explained 53% to 64% of variance
unique to the Time 2 ratings. For emotionality, change was entirely
explained by shared environmental factors.
Discussion
Adolescence is a key transition point in a person’s life, and an
important determinant of adjustment at this time is temperament.
Yet few studies have explored genetic and environmental contri-
butions to stability and change in temperament during adolescence.
The current study explored genetic and environmental contribu-
tions to temperament from early to late adolescence within a
sample that included twin and nontwin siblings. We also examined
whether sibling-interaction effects influenced estimates of genetic
and environmental contributions.
The first set of analyses explored genetic and environmental
contributions to mothers’ and fathers’ reports of their teenagers’
temperaments across adolescence, without considering sibling-
interaction effects. Genetic factors and unique experiences primar-
ily accounted for variance in emotionality, activity levels, shyness,
and sociability during early and late adolescence. Furthermore,
genetic factors present during early adolescence accounted for
Table 6
Parameter Estimates for the Sibling-Interaction Model for Fathers’ Reports of Adolescent Temperament (Sibling-Interaction Models)
Model
Activity
Emotionality
Shyness
Sociability
df
⫺2LL
df
⫺2LL
df
⫺2LL
df
⫺2LL
GCE
2821
6666.37
2821
6734.05
2821
6758.20
2821
6867.86
CE-B
1
B
2
2822
6801.60
b
2822
6798.53
b
2822
6842.58
b
2822
6950.04
b
GCE-B
1
B
2
2819
6635.32
a
2819
28.97
2819
6731.65
a
2819
6856.45
a
GCE-B
1
B
2
B
3
B
4
2817
6654.84
a
2817
6711.14
ac
2817
6756.07
2817
6850.48
a
Path
Path estimates for the best-fitting models
Activity (CI)
Emotionality (CI)
Shyness (CI)
Sociability (CI)
Time 1
g
11
.76 (.38, .93)
.19 (.01, .67)
.81 (.57, .88)
.01 (.01, .73)
c
11
.57 (
⫺.91, .91)
.97 (.60, .97)
.29 (
⫺.75, .75)
1.0 (.47, 1.0)
e
11
.30 (.15, .41)
.13 (.003, .47)
.50 (.34, .62)
.004 (.004, .52)
B
1
all siblings
⫺.23 (⫺.56, ⫺.06)
—
⫺.08 (⫺.32, .001)
⫺.99 (⫺.99, ⫺.14)
B
1
twins only
—
⫺.66 (⫺.99, ⫺.11)
—
—
B
3
nontwins only
—
⫺.68 (⫺.99, ⫺.12)
—
—
Time 1
3 2
g
21
.45 (.36, .53)
.004 (.003, .004)
.48 (.37, .57)
.41 (.29, .54)
c
21
.10 (
⫺.27, .25)
.37 (.30, .52)
⫺.03 (⫺.37, .17)
.27 (.06, .32)
e
21
.10 (.02, .18)
.001 (.0004, .001)
.11 (.01, .21)
⫺.02 (⫺.11, .08)
Time 2
g
22
.80 (.67, .84)
.007 (.006, .007)
.73 (.64, .79)
.74 (.60, .81)
c
22
.00 (
⫺.50, .50)
.93 (.93, .95)
.00 (
⫺.37, .37)
.00 (
⫺.53, .53)
e
22
.38 (.31, .46)
.003 (.003, .0032)
.48 (.41, .57)
.46 (.37, .54)
B
2
all siblings
⫺.08 (⫺.21, ⫺.04)
—
⫺.10 (⫺.14, ⫺.15)
⫺.06 (⫺.19, ⫺.001)
B
2
twins only
—
⫺.99 (⫺.99, ⫺.99)
—
—
B
4
nontwins only
—
⫺.99 (⫺.99, ⫺.98)
—
—
Note.
