ABSTRACT
The administration of ephedrine and caffeine
(E+C) has been proposed to promote weight loss by increasing
energy expenditure and decreasing food intake. We tested this
hypothesis in six lean (4–9% body fat) and six mildly to moder-
ately obese (13–44% body fat) monkeys studied during a 7-wk
control period, an 8-wk drug treatment period, and a 7-wk place-
bo period. During the drug treatment period, the monkeys were
given ephedrine (6 mg) and caffeine (50 mg) orally three times
per day. At the end of each period, a glucose tolerance test was
performed, energy expenditure was measured, and body compo-
sition was determined. Treatment with E+C resulted in a
decrease in body weight in the obese animals (P = 0.06). This
loss in weight was primarily the result of a 19% reduction in
body fat. Drug treatment also resulted in a decrease in body fat
in the lean group (P = 0.05). Food intake was reduced by E+C
only in the obese group (P < 0.05). Nighttime energy expenditure
was increased by 21% (P < 0.03) in the obese group and 24%
(P < 0.01) in the lean group with E+C treatment. Twenty-
four–hour energy expenditure was higher in both groups during
drug treatment. E+C did not produce systematic changes in glu-
coregulatory variables, whereas plasma leptin concentrations
decreased in both groups with drug treatment. Overall, these
results show that E+C treatment can promote weight loss
through an increase in energy expenditure, or in some individu-
als, a combination of an increase in energy expenditure and a
decrease in food intake.
Am J Clin Nutr 1998;68:42–51.
KEY WORDS
Rhesus monkeys, ephedrine, caffeine, oxy-
gen consumption, body composition, insulin, glucose, leptin,
weight loss
INTRODUCTION
Obesity is characterized by an imbalance between energy
intake and expenditure, resulting in a net increase in the storage
of body energy primarily as fat. This imbalance is the result of
either a high energy intake, low energy expenditure, or a mixture
of the two conditions. A reduced rate of energy expenditure has
been shown to contribute to the development of obesity in
humans (1) and genetically obese strains of rodents (2). The
mechanism for this decreased energy expenditure has not been
fully established, but one contributing factor may be low sympa-
thetic nervous system (SNS) activity. Studies with rodents (3)
and human subjects (4) have shown that low SNS activity can
contribute to the development of obesity. These observations
suggest that obesity may be treated effectively by increasing
energy expenditure with the use of sympathomimetic agonists.
The use of an ephedrine and caffeine mixture (E+C) is one treat-
ment that has shown promise as an antiobesity agent, presum-
ably acting at least in part by SNS-mediated stimulation of
energy expenditure (5, 6).
Ephedrine has been shown to increase energy expenditure in
humans (7). The mechanism for this effect appears to be related
to stimulation of release of norepinephrine (8) and direct agonis-
tic stimulation of adrenoreceptors (9). Both of these actions may
stimulate energy expenditure through increases in intracellular
concentrations of cyclic AMP. The ephedrine-induced stimula-
tion of energy expenditure is further enhanced when ephedrine is
administered in combination with methylxanthines such as caf-
feine (10). The mechanism for methylxanthine enhancement of
ephedrine is likely the result of inhibition of phosphodiesterase
enzyme activity and antagonism of the inhibitory effect of
adenosine on norepinephrine release (11). The presence of plau-
sible modes of action for ephedrine- and caffeine-induced
increases in energy expenditure suggest that continued adminis-
tration of a combination of the 2 drugs may have potent antiobe-
sity actions.
A study in postobese and lean volunteers first showed in
humans that E+C was more effective than ephedrine alone at
raising energy expenditure and E+C could effectively raise
energy expenditure in individuals predisposed to obesity (12).
Several studies have shown that E+C can effectively cause
weight loss in humans (13–15). The contribution of increased
energy expenditure or decreased food intake to weight loss with
E+C treatment is still not firmly established, with studies report-
Energy expenditure, body composition, and glucose metabolism
in lean and obese rhesus monkeys treated with ephedrine
and caffeine
1–4
Jon J Ramsey, Ricki J Colman, Andrew G Swick, and Joseph W Kemnitz
1
From the Wisconsin Regional Primate Research Center, University of
Wisconsin, Madison, and the Department of Metabolic Diseases, Pfizer Cen-
tral Research, Groton, CT.
2
This is publication number 37-027 of the Wisconsin Regional Primate
Research Center.
3
Supported by Pfizer Inc and NIH grants RR00167, AG11913, and
AG07831.
4
Address reprint requests to JW Kemnitz, Wisconsin Regional Primate
Research Center, University of Wisconsin, 1220 Capitol Court, Madison, WI
53715-1299. E-mail: kemnitz@primate.wisc.edu.
Received June 17, 1997.
Accepted for publication January 9, 1998.
Am J Clin Nutr 1998;68:42–51. Printed in USA. © 1998 American Society for Clinical Nutrition
42
ajcn.nutrition.org
Downloaded from

ing either food intake (10) or energy expenditure (16) changes as
the major contributors to weight loss. Additional research has
also been needed to determine the role these drugs may play in
altering glucose metabolism. The purpose of the present study
was to determine the effect of administration of E+C on food
intake and energy expenditure in lean or mildly to moderately
obese rhesus monkeys under carefully controlled conditions.
Additionally, frequently sampled glucose tolerance tests were
performed to determine the effect of E+C on glucose metabo-
lism, and dual-energy X-ray absorptiometry (DXA) measure-
ments were used to assess body composition.
MATERIALS AND METHODS
Experimental design
Twelve adult male rhesus monkeys (Macaca mulatta) ranging
in age from 8 to 20 y were selected for this experiment. The
monkeys were then assigned to 1 of 2 groups based on percent-
age body fat: lean (4–9% body fat) or obese (13–44% body fat).
All monkeys were individually housed in stainless steel cages
with inside dimensions of 89 cm wide
3 86 cm deep 3 86 cm
high. The cages contained food hoppers and spigots to allow the
animals continuous access to water. The rooms were maintained
at
<21 8C and lighted from 0600 to 1800. The animals were
allowed to eat commercial, nonpurified biscuits (#5037; Ralston
Purina Co, St Louis: 15% of energy as protein and 12% as fat,
with beef tallow as the primary fat source) ad libitum between
0800 and 1500. Intake, accounting for spillage, was measured
daily.
The monkeys were tested in a 7-wk control period followed
by an 8-wk drug treatment phase, and, finally, a 7-wk placebo
period. During the drug treatment phase, the animals were given
ephedrine hydrochloride (6 mg, Efedrin; Hammer Corp, Atlanta)
and caffeine (50 mg, Jet-Alert; Wendt Laboratories, Belle Plaine,
MN) orally 3 times/d. The ephedrine and caffeine capsules were
hidden in a small piece of fruit for administration to the mon-
keys. The E+C dose used in this experiment was 18 mg
ephedrine and 150 mg caffeine/d. This dose was similar to the
dose used in many human studies when normalized by using
weight
3/4
or weight
2/3
to adjust for interspecies differences in
body weight (17, 18). Previous tests of the dose in the monkeys
had shown no evidence of unusual behavior or harmful side
effects. Placebo was given 3 times per day during the final phase
of the experiment. During all of the phases, food intake was
measured daily and blood samples and body weights were taken
weekly. Additionally, oxygen consumption was measured in the
fourth and fifth weeks of all phases, and frequently sampled glu-
cose tolerance tests and body-composition measurements were
completed in the sixth and seventh weeks of all phases. This pro-
tocol was approved by the Institutional Animal Care and Use
Committee at the University of Wisconsin–Madison.
