
TECHNICAL NOTE
Exergetic ef
ficiency of high-temperature-lift chemical
heat pump (CHP) based on CaO/CO
2
and CaO/H
2
O
working pairs
Mehdi Arjmand
1,2,
*
,
†
, Longcheng Liu
1
and Ivars Neretnieks
1
1
Division of Chemical Engineering, Department of Chemical Engineering and Technology, Royal Institute of Technology (KTH),
Stockholm SE-100 44, Sweden
2
Division of Environmental Inorganic Chemistry, Department of Chemical and Biological Engineering, Chalmers University of
Technology, Göteborg SE-412 96, Sweden
SUMMARY
The use of reversible chemical reactions in recuperation of heat has gained signi
ficant interest due to higher magnitude of
reaction heat compared to that of the latent or sensible heat. To implement chemical reactions for upgrading heat, a
chemical heat pump (CHP) may be used. A CHP uses a reversible chemical reaction where the forward and the reverse
reactions take place at two different temperatures, thus allowing heat to be upgraded or degraded depending on the mode
of operation. In this work, an exergetic ef
ficiency model for a CHP operating in the temperature-level amplification mode
has been developed. The
first law and the exergetic efficiencies are compared for two working pairs, namely, CaO/CO
2
and
CaO/H
2
O for high-temperature high-lift CHPs. The exergetic ef
ficiency increases for both working pairs with increase in
task, T
H
, decrease in heat source, T
M
, and increase in condenser, T
L
, temperatures. It is also observed that the difference in
reaction enthalpies and speci
fic heats of the involving reactants affects the extent of increase or decrease in the exergetic
ef
ficiency of the CHP operating for temperature-level amplification. Copyright © 2012 John Wiley & Sons, Ltd.
KEY WORDS
chemical heat pump (CHP);
first law efficiency; second law (exergetic) efficiency; temperature amplification; heat transformer; CaO/
CO
2
; CaO/H
2
O
Correspondence
*Mehdi Arjmand, Division of Chemical Engineering, Department of Chemical Engineering and Technology, Royal Institute of Technology
(KTH), Stockholm SE-100 44, Sweden.
†
E-mail: arjmand@kth.se
Received 25 September 2011; Revised 18 February 2012; Accepted 1 March 2012
1. INTRODUCTION
Depending on the type of the process, waste heat may be
released at any temperature in the range of chilled cooling
water to high-temperature gases from an industrial furnace
[1]. High-temperature waste heat provides a higher recov-
ery rate and thus can be often effectively recovered using
conventional and physical recovery solutions (e.g. using
a series of heat exchangers). However, most waste heat
streams in the industry have a low temperature (
<400 K)
and therefore are called low-grade waste heat [2]. In this
case, physical recovery may not be effective in retrieving
the lost energy.
A low-temperature waste heat may be upgraded using a
vapor compression heat pump, which requires electricity,
and/or sorption heat pumps, which uses the heat of (de)
sorption of a medium [3
–10]. In recent years, however,
engaging reversible chemical reactions for recuperation
of heat has gained signi
ficant interest because of the higher
magnitude of reaction heat compared with that of the latent
or sensible heat as retrieved in physical recovery techni-
ques or vapor compression and sorption heat pumps [11].
To use chemical reactions for upgrading low-grade waste
heat, a chemical heat pump (CHP) may be used which
offers a wider range of operating temperature and versatil-
ity in comparison with the conventional vapor compression
or (de)sorption heat pump. In practice, temperatures as low
as 230 K in refrigeration or freezing systems, and up to 870
K in heat generation systems can be supplied using CHPs
[12
–20].
In theory, a CHP operates in either of three different
modes: (a) heat generation, (b) refrigeration, or (c)
temperature-level ampli
fication; also known as chemical
heat transformer (CHT) [21
–23]. First and/or second
INTERNATIONAL JOURNAL OF ENERGY RESEARCH
Int. J. Energy Res. (2012)
Published online in Wiley Online Library (wileyonlinelibrary.com). DOI: 10.1002/er.2918
Copyright © 2012 John Wiley & Sons, Ltd.

law (exergetic) ef
ficiency analyses of a range of CHPs
with different working pairs (working
fluid or medium)
operating mainly for heat generation and refrigeration,
that is, modes a and b, have been carried out
[12,20,24
–31]. The kinetic aspects of some of the work-
ing pairs have also been investigated [14,17,20,32
–34].
However, the work on exergetic ef
ficiency analysis of
a CHP operating in the temperature-level ampli
fication
mode, that is, mode c, is still limited [27,35].
The temperature-level ampli
fication mode may be used
to upgrade low-grade waste heat. It also shapes the basis
for high-temperature lift CHPs particularly for power gen-
eration or other industrial applications [36], an area where
progress is to be seen [23,34,37
–40]. It is well known that
the conventional heat balance for evaluation of losses and
system ef
ficiency does not fully represent the effectiveness
of a system. On the other hand, an estimation of available
energy (exergy) is advantageous for a closer measurement
of losses and thus effective conservation of energy during
design and operation of such systems. In addition, the eval-
uation of the exergetic ef
ficiency for this mode of operation
offers a criterion to discern heat transformers that are ther-
modynamically effective from those that are not. More-
over, exergetic ef
ficiency can be used to identify systems
that have potential for improvements. A signi
ficant differ-
ence between the exergetic and the practical ef
ficiency sug-
gests that there may be room for possible performance
enhancements [41].
Thus, an exergetic ef
ficiency model for a CHP operating
in the temperature-level ampli
fication mode has been devel-
oped. The model is then used to compare the ef
ficiencies of
two working pairs, namely, CaO/CO
2
and CaO/H
2
O for
high-temperature-lift CHPs.