⫺2LL ⫽ ⫺2 log likelihood; G ⫽ genetic factors; C ⫽ shared environmental factors; E ⫽ nonshared environmental factors; CI ⫽ 95% confidence
interval. The GCE-B
1
B
2
and CE-B
1
B
2
models constrain all estimates to be equivalent for all sibling groups. In these models, B
1
corresponds to
sibling-interaction effects at Time 1, and B
2
corresponds to sibling-interaction effects at Time 2. The GCE-B
1
B
2
B
3
B
4
model permits estimates of
sibling-interaction effects to vary for twin and nontwin sibling groups. Within this model, B
1
and B
2
correspond to sibling-interaction effects at Times 1
and 2, respectively, for the twins. B
3
and B
4
correspond to sibling-interaction effects at Times 1 and 2, respectively, for the nontwin siblings. The best-fitting
and most parsimonious model for each temperament dimension italicized and in bold.
a
Model fit is significantly better than the GCE model ( p
⬍ .05).
b
Model fit is significantly worse than the GCE model ( p
⬍ .05).
c
Model fit is significantly
better than the GCE-B
1
B
2
model ( p
⬍ .05).
232
GANIBAN, SAUDINO, ULBRICHT, NEIDERHISER, AND REISS

moderate variance in temperament ratings during late adolescence
and primarily explained stability in temperament over time. How-
ever, changes in temperament across adolescence were related to
the emergence of experiences and genetic factors that were not
present or did not contribute to temperament during early adoles-
cence. This pattern held true for mothers’ and fathers’ reports of
their children’s temperaments.
Previous studies have suggested that parents’ ratings of their
children’s characteristics are susceptible to sibling-contrast effects
and that such effects may lead to overestimation of genetic con-
tributions and underestimation of environmental contributions to
temperament. Consequently, in a second set of analyses, we ex-
amined whether this was true in the current study. Significant
sibling-contrast effects were observed for nearly all of the moth-
ers’ and fathers’ reports of temperament at Times 1 and 2. The
single exception to this pattern was mothers’ reports of negative
emotionality. At both time points, sibling-interaction effects were
nonsignificant for mothers’ reports of negative emotionality. Fur-
ther analyses also indicated that at Times 1 and 2, for mothers’
reports of shyness, sibling-contrast effects were nonsignificant for
twins but were significant for nontwin sibling groups. This latter
finding, however, was most likely due to reduced power to detect
sibling-contrast effects within twin-only samples, rather than the
absence of such effects for twins (Rietveld et al., 2003).
As previously reported by Saudino et al. (1995), sibling-contrast
effects influenced estimates of genetic and environmental contri-
butions to Time 1 temperament ratings. For each temperament
characteristic at Time 1, genetic contributions decreased, whereas
estimates of shared environmental contributions increased. In
some cases, this change was dramatic. These findings indicate that
if sibling-contrast effects are present but not explicitly modeled,
estimates of genetic influence are artificially inflated, whereas
estimates of shared environmental influences are deflated.
Sibling-interaction effects, however, had little impact upon the
path estimates relevant to the mothers’ and fathers’ Time 2 ratings.
The exception to this generalization was fathers’ ratings of nega-
tive emotionality. For this temperament rating alone, genetic con-
tributions fell to nearly zero, whereas shared environmental con-
tributions rose to 93% when sibling-contrast effects were
estimated. It is not clear why this was the case for fathers only.
However, this finding could reflect differences in the relationships
that evolved between mothers and fathers and their children. For
example, in previous analyses of the NEAD dataset, Reiss and
Neiderhiser (2000) noted that if a child starts to demonstrate high
levels of antisocial behavior during early adolescence, by late
adolescence, fathers reduce attention to this child and shift atten-
tion to the child who is perceived as less problematic. If this
mechanism applies to high levels of emotionality, then it is pos-
sible that fathers’ ratings of their children’s negative emotionality
continue to be influenced by their expectations, rather than by the
adolescents’ actual behaviors.
For activity, sociability, and shyness, however, most genetic
variance at Time 2 was related to nonadditive genetic factors, even
when sibling-contrast effects were taken into account. This finding
is generally consistent with other twin studies (Bouchard &
McGue, 2003). However, a large-scale study that included ex-
tended twin kinships (i.e., twins, their parents, siblings, and off-
spring) from Australia and the United States found that additive
genetic contributions to self-reported neuroticism accounted for
more variance than did nonadditive genetic contributions (Lake et
al., 2000). Thus, there is consistent evidence that nonadditive
genetic factors contribute to personality, but these factors are not
the primary source of genetic variance. In the current study, the
importance of nonadditive genetic factors relative to additive ge-
netic factors may have been exaggerated because there was insuf-
ficient power to completely differentiate between latent additive
and nonadditive genetic factors.