Oxygen consumption
The monkeys were placed individually inside a transparent
metabolic chamber with inside dimensions of
<75 cm wide 3
75 cm deep
3 80 cm high. The duration of the calorimetry
measurements was
<30 h. The animals had visual and auditory
contact with other animals the entire time they were in the cham-
ber.
The flow rate of filtered, compressed air entering the chamber
was regulated. Exhaust air was dried and continuously sampled
at a rate of 100 mL/min for analysis of oxygen content (R-1 Flow
Control Unit, S-3A Oxygen Readout/Control Unit; Applied
Electrochemistry, Inc, Sunnyvale, CA; now AMETEK, Pitts-
burgh). Outputs from the flow meter and oxygen analyzer were
recorded every 10 min by using an IDAC 1000 interface (IDAC,
Amherst, NH) and Apple Macintosh SE/30 computer (Apple
Computer, Inc, Cupertino, CA). The chamber was calibrated by
burning ethanol and measuring oxygen consumption. Accuracy
of the calorimetry system was 96%.
Energy expenditure was calculated from oxygen consumption
by using the term 20.7 kJ/L oxygen. This value was calculated
from the food quotient of the diet (the predicted respiratory quo-
tient, assuming nutrient oxidation matches diet composition).
For analysis, energy expenditure was divided into morning
(0600–1200), afternoon (1200–1800), overnight (1800–0600),
and 24-h measurements.
Behavioral observations
Thirty-minute behavioral observations were performed twice
during the control and placebo phases and 3 times during the
drug treatment period. The observations were made at 1200,
immediately after the second drug or placebo administration of
the day. The behavior of the animals was scored according to the
amount of time spent lying down, sitting, standing, moving hor-
izontally, or moving vertically. Notes were also made regarding
any unusual behaviors.
Body composition
Fat mass, lean mass, and percentage body fat were determined
by DXA (model DPX-L; Lunar Corp, Madison, WI) (19, 20).
Animals were sedated with ketamine hydrochloride (15 mg/kg,
intramuscularly) plus acepromazine (2 mg intravenously) for
additional muscle relaxation and scanned in the supine position.
Frequently sampled glucose tolerance test
Frequently sampled glucose tolerance tests were conducted
according to the Modified Minimal Model protocol (21, 22). The
animals were food deprived overnight and then anethestized with
ketamine (15 mg/kg intramuscularly) and diazapam (1 mg/kg
intramuscularly), with supplemental ketamine as needed. A
venous catheter was placed for sample collection and administra-
tion of glucose and tolbutamide. Four baseline blood samples were
taken, followed by administration of a 300-mg/kg glucose bolus
over 1 min. Blood samples were then drawn at 2, 3, 4, 5, 6, 8, 10,
12, 14, 16, 19, 22, 23, 24, 25, 27, 30, 40, 50, 60, 70, 80, 90, 100,
120, 140, 160, and 180 min after glucose administration. Tolbu-
tamide (5 mg/kg) was infused 20 min after glucose administration.
Data were analyzed by the Minimal Model Method (23).
Plasma glucose was measured by the glucose oxidase method
(model 23A; Yellow Springs Instruments, Yellow Springs, OH).
Insulin was measured by double-antibody radioimmunoassay
(Binax, South Portland, ME). Leptin was also measured by
radioimmunoassay of baseline blood samples (LINCO Research,
Inc, St Louis).
Blood samples
Weekly blood samples were taken after an overnight fast in all
of the animals. Radioimmunoassay analyses of triiodothyronine
(Diagnostic Products Corporation, Los Angeles) and cortisol
(INCSTAR, Stillwater, MN) were completed on all samples.
EPHEDRINE PLUS CAFFEINE TREATMENT AND ENERGETICS
43
ajcn.nutrition.org
Downloaded from
Statistical analysis
All results are presented as means
±
SDs. Comparisons
between the lean and obese groupings of animals at the start
of the control period were completed by using one-way analy-
sis of variance (ANOVA). Comparisons between treatment
phases were made by using repeated-measures ANOVA in
which the factors were individual animal, treatment (control,
drug, and placebo), group (obese or lean), and time. Signifi-
cant (P < 0.05) treatment-by-group interactions were present
for basal glucose, food intake, weight, fat mass, afternoon
energy expenditure, leptin, and triiodothyronine. Treatment
comparisons of these variables were analyzed within groups
by paired t test. Both groups were matched for age before the
start of the experiment and no consistent age-related effects
were noticed for any of the variables at any of the assessment
periods. All analyses were completed by using the JMP sta-
tistical program (SAS Institute, Cary, NC).
RESULTS
Characteristics of the obese and lean groups of monkeys are
summarized in Table 1. As expected, body weight (P < 0.01),
lean tissue mass (P = 0.05), and fat mass (P < 0.01) were higher
in the obese group than in the lean animals. This increased body
size was not associated with hyperphagia because food intake
was actually higher in the lean than in the obese group. Total
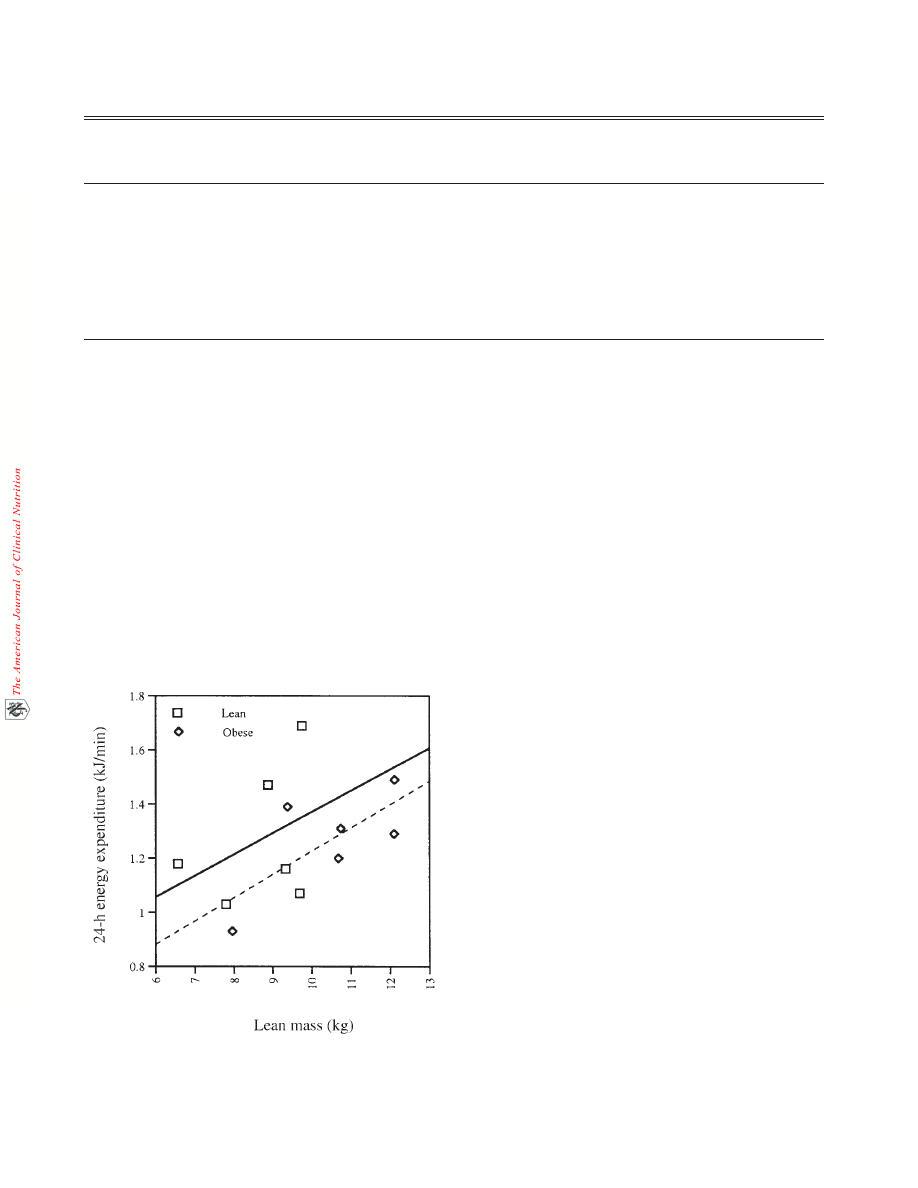
energy expenditure (kJ/min) was not different between groups
during any period of the day (P > 0.10); however, 24-h energy
expenditure expressed as a function of lean body mass was lower
in the obese animals (Figure 1).