2. CHEMICAL HEAT PUMP
In contrast to the heat engine de
fined by Carnot where
work is delivered between a high-temperature heat source
and a low-temperature heat sink, the CHP uses three (or
four) temperature levels of high, medium, and low to con-
sume or produce thermochemical energy. Thus, for a CHP,
a closed cycle in which the forward reaction is endothermic
and the reverse reaction is exothermic may be considered.
The endothermic reaction occurs at a lower temperature,
whereas the exothermic reaction is carried out at a higher
temperature. As a result, a low-temperature heat may be
absorbed by the endothermic reaction and released at a
higher temperature by the exothermic reaction.
In principal, the general reaction in a CHP may be
assumed as follows:
A
þ B↔C
(1)
It should be noted that the reaction is reversible and that
the forward and the reverse reactions are assumed to take
place at two different temperatures, thus allowing heat to
be upgraded or degraded depending on the mode of
operation (i.e. refrigeration, heat generation, or tempera-
ture-level ampli
fication) [19]. By absorbing heat through
the reverse endothermic reaction in Equation (1), C is
decomposed to A (a nonvolatile compound) and B (a vola-
tile compound). On the other hand, the forward reaction in
Equation (1) is exothermic, during which C is formed
again. Thus, in the simplest form, a CHP comprises a
decomposition reactor, a condenser, an evaporator, and a
synthesis reactor [18]. The evaporator is the source of the
volatile compound B for later formation of C in the synthe-
sis reactor. B is eventually condensed in the condenser
after C is decomposed into A and B in the decomposition
reactor. Because the chemical compound C and the con-
densate are pure phases, the pressure of the volatile com-
pound B in the reactors and the evaporator depend only
on the temperature. Thus, the operation is monovariant,
that is by specifying the pressure, the temperature will also
be determined [42,43].
The modes of heat generation or refrigeration have sim-
ilar fundamentals in using the low temperature heat from
the environment or surrounding to achieve the output
effect, that is, producing heat or cold, respectively. How-
ever, they differ in the de
finition of the user as demanding
cold (refrigerating) or hot (heat generating) streams. Such
CHPs have been thoroughly studied, and the evaluation
of both thermal ef
ficiencies has been reported in several
publications [12,20,24,27
–31].
The temperature-level ampli
fication mode on the
other hand operates on a somewhat different basis that
uses an intermediate-level heat source to generate a
high-temperature-level heat [9,43]. In order for this to
occur, the forward and the reverse directions of the
reversible reaction (Equation (1)) must both take place
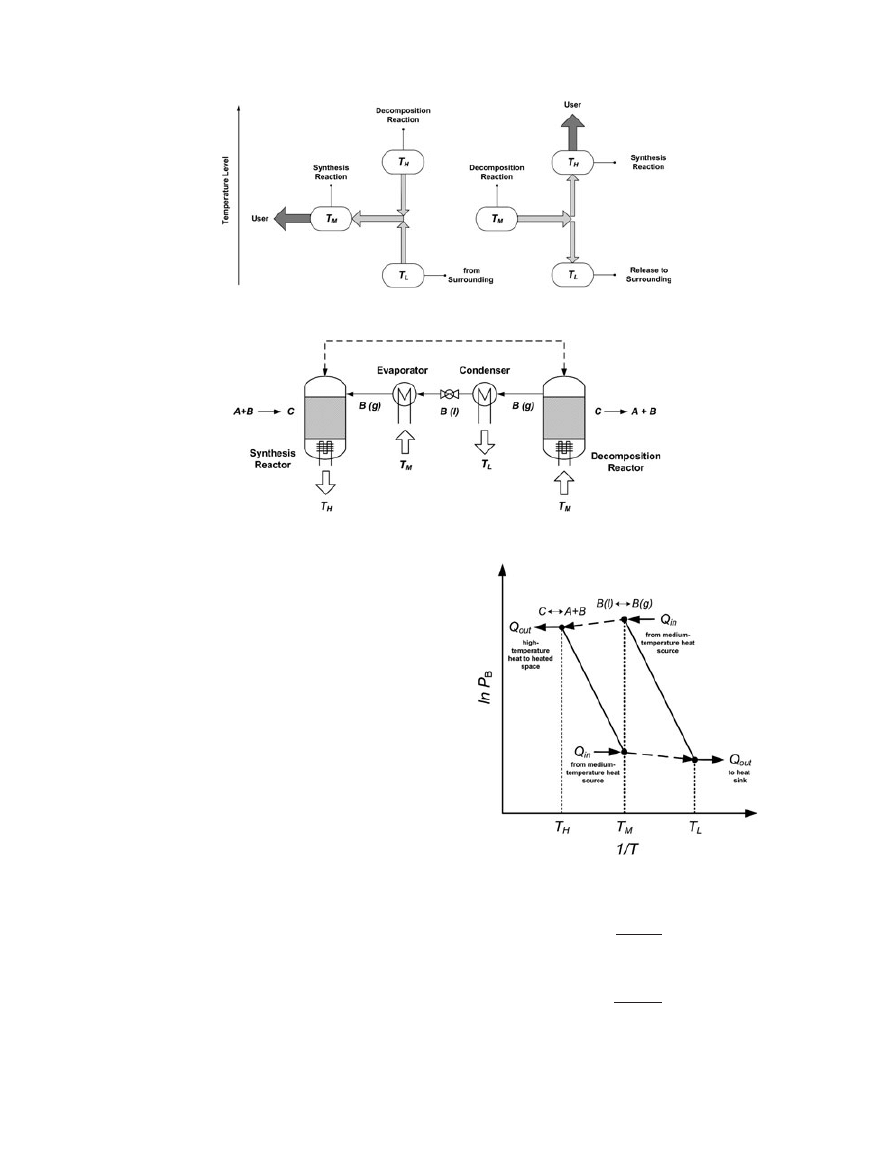
at temperatures higher than that of the environment. To
further distinguish between the heat ampli
fication and
the heat generation and cooling modes, consider T
M
and
T
L
representing different media as outlined in Figure 1.