Sibling-interaction effects also had limited impact on under-
standing continuity and change in temperament. With the excep-
tion of fathers’ ratings of emotionality, genetic factors explained
continuity in temperament, even when sibling-interactions effects
were estimated. In regard to change, genetic and nonenvironmental
factors continued to account for unique variance in temperament
ratings. These latter findings are consistent with previous longitu-
Table 7
Genetic and Environmental Contributions to Fathers’ Reports of Adolescent Temperament at Times 1 and 2 for Models That
Excluded Sibling-Interaction Paths and Models That Included Sibling-Interaction Paths
Variable
Time 1 total variance
Time 2 total variance
Time 2
Variance related
to Time 1
Variance unique
to Time 2
h
2
c
2
e
2
h
2
c
2
e
2
h
2
c
2
e
2
h
2
c
2
e
2
Nonsibling-interaction model
Activity
.83
—
.18
.79
—
.21
.18
—
.02
.61
—
.19
Emotionality
.70
—
.30
.71
—
.29
.67
—
.02
.04
—
.27
Shyness
.69
—
.31
.63
—
.37
.19
—
.02
.44
—
.35
Sociability
.59
—
.40
.75
—
.25
.19
—
.00
.56
—
.25
Sibling-interaction model
Activity
.58
.32
.09
.84
.01
.15
.20
.01
.01
.64
.00
.14
Emotionality
.04
.94
.02
.00
1.0
.00
.00
.14
.00
.00
.86
.00
Shyness
.66
.08
.25
.76
.00
.23
.23
.00
.01
.53
.00
.23
Sociability
.00
1.00
.00
.72
.07
.21
.17
.07
.00
.55
.00
.21
Note.
h
2
⫽ heritability; c
2
⫽ variance explained by shared environmental factors; e
2
⫽ variance explained by nonshared environmental factors.
233
ADOLESCENCE AND TEMPERAMENT

dinal studies conducted with adults (M. McGue et al., 1993; Viken
et al., 1994) and with adolescents (Gillespie et al., 2004). Thus, to
the extent that stability exists, it is not derived from a stable
environment but, rather, is driven by a person’s genotype.
Much variance in Time 2 ratings, however, was independent of
the Time 1 ratings, suggesting that temperament ratings also
change over time. Both nonshared environmental and genetic
factors appear to play important roles in these changes. Environ-
mental influences have been found in other longitudinal studies
(e.g., Gillespie et al., 2004; McGue et al., 1993; Viken et al.,
1994). Such changes are consistent with McCrae et al.’s (2000)
description of “characteristic adaptations” of genetically influ-
enced personality characteristics that are caused by socialization.
The finding of genetic contributions to change, however, was less
expected. A previous study that included adolescents also reported
significant “genetic innovations” (i.e., new genetic influences) for
some characteristics at ages 14 and 16 years (Gillespie et al.,
2004). However, studies with adults have found little evidence of
new genetic effects (e.g., McGue et al., 1993; Viken et al., 1994).
Consequently, new genes may become active and affect personal-
ity as children undergo puberty. But genetically evoked changes
become less likely after this period, and change becomes more
driven by one’s environment.
In summary, sibling-interaction effects were present for both
mother- and father-rated temperament during early and late ado-
lescence. However, sibling contrast affected genetic and environ-
mental contributions at Time 1 but not at Time 2. It is not clear
why this was the case. It is possible that low power at Time 2 led
to an overestimation of nonadditive genetic contributions and an
underestimation of shared environmental effects. Power analyses
indicated that there was only sufficient power to detect moderate
shared environmental effects (i.e., estimates of .40 or higher) in the
presence of moderate nonadditive genetic effects. If shared envi-
ronmental effects were present but not detected because of low
power, such effects would have been relatively small and should
not have had a dramatic effect on the Time 2 genetic estimates. It
is worthwhile noting that the shared environment does seem to
affect positive affectivity during early childhood (Goldsmith et al.,
1997) and neuroticism during adolescence (Gillespie et al, 2004).