Leptin (P < 0.01), basal insulin (P = 0.03), and glucose
(P = 0.02) were all higher in the obese than in the lean animals.
Insulin sensitivity and disposition index were lower (P < 0.10) in
the obese than the lean group, whereas other test variables did
not differ significantly between the groups.
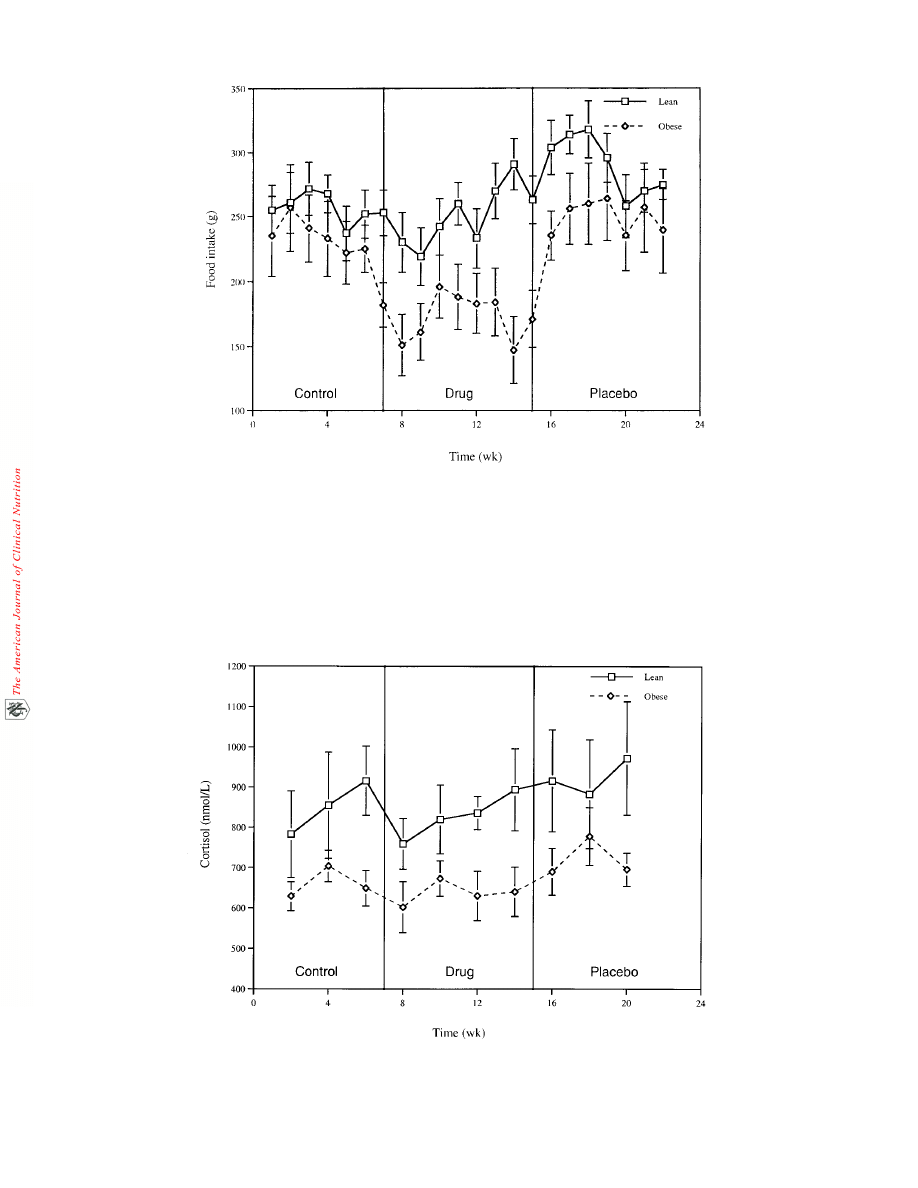
The effect of E+C treatment on food intake is illustrated in
Figure 2. Food intake, through 8 wk of drug treatment,
decreased by 24% (P = 0.01) in the obese animals, whereas E+C
treatment did not alter food intake in the lean group. Food intake
increased in both the obese (P = 0.02) and lean (P = 0.01) groups
during the placebo phase when compared with the drug treat-
ment phase. Food intake also tended to be higher in the placebo
phase than in the control period, although these differences were
not significant.
The effect of E+C treatment on body weight and composition
is summarized in Table 2. Eight weeks of drug treatment
resulted in a 7.5% decrease in body weight in the obese group
(P = 0.057) whereas no significant changes were noticed in the
lean animals. Weight loss began the first week of drug treatment
in the obese animals and continued throughout the drug treat-
ment phase. This change in body weight was primarily the result
of a 19% decrease in body fat in the obese animals (P = 0.075).
Similarly, E+C treatment resulted in a decrease in body fat in the
lean animals but this was countered by a slight increase in lean
body mass and resulted in no net change in total body mass.
After E+C treatment, the obese and lean animals gained weight;
this weight gain plateaued in the fifth week of the placebo phase.
Both groups of animals tended to increase in body weight after
cessation of the E+C treatment, which was attributable to slight
increases in both lean tissue mass and fat mass in the lean group
and increases primarily in lean mass in the obese animals. Final
44
RAMSEY ET AL
TABLE 1
Comparison of control period variables in the obese and lean groups of rhesus monkeys
1
Values greater in
Values lower in
obese than in
Values not significantly different
obese than in
lean animals
between obese and lean animals
lean animals
(P < 0.10)
(P > 0.10)
(P < 0.10)
Body weight (0.003)
Morning energy expenditure (0.179)
Food intake (0.015)
Lean tissue mass (0.053)
Afternoon energy expenditure (0.670)
Insulin sensitivity (0.047)
Fat mass (0.006)
Evening energy expenditure (0.236)
Disposition index (0.068)
Leptin (0.004)
24-hour energy expenditure (0.999)
Basal insulin (0.033)
Triiodothyronine (0.978)
Basal glucose (0.023)
Glucose effectiveness (0.145)
Glucose disappearance rate (0.120)
Acute insulin response to glucose (0.573)
2nd-Phase insulin response to glucose (0.455)
Insulin response to tolbutamide (0.111)
1
P values for one-way ANOVA between the obese and lean groups.
FIGURE 1. Twenty-four–hour energy expenditure plotted against
lean body mass for both the lean (n = 6) and obese (n = 6) groups of
monkeys during the control phase of the experiment.
ajcn.nutrition.org
Downloaded from
body weights in the placebo and control periods were not signi-
ficantly different in the obese group, whereas body weight was
greater in the placebo than in the control period in the lean mon-
keys (P = 0.001).
E+C treatment resulted in increased energy expenditure in
both the obese and lean animals (Table 3). Drug treatment
increased overnight energy expenditure by
≥
20% in both groups.