In the case of a CHP for heat generation, T
M
represents
the temperature of the synthesis reaction that releases
the useful heat to the user, which is generated with the
help of heat from the endothermic reaction at the higher
temperature, T
H
, (e.g. using surplus heat) and heat from
the environment at the lower temperature, T
L
. By
contrast, in the case of a CHP operating for temperature
ampli
fication, T
L
represents the condenser, which releases
nonuseful heat to the environment (from part of the sup-
plied heat) while offering the upgraded heat at the higher
temperature, T
H
, with the help of the medium-temperature
heat source, T
M
, (e.g. from surplus heat).
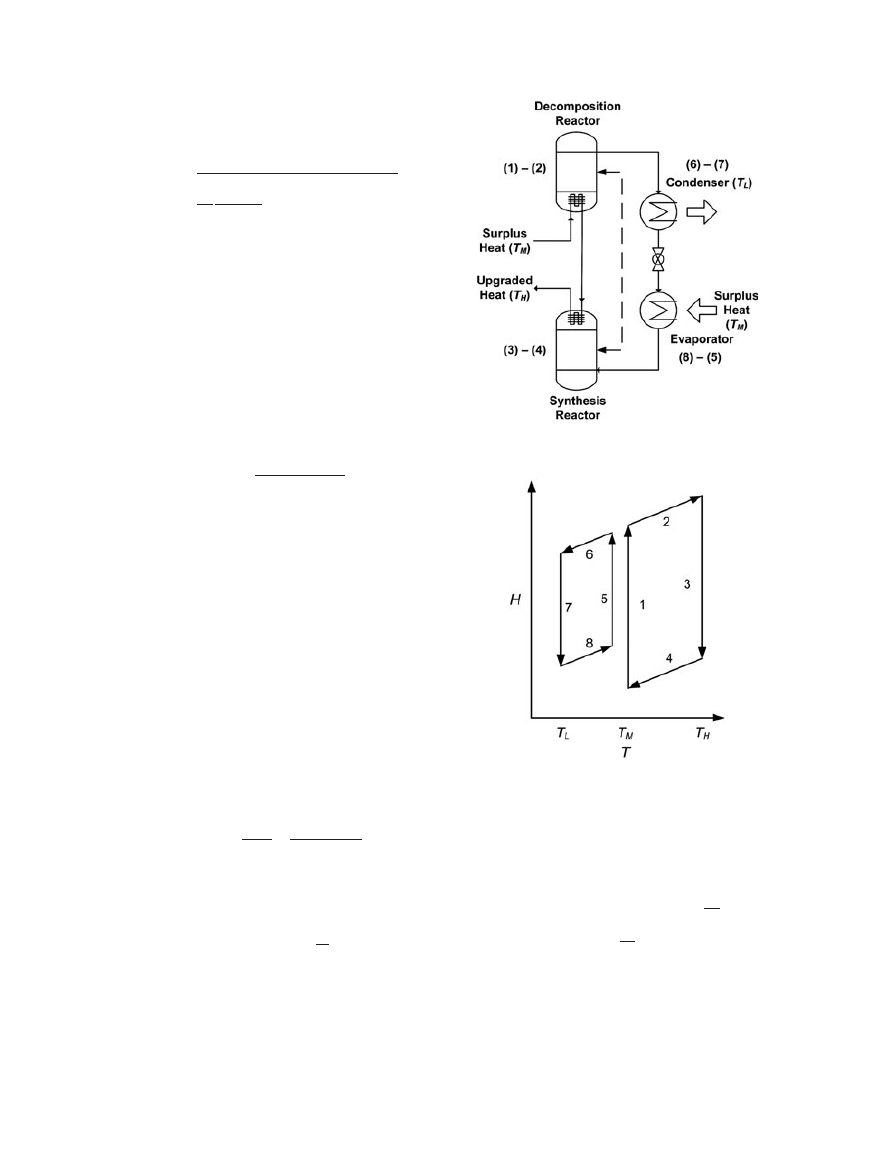
Figure 2 shows the schematic of a CHP operating in the
temperature-level ampli
fication mode. Here, an intermediate-
level heat (e.g. waste heat) at T
M
is supplied to both the
decomposition reactor to breakup C into its constituents and
later to the evaporator to vaporize the working
fluid, B. The
vapor B generated from the decomposition reactor condenses
in the condenser during the charging phase. During the dis-
charging phase, the working
fluid B is admitted to the evap-
orator, where it is vaporized and allowed to react with A in
Exergetic efficiency of high-temperature high-lift chemical heat pump
M. Arjmand, L. Liu and I. Neretnieks
Int. J. Energy Res. (2012) © 2012 John Wiley & Sons, Ltd.
DOI: 10.1002/er
the synthesis reactor. Thus, compound C is formed at a
higher temperature-level, T
H
, and with a higher quality than
that of the original source supplied to the system. The con-
denser is used to partly remove the low-temperature heat at
ambient, T
L
, and to complete the cycle. The result is that a
medium-temperature heat, T
M
(e.g. from waste heat), is
absorbed by the system through the reverse endothermic re-
action and evaporation of the working
fluid and is upgraded
to a high-temperature heat, T
H
, by the exothermic heat of
the forward reaction. Figure 3 shows the energy
flows of a
CHP operating in the temperature-level ampli
fication mode.
It can be noticed that the intermediate temperature-level heat,
T
M
, is required during both charging and discharging
modes, that is, decomposition of C and evaporation of
B. If this source is not always available, two CHPs may
be integrated [43].