However, in most adult personality studies, shared environmental
contributions are negligible (Bouchard & McGue, 2003). There-
fore, adolescence may represent a transition point that marks the
decline of family-wide effects on temperament and personality.
Another possibility is that the source of sibling-interaction ef-
fects differs for Times 1 and 2. Sibling-interaction effects at Time
1 may truly represent rater bias. But Time 2 sibling-interaction
effects may represent evocative gene– environment correlation, as
proposed by Eaves and Silberg (2005). In this latter case, sibling-
contrast effects themselves may be artifactual and emerge when
parents respond to true differences in their children’s behaviors.
Rater bias may be operative during early adolescence because
children are at the beginning stages of individuation and may not
assert their independence or even recognize their own indepen-
dence from each other. Parents, however, may expect such differ-
ences and, when those differences are not clear, impose them. But
by late adolescence, most teenagers have started to define and
assert their own identities and make their own decisions, and
phenotypic differences between siblings may become more salient.
As a result, parents may be more compelled at this time than at
earlier ages to notice and acknowledge differences between sib-
lings. If this occurs, parents may fine tune their perceptions of
what makes their children different from each other and respond to
these differences accordingly. In this case, contrast effects are
present but are based upon actual sibling dissimilarities and shaped
by the parents’ unique experiences with each child (Eaves &
Silberg, 2005), rather than upon a biased view of how they expect
their children to differ. This explanation is purely speculative, and
more research with larger samples would be needed to determine
if the meaning of sibling-interaction effects varies with age.
Implications
There are few studies that examine genetic and environmental
contributions to temperament stability and change at any age. Most
studies with adolescents have assessed temperament at a single
time point or have included only twins or adopted children. This
has led to contradictory findings and a confusing picture of the
determinants of temperament during adolescence. Previous inves-
tigators have proposed that discrepancies in the findings of twin
and nontwin sibling studies can be explained by nonadditive
genetic effects and by sibling-interaction effects. Our findings
suggest that both nonadditive genetic factors and sibling-
interaction effects are present. Furthermore, this study illustrates
that nonadditive genetic effects can be overestimated if sibling-
contrast effects are not statistically controlled and that it is more
difficult to detect significant sibling-interaction effects for twins.
Conversely, this study also consistently detected nonadditive ge-
netic contributions to temperament. Sibling samples that do not
include MZ twins are underpowered to detect nonadditive effects
and may underestimate heritability.
Temperament was also moderately stable, and this stability was
primarily explained by genetic factors. This finding has particular
implications for developmental models that cast temperament as a
risk factor for psychopathology. In some models, it is argued that
genetically influenced characteristics, such as heightened negative
emotionality, can elicit more parent hostility and coercion, which,
in turn, can foster significant behavior problems during early
childhood (e.g., Moffitt, 1993). Because emotionality appears
moderately stable and stability is genetically influenced, highly
negative children may continue to evoke negative responses from
teachers and peers throughout childhood, even as they enter new
social environments. If this occurs, constant negative feedback
from the broader world could consolidate and perpetuate behav-
ioral and emotional problems. On the other hand, our findings also
indicate that significant changes in temperament occur and that
temperament is not fixed by late adolescence. Genetic factors
contribute to change, but environmental contributions are also
significant, suggesting that temperament itself may prove to be an
important target of prevention and intervention efforts.
Although this study provided further insight into the develop-
ment of temperament during adolescence, its results are qualified
by a relatively small sample size at Time 2. As discussed previ-
ously, limited power may have caused the contributions of non-
additive genetic factors to be exaggerated at the expense of addi-
tive genetic or shared environmental factors. Additional
longitudinal studies that include twins and other sibling groups or
relatives (e.g., parents, offspring) are needed to generate true
234
GANIBAN, SAUDINO, ULBRICHT, NEIDERHISER, AND REISS

estimates of genetic and environmental influences upon personal-
ity and temperament in the presence of sibling-interaction effects.