Morning and 24-h energy expenditure were also significantly
increased by E+C treatment (P < 0.001). Afternoon energy
expenditure, however, increased only in the lean group
(P < 0.05). Energy expenditure was significantly lower during
the placebo period than the drug treatment period in both groups
and at all time periods. Placebo and control 24-h energy expen-
ditures were not significantly different (P > 0.10). No systematic
changes in behavior or activity were observed between treatment
phases in either the obese or lean group of monkeys.
Frequently sampled glucose tolerance tests were performed
on each of the animals in all phases of the experiment (Table 4).
No treatment effects (P > 0.10) were detected for basal insulin,
insulin sensitivity, glucose effectiveness, acute insulin response
to glucose, second-phase insulin response to glucose, insulin
response to tolbutamide, and disposition index when comparing
the control, drug, and placebo phases. Basal insulin did show a
trend toward a decrease during the drug treatment phase when
compared with the control (P = 0.072) and placebo (P = 0.093)
phases. Glucose disappearance rate tended to increase between
the drug and placebo phases of the experiment (P = 0.075).
Treatment response was similar between the obese and lean ani-
mals for all of the glucose-tolerance-test variables except basal
glucose. The lean group of animals showed a significant
increase in basal glucose during the placebo period when com-
pared with the control and drug phases (P < 0.05), whereas no
significant differences were observed between treatment phases
in the obese group (P > 0.10).
Cortisol concentrations (Figure 3) showed no significant dif-
ferences between the control and drug treatment phases in either
the lean or obese groups (P > 0.10). Triiodothyronine concentra-
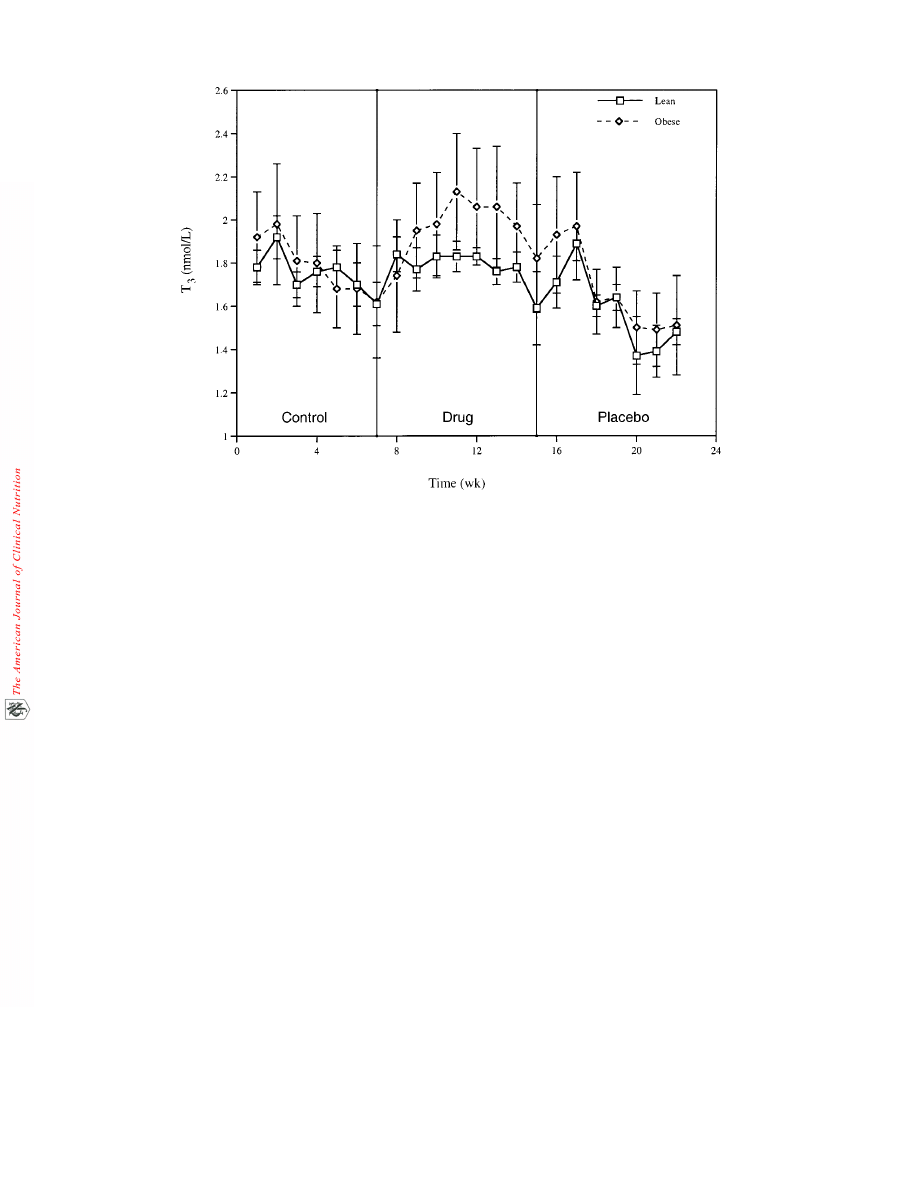
tions were increased by 10% with E+C treatment in the obese
EPHEDRINE PLUS CAFFEINE TREATMENT AND ENERGETICS
45
TABLE 2
Body composition in lean and obese rhesus monkeys receiving placebo or ephedrine + caffeine treatments
1
P value
2
Control
Drug
Placebo
C
3 D
D
3 P
C
3 P
Obese
kg
Body weight
15.57 ± 2.79
3
14.40 ± 2.45
15.11 ± 2.55
0.057
0.077
0.545
Lean tissue mass
10.50 ± 1.61
10.34 ± 1.51
10.60 ± 1.58
0.352
0.110
0.701
Fat mass
3.91 ± 2.25
3.18 ± 2.18
3.26 ± 2.11
0.075
0.719
0.238
Lean
Body weight
10.33 ± 1.60
10.26 ± 1.45
11.12 ± 1.69
0.635
0.003
0.001
Lean tissue mass
8.67 ± 1.26
8.73 ± 1.37
9.06 ± 1.29
0.641
0.010
0.015
Fat mass
0.70 ± 0.29
0.55 ± 0.21
0.95 ± 0.44
0.050
0.016
0.033
1
A significant (P < 0.05) treatment-by-group interaction was found with the body-composition measures, consequently, groups (obese or lean) were
analyzed separately.
2
Paired t tests. C, control; D, drug (ephedrine + caffeine); P, placebo.
3
x– ± SD.
TABLE 3
Energy expenditure in lean and obese rhesus monkeys receiving placebo or ephedrine + caffeine treatments
P values
1
Obese
Lean
C
3 D
D
3 P
C
3 P
Morning energy expenditure
2
kJ/min
Control
3.00 ± 0.29
3
3.73 ± 0.53
0.011
< 0.001
0.245
Drug
3.33 ± 0.23
4.04 ± 0.43
Placebo
2.40 ± 0.36
3.25 ± 0.55
Afternoon energy expenditure
4
Control
3.21 ± 0.28
a
3.02 ± 0.43
c
—
—
—
Drug
3.04 ± 0.23
a
3.54 ± 0.33
d
Placebo
2.36 ± 0.29
b
2.92 ± 0.43
e
Evening energy expenditure
2
Control
2.13 ± 0.27
1.84 ± 0.06
< 0.001
< 0.001
0.577
Drug
2.50 ± 0.22
2.38 ± 0.11
Obese
1.80 ± 0.16
1.53 ± 0.17
24-h energy expenditure
2
Control
2.63 ± 0.23
2.61 ± 0.26
0.004
< 0.001
0.675
Drug
2.84 ± 0.14
3.11 ± 0.20
Obese
2.09 ± 0.22
2.30 ± 0.27
1
Paired t tests. C, control; D, drug (ephedrine + caffeine); P, placebo.