3. THERMAL EFFICIENCIES
3.1. First law ef
ficiency
The maximum ef
ficiency of any heat engine, heat pump, or
refrigerator can be derived for cyclic reversible processes
using the well-known Carnot ef
ficiency. To derive the
Carnot ef
ficiency of a CHP, two cycles consisting of one heat
pump (operating in the higher temperature interval) and one
heat engine (operating in the lower temperature interval) may
be considered [9,42]. The maximum (or Carnot) ef
ficiency of
the heat engine may then be expressed as
max
HE
¼
T
M
T
L
T
M
(2)
and the maximum ef
ficiency of the heat pump may be
written as
max
HP
¼
T
H
T
H
T
M
(3)
The overall maximum ef
ficiency of the CHP is defined as
the ratio of the heat obtained to the heat supplied. Thus, the
Figure 1. Operation principle of a CHP operating in heat generation mode (left) and temperature-level ampli
fication mode (right).
Figure 2. Schematic of a CHP operating in the temperature-level ampli
fication mode.
Figure 3. Energy
flow of a CHP operating in the temperature-
level ampli
fication mode.
Exergetic efficiency of high-temperature high-lift chemical heat pump
M. Arjmand, L. Liu and I. Neretnieks
Int. J. Energy Res. (2012) © 2012 John Wiley & Sons, Ltd.
DOI: 10.1002/er
maximum ef
ficiency of a CHP operating as a temperature
ampli
fier is as follows
max
CHP
¼
Utilized heat at higher temperature
Supplied heat
¼
T
H
T
M
T
M
T
L
T
H
T
L
(4)
Equation (4) shows that the ideal performance of a CHP
depends only on the cycle temperature boundaries [19]. An
investigation of different working pairs and optimum operat-
ing temperatures has also been reported. An overview of the
working pairs used for CHPs can be found in the reviews by
Wongsuwan et al. [18] and Aristov et al. [19].
However, the actual ef
ficiency of a CHP is not depen-
dent on the temperature levels but on the enthalpy changes
in the high and low temperature cycle. Thus, assuming
negligible variation in reaction enthalpy with temperature
and neglecting sensible heat, the actual ef
ficiency of a CHP
in operation for temperature-level ampli
fication is [43]
CHP
;I
¼
ΔH
H
H
ΔH
L
M
þ Δ
H
H
M
(5)
In Equation (5), the superscripts refer to either the high- or
the low-temperature reaction (or reactor) and the subscripts
indicate the temperature level. It should be mentioned that
the temperatures of the CHP cycle are not independent of
each other, and with the determination of one, the other
two temperatures are also set. Consequently, the actual ef
fi-
ciency of the CHP (Equation (5)) can theoretically reach
the Carnot ef
ficiency (Equation (4)), irrespective of the
chemical nature of the working pair [19].
3.2. Second law (exergetic) ef
ficiency
In contrast to the
first law, the second law analysis uses the
concept of available energy (exergy) and irreversibility
[44]. Exergy analysis provides the means for evaluation
of the degree of thermodynamic perfection of a process.
On the basis of the exergy function, the ef
ficiency of a heat
pump can be expressed as [45]
CHP
;II
¼
E
X
out
E
X
avail
:
¼
E
X
avail
:
E
X
loss
E
X
avail
:
(6)
For a heat pump receiving heat at T
i
, the available
exergy is given as per the following equation [45]:
E
X
avail
:
¼ ΔH
i
1
T
0
T
i
(7)
where T
0
represents the reference (or ambient) temperature
in Equation (7).
Figure 4 shows the scheme of the processes con
figura-
tion for a CHP operating in the temperature ampli
fication
mode, and Figure 5 represents the corresponding cycle
path of such CHP. It can be observed in Figure 5 that the
available exergy is provided as heat input during processes
1, 2, 5 and 8; thus,
E
X
avail
:
¼ ΔH
1
þ ΔH
5
þ ΔH
8
ð
Þ 1
T
L
T
M
þ ΔH
2
ð
Þ 1
T
L
T
H
(8)
To account for the exergy losses, the irreversibilities of
the process should also be determined. For this, irrevers-
ibility can be expressed in terms of entropy and enthalpy
change with respect to the reference temperature (T
0
) for
each cycle path as [46]
Figure 4. Process con
figuration of a CHP operating in the
temperature-level ampli
fication mode.
Figure 5. Cycle path of a CHP operating in the temperature-
level ampli
fication mode.
Exergetic efficiency of high-temperature high-lift chemical heat pump
M. Arjmand, L. Liu and I. Neretnieks
Int. J. Energy Res. (2012) © 2012 John Wiley & Sons, Ltd.