References
Blom, G. (1958). Statistical estimates and transformed beta variables.
New York: Wiley.
Bouchard, T. J., & McGue, M. (2003). Genetic and environmental influ-
ences on human psychological differences. Journal of Neurobiology, 54,
4 – 45.
Buss, A. H., & Plomin, R. (1984). Temperament: Early developing per-
sonality traits. Hillsdale, NJ: Erlbaum.
Carey, G. (1986). Sibling imitation and contrast effects. Behavior Genetics,
16, 319 –341.
Caspi, A. (1998). Personality development across the lifespan. In N.
Eisenberg (Ed.), Handbook of child psychology, Vol. 3: Social, emo-
tional, and personality development (5th ed., pp. 311–388). New York:
Wiley.
Cloninger, C. R., Sigvardsson, S., & Bohman, M. (1988). Childhood
personality predicts alcohol abuse in young adults. Alcoholism Clinical
and Experimental Research, 12, 494 –505.
Colder, C. R., & Chassin, L. (1997). Affectivity and impulsivity: Temper-
ament risk for adolescent alcohol involvement. Psychology of Addictive
Behaviors, 11, 83–97.
Davies, P. T., & Windle, M. (2001). Interparental discord and adolescent
adjustment trajectories: The potentiating and protective role of intra-
personal attributes. Child Development, 72, 1163–1178.
Eaves, L. J. (1976). A model for sibling effects in man. Heredity, 36,
205–214.
Eaves, L. J., Heath, A. C., Martin, N., Maes, H., Neale, M., Kendler, K., et
al. (1999). Comparing the biological and cultural inheritance of person-
ality and social attitudes in the Virginia 30,000 Study of twins and their
relatives. Twin Research, 2, 62– 80.
Eaves, L. J., Heath, A. C., Neale, M. C., Hewitt, J. K., & Martin, N. G.
(1998). Sex differences and non-additivity in the effects of genes on
personality. Twin Research, 1, 131–137.
Eaves, L. J., & Silberg, J. L. (2005). Parent– child feedback predicts sibling
contrast: Using twin studies to test theories of parent– offspring interac-
tion in infant behavior. Twin Research and Human Genetics, 8, 1– 4.
Eaves, L. J., Silberg, J. L., Maes, H. H., Simonoff, E., Pickles, A., Rutter,
M., et al. (1997). Genetics and developmental psychopathology: 2. The
main effects of genes and environment on behavioral problems in the
Virginia Twin Study of Adolescent Behavioral Development. Journal of
Child Psychology and Psychiatry and Allied Disciplines, 38, 965–980.
Finkel, D., & McGue, M. (1997). Sex differences and nonadditivity in
heritability of the Multidimensional Personality Questionnaire Scales.
Journal of Personality and Social Psychology, 72, 929 –938.
Gagne, J., Saudino, K. J., & Cherny, S. (2003). Genetic influences on
temperament in early adolescence: A multimethod perspective. In S. A.
Petrell, R. Plomin, J. C. DeFries, & J. K. Hewitt (Eds), Nature, nurture,
and the transition to early adolescence (pp. 166 –184). Oxford, United
Kingdom: Oxford University Press.
Gillespie, N. A., Evans, D. E., Wright, M. M., & Martin, N. G. (2004).
Genetic simplex modeling of Eysenck’s dimensions of personality in a
sample of young Australian twins. Twin Research, 6, 637– 648.
Goldsmith, H. H., Buss, K. A., & Lemery, K. (1997). Toddler and child-
hood temperament: Expanded content, stronger genetic evidence, new
evidence for the importance of environment. Developmental Psychol-
ogy, 33, 891–905.
Keller, M. C., Coventry, W. L., Heath, A. C., & Martin, N. G. (2005).
Widespread evidence for non-additive genetic variation in Cloninger’s
and Eysenck’s personality dimensions using a twin plus sibling design.
Behavior Genetics, 35, 707–721.
Lake, R. I. E., Eaves, L. J., Maes, H. H. M., Heath, A. C., & Martin, N. G.
(2000). Further evidence against the environmental transmission of
individual differences in neuroticism from a collaborative study of
45,850 twins and relatives on two continents. Behavior Genetics, 30,
223–233.