2
Overall treatment effect significantly different from the other treatments, P < 0.001 (repeated-measures ANOVA).
3
x– ± SD.
4
Within groups, values with different letter superscripts are significantly different and show a significant treatment-by-group interaction, P < 0.05
(paired t test).
ajcn.nutrition.org
Downloaded from

monkeys (P = 0.02), whereas concentrations of this hormone
were not altered with drug treatment in the lean animals (Figure
4). Triiodothyonine concentrations also decreased (P < 0.05)
after cessation of drug treatment in the obese and lean groups.
Triiodothyronine concentrations during the last 5 wk of the
placebo period were not different from values measured during
the control period in both the lean and obese monkeys
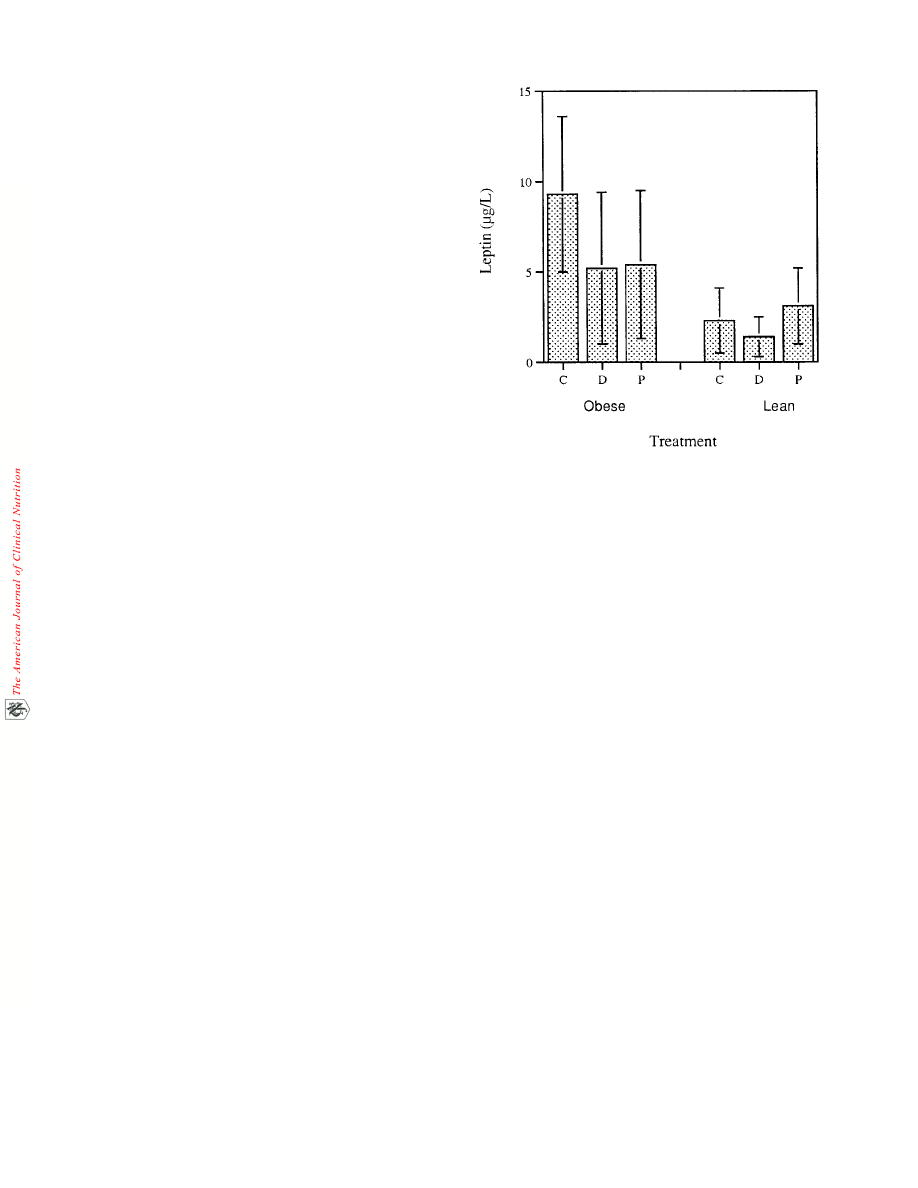
(P > 0.10). Plasma leptin concentrations were lower in the drug
treatment phase in both the lean (P = 0.10) and obese (P < 0.09)
groups of animals (Figure 5). Plasma leptin concentrations also
tended to increase after cessation of E+C treatment in both
groups of animals.
DISCUSSION
This study showed that E+C given for 8 wk induced weight
loss in obese rhesus monkeys, whereas body weight was not
significantly altered in lean monkeys. Several studies with
human volunteers (13, 15, 24) have shown that administration of
E+C along with a 5-MJ/d diet causes a significant decrease in
body weight in obese individuals. Similarly, studies in obese
mice (16) and rats (25) have shown that E+C induces a signifi-
cant decrease in body weight without concurrent food restric-
tion. These results agree with the present finding that E+C
decreased body weight in obese monkeys allowed free consump-
tion of food. Changes in body weight during treatment with these
drugs, however, appeared to be dependent on the initial body
composition of the animals. In the present study, body weight
was not significantly changed in the lean group. This probably
reflects the fact that the lean group had a very low body fat con-
tent and despite a significant decrease in body fat, changes in
total body weight were not large enough for significant differ-
ences to be detected. The observation of increases in body
weight in both the lean and obese groups of animals after the
46
RAMSEY ET AL
TABLE 4
Glucose tolerance test values in lean and obese rhesus monkeys receiving placebo or ephedrine + caffeine treatments
1
P values
2
Obese
Lean
C
3 D
D
3 P
C
3 P
Basal insulin (pmol/L)
3
Control
702 ± 420
4
266 ± 99
0.072
0.093
0.215
Drug
433 ± 328
249 ± 114
Placebo
581 ± 445
312 ± 147
Basal glucose (mmol/L)
Control
3.49 ± 0.43
2.94 ± 0.25
—
—
—
Drug
3.31 ± 0.28
2.96 ± 0.24
Placebo
3.20 ± 0.22
3.21 ± 0.12
5
Insulin sensitivity (
310
4
min · pmol/L)
6
Control
0.210 ± 0.146
0.726 ± 0.530
0.649
0.648
0.334
Drug
0.276 ± 0.152
0.711 ± 0.305
Placebo
0.236 ± 0.139
0.783 ± 0.496
Glucose effectiveness (
310/min)
7
Control
0.039 ± 0.023
0.056 ± 0.015
0.367
0.112
0.887
Drug
0.024 ± 0.006
0.055 ± 0.044
Placebo
0.038 ± 0.015
0.069 ± 0.026
Glucose disappearance rate (%/min)
8
Control
5.79 ± 2.44
12.43 ± 9.25
0.476
0.075
0.657
Drug
4.43 ± 2.17
11.68 ± 4.73
Placebo
5.91 ± 3.78
16.45 ± 7.95
AIR (pmol/L)
9
Control
1338 ± 542
1140 ± 635
0.380
0.176
0.753
Drug
1200 ± 357
1040 ± 369
Placebo
1409 ± 638
1174 ± 500
f
2
(pmol/L)
10
Control
1418 ± 581
1146 ± 631
0.903
0.793
0.975
Drug
1353 ± 547
1173 ± 573
Placebo
1395 ± 733
1222 ± 668
Tol (pmol/L)
11
Control
1905 ± 739
1194 ± 661
0.423
0.287
0.711
Drug
1161 ± 600
1217 ± 583
Placebo
1917 ± 880
1060 ± 787
Disposition index
12
Control
731 ± 150
1062 ± 357
0.629
0.661
0.185
Drug
697 ± 401
1259 ± 770
Placebo
690 ± 292
1693 ± 552
1
AIR, acute insulin response to glucose;
f
2
, second-phase insulin response to glucose; Tol, insulin response to tolbutamide.