DOI: 10.1002/er
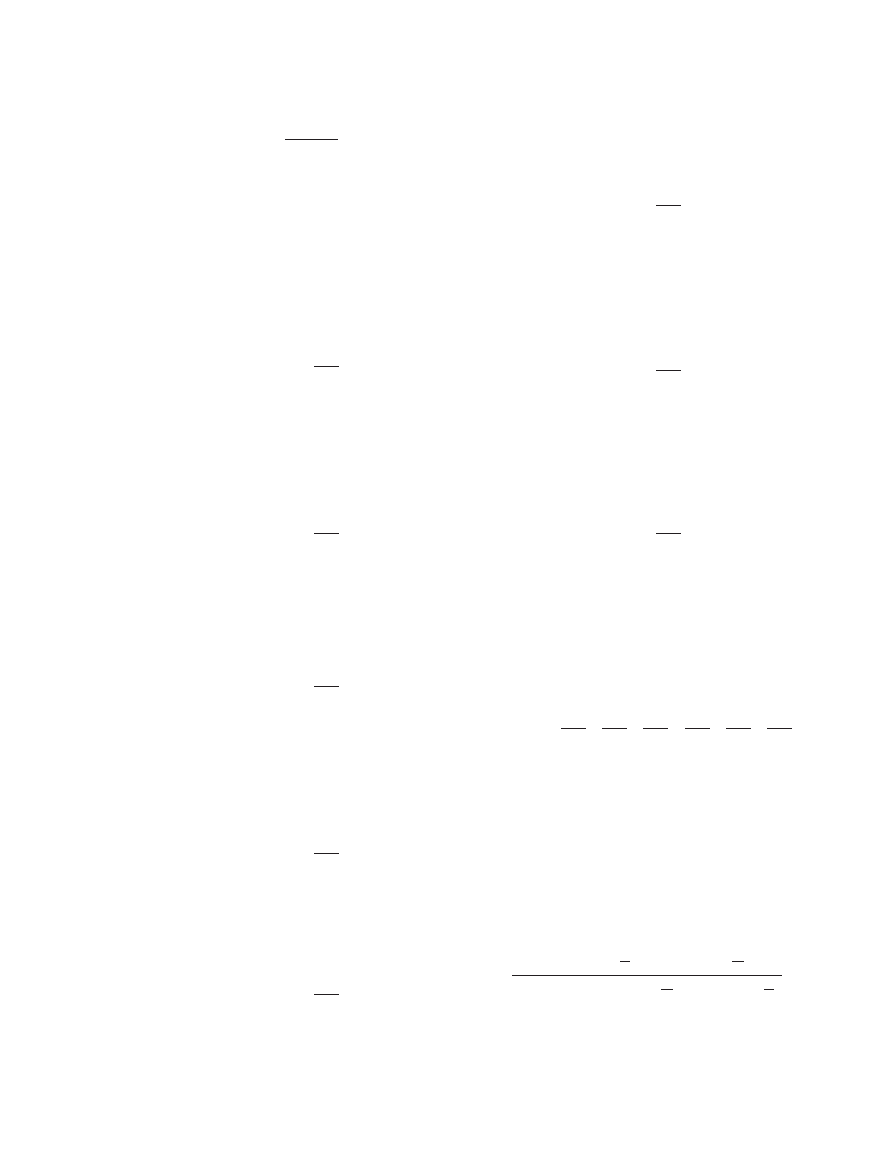
I
¼ T
0
ΔS
i
ΔH
i
T
sink
=source
(9)
In Equation (9), i refers to the ith cycle path and T
sink/source
is the temperature at the end of the cycle path of the process,
which can either be a sink or a source depending on the cycle
path.
Considering Equation (1) for the CHP, the cycle paths
in the processes involved can be described as follows:
(1) Dissociation of compound C at T
M
,
ΔH
H
M
is supplied
ΔH
1
¼ ΔH
H
M
(10)
I
1
¼ T
0
ΔS
1
ΔH
1
T
M
(11)
(2) Temperature of the constituting compounds A and B
increases to T
H
, absorbing sensible heat
ΔH
2
ΔH
2
¼ C
P
A
þ C
P
B
ð
Þ T
H
T
M
ð
Þ
(12)
and
I
2
¼ T
0
ΔS
2
ΔH
2
T
H
(13)
(3) Formation of compound C at T
H
,
ΔH
H
H
is released
ΔH
3
¼ ΔH
H
H
(14)
and
I
3
¼ T
0
ΔS
3
ΔH
3
T
H
(15)
(4) Temperature of compound C decreases to T
M
, releasing
sensible heat
ΔH
4
ΔH
4
¼ C
P
C
T
H
T
M
ð
Þ
(16)
and
I
4
¼ T
0
ΔS
4
ΔH
4
T
M
(17)
(5) B evaporates at T
M
, absorbing latent heat
ΔH
5
ΔH
5
¼ ΔH
L
M
(18)
and
I
5
¼ T
0
ΔS
5
ΔH
5
T
M
(19)
(6) Temperature of B decreases from T
M
to T
L
, releasing
sensible heat
ΔH
6
ΔH
6
¼ C
P
B
T
M
T
L
ð
Þ
(20)
and
I
6
¼ T
0
ΔS
6
ΔH
6
T
L
(21)
(7) B condenses at T
L
, releasing latent heat
ΔH
7
ΔH
7
¼ ΔH
L
L
(22)
and
I
7
¼ T
0
ΔS
7
ΔH
7
T
L
(23)
(8) B absorbs sensible heat from the medium at T
M
ΔH
8
¼ C
P
B
T
M
T
L
ð
Þ
(24)
and
I
8
¼ T
0
ΔS
8
ΔH
8
T
M
(25)
because for the entire process, it is known that
X
ΔH
i
¼ 0 ;
X
ΔS
i
¼ 0
(26)
the total irreversibility of the process can be written as
E
X
loss
¼
X
I
i
¼ T
L
ΔH
1
T
M
þ
ΔH
2
T
H
þ
ΔH
3
T
H
þ
ΔH
4
T
M
þ
ΔH
5
T
M
þ
ΔH
8
T
M
ΔH
6
ΔH
7
(27)
Considering that the overall enthalpy change of a cycle is
zero, that is,
ΔH
1
þ ΔH
2
¼ ΔH
3
ð
Þ þ ΔH
4
ð
Þ
(28)
using Equations (8) and (27), Equation (6) can be written as
CHP
;II
¼
ΔH
3
ð
Þ 1
T
L
T
H
þ ΔH
4
ð
Þ 1
T
L
T
M
ΔH
1
þ ΔH
5
þ ΔH
8
ð
Þ 1
T
L
T
M
þ ΔH
2
ð
Þ 1
T
L
T
H
(29)
which by substituting
ΔH
i
, the
CHP,II
is obtained as
Exergetic efficiency of high-temperature high-lift chemical heat pump
M. Arjmand, L. Liu and I. Neretnieks
Int. J. Energy Res. (2012) © 2012 John Wiley & Sons, Ltd.
DOI: 10.1002/er

where subscript II indicates the second law (exergetic) ef
fi-
ciency of the CHP operating for temperature-level
ampli
fication.