Lemery, K., Goldsmith, H. H., Klinnert, M., & Mrazek, D. (1999). Devel-
opmental models of infant and childhood temperament. Developmental
Psychology, 35, 189 –204.
Loehlin, J., Neiderhiser, J. M., & Reiss, D. (2003). The behavior genetics
of personality and the NEAD study. Journal of Research in Personality,
37, 373–387.
Lynam, D. R., Caspi, A., Moffitt, T. E., Wikstron, P. O., Loeber, R., &
Novak, S. (2000). The interaction between impulsivity and neighbor-
hood context on offending: The effects of impulsivity are stronger in
poorer neighborhoods. Journal of Abnormal Psychology, 109, 563–574.
McCrae, R. R., Costa, P. T., Ostendorf, F., Angleitner, A., Hrebickiova,
M., Avia, M. D., Sanz, J., & Sanchez-Bernardos, M. L. (2000). Nature
over nurture: Temperament, personality, and life span development.
Journal of Personality and Social Psychology, 78, 173–186.
McGue, M., Bacon, S., & Lykken, D. T. (1993). Personality stability and
change in early adulthood: A behavioral genetic analysis. Developmental
Psychology, 29, 96 –109.
McGue, M., & Bouchard, T. J. (1984). Adjustment of twin data for the
effects of age and sex. Behavior Genetics, 14, 325–343.
Moffitt, T. E. (1993). “Life-course persistent” and “adolescent-limited”
antisocial behavior: A developmental taxonomy. Psychological Review,
100, 674 –701.
Neale, M., & Cardon, L. (1992). Methodology for genetic studies of twins
and families. Boston: Kluwer Academic.
Neale, M. C., Baker, S. M., Xie, G., & Maes, H. H. (2003). Mx: Statistical
modeling (6th ed.) [Computer software]. Richmond: Medical College of
Virginia, Department of Psychiatry.
Neale, M. C., & Stevenson, J. (1989). Rater bias in the EASI Temperament
scales: A twin study. Journal of Personality and Social Psychology, 56,
446 – 455.
Newman, D. L., Caspi, A., Moffitt, T. E., & Silva, P. A. (1997). Anteced-
ents of adult interpersonal functioning: Effects of individual differences
in Age 3 temperament. Developmental Psychology, 33, 206 –217.
Nichols, R. C., & Bilbro, W. C. (1966). The diagnosis of twin zygosity.
Acta Genetica, 16, 265–275.
Nigg, J. T., & Goldsmith, H. H. (1994). Genetics of personality disorders:
Perspectives from personality and psychopathology research. Psycho-
logical Bulletin, 115, 346 –380.
Plomin, R., Corley, R., Caspi, A., Fulker, D. W., & DeFries, J. (1998).
Adoption results for self-reported personality: Evidence for nonadditive
genetic effects? Journal of Personality and Social Psychology, 75,
211–218.
Price, T. S., Simonoff, E., Asherson, P., Curran, S., Kuntsi, J., Waldman,
I., & Plomin, R. (2005). Continuity and change in preschool ADHD
symptoms: Longitudinal genetic analysis with contrast effects. Behavior
Genetics, 35, 121–132.
Reiss, D., & Neiderhiser, J. M. (2000). The interplay of genetic influences
and social processes in developmental theory: Specific mechanisms are
coming into view. Development and Psychopathology, 12, 357–374.
Reiss, D., Neiderhiser, J. M., Hetherington, E. M., & Plomin, R. (2000).
The relationship code: Deciphering genetic and social influences on
adolescent development. Cambridge, MA: Harvard University Press.
Rietveld, M. J. H., Posthuma, D., Dolan, C. V., & Boomsa, D. I. (2003).
ADHD: Sibling interaction or dominance: An evaluation of statistical
power. Behavior Genetics, 33, 247–255.
Roberts, B. W., Caspi, A., & Moffitt, T. E. (2001). The kids are alright:
Growth and stability in personality development from adolescence to
adulthood. Journal of Personality and Social Psychology, 81, 670 – 683.