2
Paired t tests comparisons. C, control; D, drug (ephedrine + caffeine); P, placebo.
3, 6–12
Overall treatment effect (repeated-measures ANOVA):
3
P = 0.210,
6
P = 0.362,
7
P = 0.209,
8
P = 0.070,
9
P = 0.359,
10
P = 0.948,
11
P = 0.550,
12
P = 0.200.
4
x
– ± SD.
5
Significant treatment-by-group interaction; for group, placebo significantly different from drug and control, P < 0.05 (paired t test).
ajcn.nutrition.org
Downloaded from
drug treatment phase also supports the idea that E+C reduces
body weight.
Changes in body weight during E+C treatment were primarily
the result of decreases in fat mass. Fat mass decreased in both
lean and obese groups of monkeys whereas lean tissue mass was
not changed significantly with drug treatment. This observation
agrees with previous animal studies that show E+C treatment
induces a dramatic decrease in total body fat. Dulloo and
Miller (26) showed that body fat was reduced 75% in obese
(fa/fa) Zucker rats after E+C treatment for 15 wk. Inclusion of
EPHEDRINE PLUS CAFFEINE TREATMENT AND ENERGETICS
47
FIGURE 3. Cortisol plotted against time during the 3 treatment phases. Repeated-measures ANOVA was used to test for differences in cortisol
concentrations between the treatment phases. No significant differences were detected between the treatment phases (P > 0.10).
FIGURE 2. Food intake plotted against time during the 3 treatment phases. Repeated-measures ANOVA was used to test for differences in food
intake between the treatment phases. Food intake was significantly lower in the drug treatment phase than in the control and placebo phases in the
obese animals (P < 0.05). In the lean animals, food intake was significantly greater during the placebo phase than during the drug treatment phase
(P < 0.05).
ajcn.nutrition.org
Downloaded from
E+C in the diets of growing pigs was also shown to cause a
27% decrease in lipid accretion and to accelerate muscle pro-
tein deposition over a 6-wk period (27). After cessation of
E+C, weight gain was the result of increases in both lean and
fat mass in both groups of monkeys.
The mechanism for the preferential decrease in fat mass dur-
ing administration of E+C remains largely unknown. However,
some evidence suggests that direct stimulation of
b-adrenergic
receptors is a major contributor to this response. Ephedrine has
both indirect sympathomimetic activity, by causing release of
norepinephrine from sympathetic nerve terminals, and direct
agonist activity on
b-adrenergic receptors (5). b-Adrenergic
agonists have been studied extensively and shown to decrease
carcass fat content (28). Research with
b
3
-agonists has also
shown a decrease in carcass adipose tissue in rats after treatment
with a highly selective agonist (29). These results suggest that
the decreases in body fat associated with E+C treatment may be
the result of interaction between ephedrine and
b
3
or other
b-
receptors.
Ultimately, changes in body composition are the result of an
imbalance between energy intake and energy expenditure. E+C
treatment significantly changed food intake in only the obese
group of monkeys, whereas energy expenditure tended to
increase in both groups during drug treatment. These results show
that changes in energy expenditure contributed to changes in
body weight in both groups, whereas a decrease in food intake
also contributed to weight loss in the obese animals.
The effect of E+C on food intake, however, is variable,
with some experiments reporting a major decrease and others
reporting no change in intake during drug treatment. Chronic
treatment of obese human patients with E+C resulted in weight
loss, which was attributed primarily to the anorectic effect of
the drug mixture (6). In another study, a decrease in food intake
was estimated to account for 80% of the weight loss in human
volunteers after 8 wk of E+C treatment (30). Food intake has
also been shown to decrease in obese (fa/fa) Zucker rats after
administration of E+C (26). Studies with monosodium gluta-
mate–induced obese mice, however, have shown weight loss
without a change in food intake after administration of E+C (16).
The different findings among studies may reflect the type of obe-
sity the subjects had. The obese group of monkeys, similar to the
human (6) and Zucker rat (26) studies, showed a decrease in food
intake during E+C treatment whereas food intakes in the lean
group remained unaltered. Curiously, the maintenance of obesity
in the present study does not appear to be the result of hyper-
phagia because the obese group of monkeys actually had lower
daily food intakes than the lean group, despite having a larger
body size. Total energy expenditure was not different between
the lean and obese groups, despite the fact that the obese animals
had a larger total mass and lean body mass than the lean animals.
When energy expenditure was expressed as a function of lean
body mass (Figure 1) the obese animals had a lower energy
expenditure than the lean animals. This suggests that a low
energy expenditure was probably a major contributor to the obe-
sity of this group of monkeys. It is possible that the different
responses in food intake noticed between studies may reflect
obesity of different etiology and differences in sensitivity or
activity of food intake regulatory systems.
E+C treatment resulted in a decrease in serum leptin concen-
trations in both the lean and obese groups of monkeys. Leptin
48
RAMSEY ET AL
FIGURE 4. Triiodothyronine (T
3
) plotted against time during the 3 treatment phases. Repeated-measures ANOVA was used to test for differences
in T
3
concentrations between the treatment phases. T
3
was significantly greater in the drug treatment phase than in the control and placebo phases in
the obese animals (P < 0.05). In the lean animals, T
3
was significantly reduced during the placebo phase when compared with the drug treatment phase
(P < 0.05).
ajcn.nutrition.org
Downloaded from
administration to ob/ob mice (31) and diet-induced obese mice
(32) has been shown to decrease food intake. Decreases in serum
leptin concentrations in both groups of monkeys suggest that at
least one mechanism is induced by E+C treatment that may
counteract processes that would decrease food intake. The
decrease in serum leptin concentrations during E+C administra-
tion may be the result of stimulation of
b
3
-adrenergic receptors
by ephedrine because previous research showed that activation
of
b
3
-adrenergic receptors suppresses leptin gene expression
(33). The changes in serum leptin concentrations may also sim-
ply reflect changes in body fat content during drug treatment.
Experiments measuring leptin concentrations in rodents (34, 35),
humans (35, 36), and monkeys (37) have shown that leptin has a
strong positive correlation with body fat. Decreased plasma lep-
tin concentrations in the monkeys with E+C treatment are prob-
ably related to the decreased body fat measured in these animals
during drug treatment. Similarly, increases in plasma leptin con-
centrations in the lean animals after cessation of E+C treatment
may reflect increases in body fat during this period.
In contrast with the mixed results obtained regarding food
intake with E+C, this drug combination has been consistently
shown to increase energy expenditure. Studies with rodents (16,
26) and short-term measurements in humans (38) showed that
E+C increases total energy expenditure. Additionally, E+C was
shown to increase resting metabolic rate (25) and meal-induced
thermogenesis (39, 40). These results are consistent with the
present observation that E+C tended to increase energy expendi-
ture in both the lean and obese groups of monkeys. Evening or
resting energy expenditure was significantly increased in both
the lean and obese animals. The thermic effect of meals was not
specifically measured in this experiment, but drug-treated ani-
mals did tend to have higher oxygen consumption in the morn-
ing, when most of the food was consumed. Total or 24-h energy
expenditure was also higher in E+C–treated monkeys than in con-
trols. The fact that energy expenditure decreased in both groups at
all time points after E+C was stopped also supports increased
energy expenditure as a major effect of treatment with this combi-
nation of drugs. Overall, resting and 24-h energy expenditure were
increased by E+C treatment.