4. CASE STUDY
In what follows, the results from the
first law efficiency
and the exergetic ef
ficiency model developed here for a
CHP operating in the temperature-level ampli
fication mode
will be presented and discussed for two working pairs,
namely, CaO/CO
2
and CaO/H
2
O. These working pairs
were selected among a series of potential sets by the crite-
rion that they offer a high-lift temperature increase (773 to
1273 K and 673 to 873 K, respectively) [26,29].
4.1. CaO/CO
2
working pair
The principle of operation using the CaO/CO
2
working
pair is similar to what is shown in Figure 2. Energy is
absorbed with the dissociation of CaCO
3
and is released
again with the reaction of dissociated products, that is,
CaO and CO
2
. If the pressure of CO
2
during the carbon-
ation process is higher than the pressure of CO
2
during
the de-carbonation process, then the former reaction can
occur at a higher temperature than the latter. Thus, to
increase the CO
2
pressure, it can either be (i) reacted with
another metal oxide and stored in the form of a metal car-
bonate (i.e. using chemical looping [47], (ii) compressed
and stored, (iii) adsorbed (e.g. using zeolites or activated
carbon), or (iv) absorbed with an appropriate solvent (e.g.
using amines) [26].
The compression of the CO
2
gas is less attractive be-
cause of additional energy penalty and the fact that it is
only a modi
fication of the conventional vapor compression
heat pump. CO
2
separation by chemical looping increases
the complexity of the process, and absorption using amine
is an existing process with associated energy cost. There-
fore, this work further investigates the process involving
the adsorption of CO
2
gas. Restuccia et al. [22] showed
that the zeolite 13X is the most suitable adsorbent for
CO
2
in this case because of the highest energy density of
this particular zeolite. Thus, the data for the zeolite 13X
are used in the following. The process description is as
follows: heat is absorbed at the intermediate temperature
level, T
M
, in the decomposition reactor thus producing
CO
2
as CaCO
3
decomposes. The produced CO
2
gas is
cooled and is removed by the zeolite at the lower tempera-
ture, T
L
. The vessel is sealed and is heat to the intermediate
temperature, T
M
. This increases the partial pressure of the
CO
2
above the zeolite as more CO
2
is desorbed. The
CO
2
gas is then sent to the synthesis reactor to form
CaCO
3
, producing a higher-temperature heat, T
H
.
The parameters and values were analyzed using
MATLAB
W
to evaluate the ef
ficiencies based on the previ-
ously mentioned formulation (summarized in Table I). It
should be noted that for the CaO/CO
2
working pair, CO
2
is assumed to be stored at an average temperature of 273
K, which is equivalent to storing energy at 773 K [26]. It
is also known that regardless of the number of moles
adsorbed, an isosteric enthalpy of
40 kJ/mol may be
assumed [48]. Given this and other data sets [25,49
–51]
as listed in Table I, the results for the
first law and exergetic
ef
ficiencies obtained using Equations (5) and (30) are
shown in Figure 6.
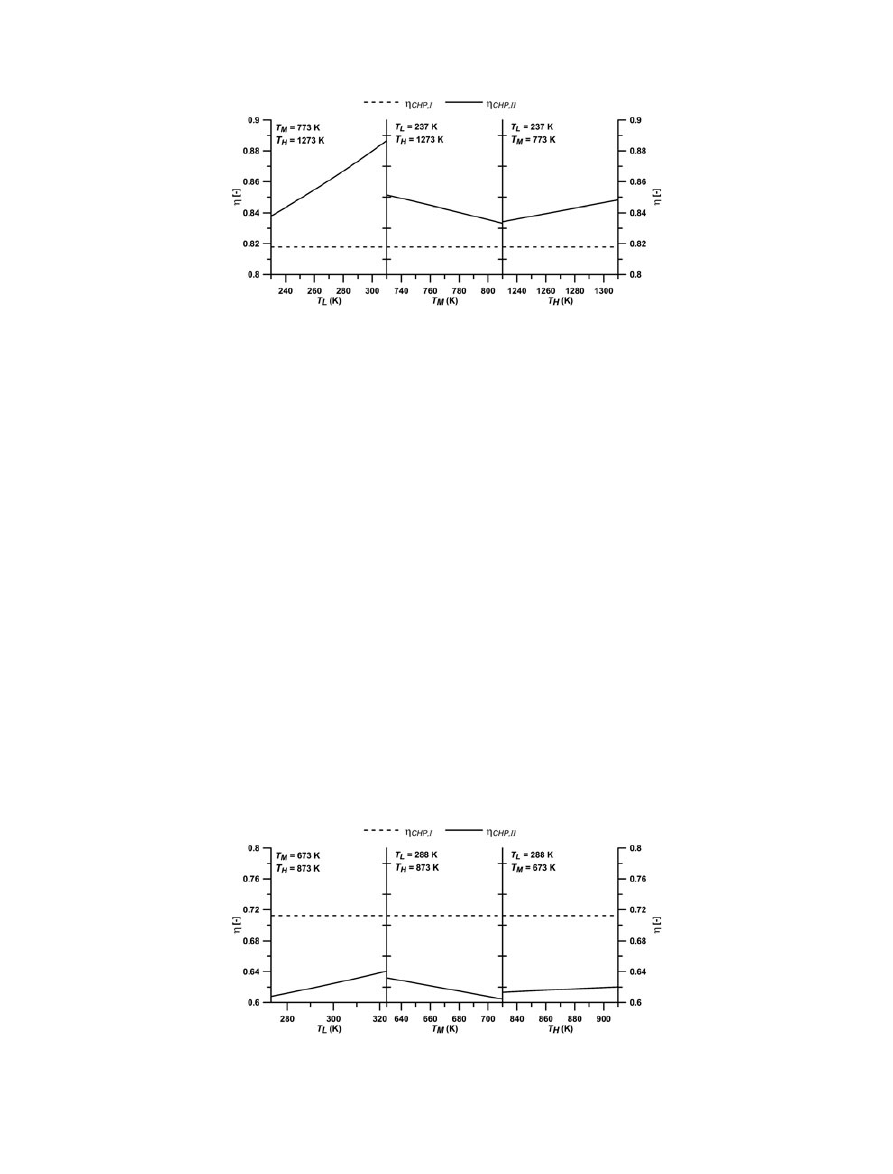
To observe the effect of heat source, condenser and task
temperatures on the ef
ficiencies, a range of temperatures
has to be considered. As shown in Figure 6, it can be inferred
that the exergetic ef
ficiency is dependent on the temperatures
of the heat source, T
M
, condenser, T
L
, and the task, T
H
,
whereas the
first law efficiency remains constant irrespective
of the temperatures. Calculated
first law efficiency is ap-
proximated at 0.82, which is slightly higher than the
corresponding value obtained experimentally [26] for an
upgraded temperature of 1173 K. This justi
fies the validity
of the
first law efficiency (Equation (5)).