Roberts, B. W., & DelVecchio, W. F. (2000). The rank-order consistency
235
ADOLESCENCE AND TEMPERAMENT
of personality traits from childhood to old age: A quantitative review of
longitudinal studies. Psychological Bulletin, 126, 3–25.
Rothbart, M. K., Ahadi, S. A., & Evans, D. E. (2000). Temperament and
personality: Origins and outcomes. Journal of Personality and Social
Psychology, 78, 122–135.
Rothbart, M. K., & Bates, J. E. (1998). Temperament. In N. Eisenberg
(Ed.), Handbook of child psychology, Vol. 3: Social, emotional, and
personality development (5th ed., pp. 105–176). New York: Wiley.
Saudino, K. J., Cherny, S. S., & Plomin, R. (2000). Parent ratings of
temperament in twins: Explaining the “too low” DZ correlations. Twin
Research, 3, 224 –233.
Saudino, K. J., McGuire, S., Reiss, D., Hetherington, E. M., & Plomin, R.
(1995). Parent ratings of EAS temperaments in twins, full siblings, half
siblings, and step siblings. Journal of Personality and Social Psychol-
ogy, 68, 723–733.
Saudino, K. J., Wertz, A. E., Gagne, J. R., & Chawla, S. (2004). Night and
day: Are siblings as different in temperament as parents say they are?
Journal of Personality and Social Psychology, 87, 698 –706.
Shiner, R., Masten, A., & Tellegen, A. (2002). A developmental perspec-
tive on personality in emerging adulthood: Childhood antecedents and
concurrent adaptation. Journal of Personality and Social Psychology,
83, 1168 –1188.
Silberg, J. L., San Miguel, V. F., Murrelle, E. L., Prom, E., Bates, J. E.,
Canino, G., et al. (2005). Genetic and environmental influences on
temperament in the first year of life: The Puerto Rico Infant Twin Study
(PRINTS). Twin Research and Human Genetics, 8, 328 –336.
Spitz, E., Moutier, R., Reed, T., Busnel, M. C., Marchaland, C., Rouber-
toux, P. L., & Carlier, M. (1996). Comparative diagnoses of twin
zygosity by SSLP variant analysis, questionnaire, and dermatoglyphic
analysis. Behavior Genetics, 26, 55– 63.
Viken, R. J., Rose, R. J., Kaprio, J., & Koskenvuo, M. (1994). A devel-
opmental genetic analysis of adult personality: Extraversion and neurot-
icism from 18 to 59 years of age. Journal of Personality and Social
Psychology, 66, 722–730.
Received January 3, 2007
Revision received January 29, 2008
Accepted January 31, 2008
䡲
236
GANIBAN, SAUDINO, ULBRICHT, NEIDERHISER, AND REISS
Wyszukiwarka
Podobne podstrony:
Changes in passive ankle stiffness and its effects on gait function in
improvment of chain saw and changes of symptoms in the operators
Changes in Brain Function of Depressed Subjects During
3 T Proton MRS Investigation of Glutamate and Glutamine in Adolescents at High Genetic Risk for Schi
20 Seasonal differentation of maximum and minimum air temperature in Cracow and Prague in the period
16 Changes in sea surface temperature of the South Baltic Sea (1854 2005)
Changes in passive ankle stiffness and its effects on gait function in
Audio CD changer In Dash and Hide Away LHD
Changes in Negative Affect Following Pain (vs Nonpainful) Stimulation in Individuals With and Withou
improvment of chain saw and changes of symptoms in the operators
2004 Variation and Morphosyntactic Change in Greek From Clitics to Affixes Palgrave Studies in Langu
Influence of different microwave seed roasting processes on the changes in quality and fatty acid co
Karger Howard Short Changed Life And Debt In The Fringe Economy
Barwiński, Marek Changes in the Social, Political and Legal Situation of National and Ethnic Minori
Feltynowski, Marcin The change in the forest land share in communes threatened bysuburbanisation an
Kwiek, Marek; Maassen, Peter Changes in Higher Education in European Peripheries and Their Contexts
Fr hlich Stability of Pulegone and Thujone in Ethanolic Solution
więcej podobnych podstron