Increases in energy expenditure during E+C treatment appear to
be mediated through ephedrine-induced stimulation of
b-adrenergic
receptors.
b
1
-,
b
2
-, and
b
3
-adrenoceptors all may play a role in the
thermogenic action of ephedrine but the
b
3
-adrenoceptor has been
proposed to be responsible for
≥
40% of this thermogenic response
in humans (41). Experiments using specific
b
3
-adrenergic agonists
have shown that stimulation of this receptor results in increased
thermogenesis (42, 43) primarily by increasing resting metabolic
rate (29). This is consistent with the finding that the most dramatic
changes in energy expenditure in the monkeys occurred in resting
energy expenditure.
The site of action for ephedrine-induced thermogenesis has not
been firmly established, but one study in humans showed that
<50% of the increase in oxygen consumption occurs in skeletal
muscle (44). It is probable that skeletal muscle is also a significant
contributor to the increased energy expenditure observed during
drug treatment in the monkeys. Adult rhesus monkeys have also
been shown to have functional brown adipose tissue (45), so it is
possible that this tissue may also contribute to the increased energy
expenditure with E+C treatment. Additional research will have to be
performed, however, to determine the cellular mechanisms respon-
sible for the increased energy expenditure with E+C treatment.
Blood concentrations of triiodothyronine were elevated during
E+C treatment in the obese group of monkeys. Previous experi-
ments have shown that treatment with ephedrine (46) or E+C
(47) at least transiently increase the ratio of triiodothyronine to
thyroxine. This information suggests that changes in thyroid hor-
mone concentrations may partially contribute to the elevated
energy expenditure rate in the obese animals during drug treat-
ment. The lack of a significant increase in triiodothyronine con-
centrations with E+C treatment in the lean group, however, show
that changes in this hormone are not essential for E+C–induced
elevations of energy expenditure.
Frequently sampled glucose tolerance tests were performed
on the monkeys to determine the role E+C may play in carbohy-
drate metabolism. At the start of the experiment, the obese group
had higher basal insulin and basal glucose concentrations and
lower insulin sensitivity than the lean group. These results agree
with previous reports of hyperinsulinemia and insulin insensitiv-
ity for obese compared with lean rhesus monkeys (48, 49) and
humans (50). A trend toward a decrease in basal insulin and glu-
cose disappearance rate were the only changes that occurred
between the control and drug treatment phases. Previous experi-
ments have shown that ephedrine acutely increases plasma glu-
cose and insulin (6, 7) in a dose-dependent manner in humans. In
the present study, it is likely that short-term changes in plasma
insulin and glucose had disappeared by the time that these vari-
ables were measured. The decrease in insulin in the monkeys
was probably not a direct effect of drug treatment but may have
been the result of a decrease in body fat during the experiment.
Similarly, slight changes in other glucose tolerance values
EPHEDRINE PLUS CAFFEINE TREATMENT AND ENERGETICS
49
FIGURE 5. Plasma leptin concentrations during the control (C), drug
(D), and placebo (P) phases of the experiment in both the lean and obese
groups of animals. Plasma leptin concentrations were measured during
the sixth or seventh week of each treatment phase. P values for paired
t test comparisons between the treatment phases are as follows: obese,
C compared with D = 0.09, P compared with D = 0.11, C compared with
P = 0.11; lean, C compared with D = 0.10, P compared with D = 0.05, C
compared with P = 0.07.
ajcn.nutrition.org
Downloaded from
50
RAMSEY ET AL
tended to occur during periods of greatest weight change in both
the lean and obese animals, and these changes were probably the
result of changes in body fat mass. Additional experiments of
longer duration are needed to determine whether this treatment
would have been beneficial in the treatment of hyperinsulinemia
in the obese group of monkeys.
Treatment with a mixture of ephedrine and caffeine effec-
tively decreased fat mass in both lean and obese rhesus monkeys.
The decrease in fat mass was primarily due to an increase in rest-
ing energy expenditure in the lean animals, whereas increased
resting energy expenditure and a decrease in food intake both
contributed to loss of fat mass in the obese group. Overall, E+C
promoted weight loss by increasing energy expenditure in both
groups, although additional research is needed to determine
whether this drug mixture also has beneficial long-term antidia-
betic effects.
We gratefully acknowledge the expert technical assistance provided by
Scott T Baum, Lori Mason, John Hudson, Gregory Ziegert, and Craig Goebel.
REFERENCES
1. Ravussin E, Lillioja S, Knowler WC, et al. Reduced rate of energy
expenditure as a risk factor for body-weight gain. N Engl J Med
1988;318:467–72.
2. Romsos DR. Efficiency of energy retention in genetically obese
animals and in dietary-induced thermogenesis. Fed Proc
1981;40:2524–9.
3. Bray GA, York DA, Fisler JS. Experimental obesity: a homeostatic
failure due to defective nutrient stimulation of the sympathetic ner-
vous system. Vitam Horm 1989;45:1–125.
4. Spraul M, Ravussin E, Fontvieille AM, Rising R, Larson DE,
Anderson EA. Reduced sympathetic nervous system. A potential
mechanism predisposing to body weight gain. J Clin Invest
1993;92:1730–5.
5. Astrup A, Breum L, Toubro S. Pharmacological and clinical stud-
ies of ephedrine and other thermogenic agonists. Obes Res
1995;3(suppl):537s–40s.
6. Astrup A, Toubro S, Christensen NJ, Quaade F. Pharmacology of
thermogenic drugs. Am J Clin Nutr 1992;55(suppl):246S–8S.
7. Astrup A, Toubro S, Cannon S, Hein P, Madsen J. Thermogenic,
metabolic, and cardiovascular effects of a sympathomimetic agent,
ephedrine. Curr Ther Res 1990;48:1087–100.
8. Dulloo AG, Seydoux J, Girardier L. Peripheral mechanisms of
thermogenesis induced by ephedrine and caffeine in brown adipose
tissue. Int J Obes 1991;15:317–26.
9. Bukowiecki L, Jahjah L, Follea N. Ephedrine, a potential slimming
drug, directly stimulates thermogenesis in brown adipocytes via
beta-adrenoceptors. Int J Obes 1982;6:343–50.
10. Astrup A, Toubro S. Thermogenic, metabolic, and cardiovascular
responses to ephedrine and caffeine in man. Int J Obes
1993;17(suppl):41S–3S.
11. Dulloo AG, Seydoux J, Girardier L. Potentiation of the thermo-
genic antiobesity effects of ephedrine by dietary methylxanthines:
adenosine antagonism or phosphodiesterase inhibition? Metabo-
lism 1992;41:1233–41.
12. Dulloo AG,
Miller DS. The thermogenic properties of
ephedrine/methylxanthine mixtures: human studies. Int J Obes
1986;10:467–81.
13. Toubro S, Astrup A, Breum L, Quaade F. The acute and chronic effects
of ephedrine/caffeine mixtures on energy expenditure and glucose
metabolism in humans. Int J Obes 1993;17(suppl):73S–7S.
14. Daly PA, Krieger DR, Dulloo AG, Young JB, Landsberg L. Ephedrine,
caffeine and aspirin: safety and efficacy for treatment of human obe-
sity. Int J Obes 1993;17(suppl):73S–8S.