However, the interpretation of the exergetic ef
ficiency
should not be carried out as equivalent to the
first law effi-
ciency or performance but rather only to understand the
degree of the available energy to the system for the desired
task (in this case, upgrading heat from a lower to a higher
temperature). As shown in Figure 6, a higher condenser
temperature, T
L
, offers a higher availability of energy to
CHP
;II
¼
ΔH
H
H
1
T
L
T
H
þ C
P
C
T
H
T
M
ð
Þ 1
T
L
T
M
½
ΔH
H
M
þ ΔH
L
M
þ C
P
B
T
M
T
L
ð
Þ
1
T
L
T
M
þ C
P
A
þ C
P
B
ð
Þ T
H
T
M
ð
Þ
½
1
T
L
T
H
(1)
(30)
Table I. Summary of the parameters used for the ef
ficiencies of the CaO/CO
2
and CaO/H
2
O working pairs.
Reactant
Reaction temperature (K)
Speci
fic heat (C
P
) (kJ/mol K)
Reaction
Reaction enthalpy (kJ/mol)
CaO
773
0.0522 [49]
–
–
673
0.0513 [49]
CaCO
3
1273
0.1230 [51]
CaO
(s)
+ CO
2 (g)
↔ CaCO
3(s)
178.3
CO
2
237
0.0314 [50]
CO
2 (g)
+ zeolite 13X
↔ CO
2 (ads.)
42
Ca(OH)
2
873
0.0930 [25]
CaO
(s)
+ H
2
O
(g)
↔ Ca(OH)
2(s)
104
H
2
O
288
0.0757 [50]
H
2
O
(g)
↔ H
2
O
(l)
40
Exergetic efficiency of high-temperature high-lift chemical heat pump
M. Arjmand, L. Liu and I. Neretnieks
Int. J. Energy Res. (2012) © 2012 John Wiley & Sons, Ltd.
DOI: 10.1002/er
the system for a constant heat source, T
M
, and task, T
H
,
temperatures, which is expected as the condensation in
the cooler imposes that a higher degree of energy is to be
removed from the system. On the contrary, with the
increase of heat source temperature, T
M
, a lower amount
of energy will be available to the system as the exergetic
ef
ficiency is reduced. In other words, if the quality of the
heat source, T
M
, is increased while the task temperature,
T
H
, is remained constant, the system ef
ficiency will
decrease. Accordingly,
CHP,II
increases by increasing the
task temperature, T
H
, under
fixed condenser, T
L
, and heat
source, T
M
, temperatures. This can also be interpreted as
that at lower task temperature, T
H
, the system ef
ficiency
will also be lower.
4.2. CaO/H
2
O working pair
The basis of operation for the CaO/H
2
O set does not differ
from the con
figuration of the CHP operating for tempera-
ture ampli
fication as described earlier. In this case, the
difference in the partial pressure of water in the liquid
and the gaseous form is the driving force between the reac-
tors. The ef
ficiencies of the CaO/H
2
O working pair operat-
ing in the temperature-level ampli
fication mode are shown
in Figure 7. Here, the
first law efficiency obtained is also in
agreement with the corresponding experimental value [29].
The trends of dependencies on the heat source and task
temperatures are rather similar to that of the CaO/CO
2
working pair. However, the extent in increase or decrease
in the exergetic ef
ficiency of the CaO/H
2
O working pair
differs from that of the CaO/CO
2
. It is worth noting that
the speci
fic heats of CaCO
3
and Ca(OH)
2
compounds are
only slightly different (0.1230 and 0.093 kJ/mol K, respec-
tively), whereas a larger difference exists for the respective
values of CO
2
and H
2
O in addition to the endothermic re-
action enthalpies of the involving pairs (
ΔH = 104kJ/mol
for CaO/H
2
O versus
ΔH = 178.3kJ/mol for CaO/CO
2
).
5. CONCLUSIONS
Analyses of the
first law and exergetic efficiencies of a
CHP operating in the temperature-level ampli
fication mode
have been carried out. The exergetic ef
ficiency model de-
rived was used for two working pairs, namely, CaO/CO
2
and CaO/H
2
O for high-temperature high-lift CHPs. The
exergetic ef
ficiency increases for both working pairs with
increase in task, T
H
, decrease in heat source, T
M
, and
increase in condenser, T
L
, temperatures. However, the
difference in reaction enthalpies and speci
fic heats of the
involving reactants affects the extent of increase or
decrease in the exergetic ef
ficiency of the CHP operating
in the temperature-level ampli
fication mode.
Figure 6. Dependencies of
first law (
CHP,I
) and exergetic (
CHP,II
) ef
ficiency on T
H
, T
L
and T
M
for the CaO/CO
2
working pair.
Figure 7. Dependencies of
first law (
CHP,I
) and exergetic (
CHP,II
) ef
ficiency on T
H
, T
L
and T
M
for the CaO/H
2
O working pair.
Exergetic efficiency of high-temperature high-lift chemical heat pump
M. Arjmand, L. Liu and I. Neretnieks
Int. J. Energy Res. (2012) © 2012 John Wiley & Sons, Ltd.