15. Breum L, Pedersen JK, Ahlstrøm F, Frimodt-Møller J. Comparison
of an ephedrine/caffeine combination and dexfenfluramine in the
treatment of obesity. A double-blind multi-centre trial in general
practice. Int J Obes 1994;18:99–103.
16. Dulloo AG,
Miller DS. The thermogenic properties of
ephedrine/methylxanthine mixtures: animal studies. Am J Clin Nutr
1986;43:388–94.
17. Kleiber M. The fire of life. New York: Wiley, 1961.
18. Harwood PD. Therapeutic dosage in small and large mammals. Sci-
ence 1963;139:684–5.
19. Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray
absorptiometry for total-body and regional bone-mineral and soft-
tissue composition. Am J Clin Nutr 1990;51:1106–12.
20. Pierson RN, Wang J, Heymsfield SB, et al. Measuring body fat: cal-
ibrating the rulers. Am J Physiol 1991;261:E103–8.
21. Kemnitz JW, Roecker EB, Weindruch R, Elson DF, Baum ST,
Bergman RN. Dietary restriction increases insulin sensitivity and
lowers blood glucose in rhesus monkeys. Am J Physiol 1994;
266:E540–7.
22. Yang YJ, Youn JH, Bergman RN. Modified protocols improve
insulin sensitivity estimation using the minimal model. Am J Phys-
iol 1987;253:E595–602.
23. Bergman RN. Toward physiological understanding of glucose toler-
ance. Minimal Model approach. Diabetes 1989;38:1512–27.
24. Malchow-Møller A, Larsen S, Hey H, Stokholm KH, Juhl E,
Quaade F. Ephedrine as an anorectic: the story of the ‘Elsinore pill.’
Int J Obes 1981;5:183–7.
25. Tulp OL, Buck CL. Caffeine and ephedrine stimulated thermogene-
sis in LA-corpulent rats. Comp Biochem Physiol C 1986;85:17–9.
26. Dulloo AG, Miller DS. Reversal of obesity in the genetically obese
fa/fa Zucker rat with an ephedrine/methylxanthines thermogenic
mixture. J Nutr 1987;117:383–9.
27. Oksbjerg N, Sørensen MT. Separate and combined effects of
ephedrine and caffeine on protein and lipid deposition in finishing
pigs. Anim Sci 1995;60:299–305.
28. Yen TT.
b-Agonists as antiobesity, antidiabetic and nutrient parti-
tioning agents. Obes Res 1995;3(suppl):531S–6S.
29. Himms-Hagen J, Cui J, Danforth E, et al. Effect of CL-316,243, a
thermogenic
b
3
-agonist, on energy balance and brown and white
adipose tissues in rats. Am J Physiol 1994;266:R1371–82.
30. Astrup A, Buemann B, Christensen NJ, et al. The effect of
ephedrine/caffeine mixture on energy expenditure and body compo-
sition in obese women. Metabolism 1992;41:686–8.
31. Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the
obese gene product on body weight regulation in ob/ob mice. Sci-
ence 1995;269:540–3.
32. Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant
mouse OB protein: evidence for a peripheral signal linking adipos-
ity and central neural networks. Science 1995;269:546–9.
33. Mantzoros CS, Qu D, Frederich RC, et al. Activation of
b
3
adrener-
gic receptors suppresses leptin expression and mediates a leptin-
independent inhibition of food intake in mice. Diabetes
1996;45:909–14.
34. Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB,
Flier JS. Leptin levels reflect body lipid content in mice: evidence
for diet-induced resistance to leptin action. Nat Med 1995;
1:1311–4.
35. Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and
rodent: measurement of plasma leptin and ob RNA in obese and
weight-reduced subjects. Nat Med 1995;1:1155–61.
36. Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreac-
tive-leptin concentrations in normal-weight and obese humans. N
Engl J Med 1996;334:292–5.
37. Ramsey JJ, Colman RJ, Kemnitz JW. Body composition, insulin
and leptin in rhesus monkeys following long term dietary restric-
tion. Obes Res 1996;4(suppl 1):41S (abstr).
ajcn.nutrition.org
Downloaded from

EPHEDRINE PLUS CAFFEINE TREATMENT AND ENERGETICS
51
38. Vallerand AL, Jacobs I, Kavanagh MF. Mechanism of enhanced
cold tolerance by an ephedrine-caffeine mixture in humans. J
Appl Physiol 1989;67:438–44.
39. Horton TJ, Geissler CA. Post-prandial thermogenesis with
ephedrine, caffeine and aspirin in lean, pre-disposed obese and
obese women. Int J Obes 1996;20:91–7.
40. Astrup A, Madsen J, Holst JJ, Christensen NJ. The effect of
chronic ephedrine treatment on substrate utilization, the sympa-
thoadrenal activity, and energy expenditure during glucose-
induced thermogenesis in man. Metabolism 1986;35:260–5.
41. Liu YL, Toubro S, Astrup A, Stock MJ. Contribution of
b
3
-
adrenoceptor activation to ephedrine-induced thermogenesis in
humans. Int J Obes 1995;19:678–85.
42. Ye JM, Clark MG, Colquhoun EQ. Constant-pressure perfusion of
rat hindlimb shows
a-and b-adrenergic stimulation of oxygen
consumption. Am J Physiol 1995;269:E960–8.
43. Susulic VS, Frederich RC, Lawitts J, et al. Targeted disruption of
the
b
3
-adrenergic receptor gene. J Biol Chem 1995; 270:29483–92.
44. Astrup A, Bülow J, Madsen J, Christensen NJ. Contribution of BAT
and skeletal muscle to thermogenesis induced by ephedrine in man. Am
J Physiol 1985;248:E507–15.
45. Swick AG, Kemnitz JW, Houser WD, Swick RW. Norepinephrine
stimulates activity of brown adipose tissue in rhesus monkeys. Int J
Obes 1986;10:241–4.
46. Astrup A, Lundsgaard C, Madsen J, Christensen NJ. Enhanced ther-
mogenic responsiveness during chronic ephedrine treatment in man.
Am J Clin Nutr 1985;42:83–94.
47. Buemann B, Marckmann P, Christensen NJ, Astrup A. The effect of
ephedrine plus caffeine on plasma lipids and lipoproteins during a
4.2 MJ/day diet. Int J Obes 1994;18:329–32.
48. Kemnitz JW, Goy RW, Flitsch TJ, Lohmiller JJ, Robinson JA. Obe-
sity in male and female rhesus monkeys: fat distribution, glucoreg-
ulation, and serum androgen levels. J Clin Endocrinol Metab
1989;69:287–93.
49. Kemnitz JW, Francken GA. Characteristics of spontaneous obesity
in male rhesus monkeys. Physiol Behav 1986;38:477–83.
50. Pi-Sunyer FX. Obesity. In: Shils ME, Olson JA, Shike M, eds.
Modern nutrition in health and disease. 8th ed. Malvern, PA:
Lea & Febiger, 1994:984–1006.
ajcn.nutrition.org
Downloaded from
Wyszukiwarka
Podobne podstrony:
Am J Clin Nutr 2001 Schrezenmeir 361s 4s
Am J Clin Nutr 2000 de Roos 405 11
2009 06 15 21;42;51
2009 06 15 21;42;51
2009 06 15 21;42;51
neurologia 42 45 51
51 Die Tankentluftung am strich 8
LVDA Mạng Thông Tin Di Động GSM Gv Phạm Công Hùng, 42 Trang
51 Wypowiedzenie zmieniające
AM FM SSB Empfänger Teil 1
2006 EGZ WSTĘPNY NA AM
2002 09 42
Kodeks pracy Dziennik Ustaw poz 94 nr 21 z 1998 roku
więcej podobnych podstron