DOI: 10.1002/er

NOMENCLATURE
A, B, C
= reactants
HE
= heat engine
HP
= heat pump
CHP
= chemical heat pump
C
P
= speci
fic heat
E
X
= exergy
H
= enthalpy
P
= pressure
S
= entropy
T
= temperature
= ef
ficiency
Superscripts
H
= high-temperature reactor
L
= low-temperature reactor
Subscripts
avail.
= available quantity
loss
= lost quantity
H
= high-temperature level
M
= medium-temperature level
L
= low-temperature level
g
= gas state
l
= liquid state
s
= solid state
0
= reference state
I
=
first law
II
= second law (exergetic)
ACKNOWLEDGEMENT
The authors express their gratitude to Vattenfall AB for the
financial support of this work.
REFERENCES
1. Sternlicht B. Waste energy recovery: An excellent
investment opportunity. Energy Conversion and
Management 1982;
22:361–373.
2. Spoelstra S, Haije WG, Dijkstra JW. Techno-
economic feasibility of high-temperature high-lift chem-
ical heat pumps for upgrading industrial waste heat.
Applied Thermal Engineering 2002;
22:1619–1630.
3. Cheng C-S, Shih Y-S. Exergy and energy analyses of
absorption heat pumps. International Journal of
Energy Research 1988;
12:189–203.
4. Demir H, Mobedi M, Ülkü S. A review on adsorption
heat pump: Problems and solutions. Renewable and
Sustainable Energy Reviews 2008;
12:2381–2403.
5. Tahat MA, Babushaq RF, O
’Callaghan PW, Probert
SD. Integrated thermochemical heat-pump / energy-
store. International Journal of Energy Research
1995;
19:603–613.
6. Altini
şik K, Veziroglu TN. Metal hydride heat pumps.
International
Journal
of
Energy
Research
1991;
15:549–560.
7. Dawoud B, Amer EH, Gross DM. Experimental
investigation
of
an
adsorptive
thermal
energy
storage. International Journal of Energy Research
2007;
31:135–147.
8. Morawetz E. Sorption-compression heat pumps. Inter-
national Journal of Energy Research 1989;
13:83–102.
9. Bjurström H, Raldow W. The absorption process for
heating, cooling and energy storage
—an historical
survey. International Journal of Energy Research
1981;
5:43–59.
10. Ahachad M, Charia M, Bernatchou A. Solar absorp-
tion heat transformer applications to absorption refrig-
erating machines. International Journal of Energy
Research 1993;
17:719–726.
11. Kato Y, Sasaki Y, Yoshizawa Y. Magnesium oxide/
water chemical heat pump to enhance energy utilization
of a cogeneration system. Energy 2005;
30:2144–2155.
12. Saito Y, Kameyama H, Yoshida K. Catalyst-assisted
chemical heat pump with reaction couple of acetone
hydrogenation/2
–propanol dehydrogenation for upgrad-
ing low-level thermal energy: Proposal and evaluation.
International Journal of Energy Research 1987;
11:549–558.
13. Andersson J, Azoulay M, de Pablo J. Chemical heat
pumping
—A rapid experimental procedure for investi-
gating the suitability of salt hydrates under dynamic
conditions. International Journal of Energy Research
1988;
12:137–145.
14. Matsumura Y, Yoshida K. Heat pump characteristics
of sodium carbonate dehydration/hydration system.
International
Journal
of
Energy
Research
1995;
19:253–261.
15. Cacciola G, Anikeev V, Recupero V, Kirillov V,
Parmon V. Chemical heat pump using heat of revers-
ible catalytic reactions. International Journal of
Energy Research 1987;
11:519–529.
16. Wentworth WE, Chen E. Simple thermal decomposi-
tion reactions for storage of solar thermal energy.
Solar Energy 1976;
18:205–214.
17. Mooksuwan W, Kumar S. Study on 2-propanol/
acetone/hydrogen chemical heat pump: endothermic
dehydrogenation of 2-propanol. International Journal
of Energy Research 2000;
24:1109–1122.
Exergetic efficiency of high-temperature high-lift chemical heat pump
M. Arjmand, L. Liu and I. Neretnieks
Int. J. Energy Res. (2012) © 2012 John Wiley & Sons, Ltd.
DOI: 10.1002/er

18. Wongsuwan W, Kumar S, Neveu P, Meunier F. A
review of chemical heat pump technology and
applications. Applied Thermal Engineering 2001;
21:1489–1519.
19. Aristov Y. Chemical and adsorption heat pumps:
Cycle ef
ficiency and boundary temperatures. Theoreti-
cal Foundations of Chemical Engineering 2008;
42:873–881.
20. Kim TG, Yeo YK, Song HK. Chemical heat pump
based on dehydrogenation and hydrogenation of i-
propanol and acetone. International Journal of Energy
Research 1992;
16:897–916.
21. Gandia LM, Montes M. Effect of the design variables
on the energy performance and size parameters of a
heat transformer based on the system acetone/H
2
/2-
propanol. International Journal of Energy Research
1992;
16:851–864.
22. Restuccia G, Cacciola G, Quagliata R. Identi
fication of
zeolites for heat transformer, chemical heat pump and
cooling systems. International Journal of Energy
Research 1988;
12:101–111.
23. Aristov YI, Parmon VN, Cacciola G, Giordano N.
High-temperature chemical heat pump based on
reversible
catalytic
reactions
of
cyclohexane-
dehydrogenation/benzene-hydrogenation: Comparison
of the potentialities of different
flow diagrams.
International Journal of Energy Research 1993;
17:293–303.
24. Fujii I, Tsuchiya K, Higano M, Yamada J. Studies of
an energy storage system by use of the reversible
chemical reaction: CaO + H
2
O
⇌ Ca(OH)
2
. Solar
Energy 1985;
34:367–377.
25. Azpiazu MN, Morquillas JM, Vazquez A. Heat
recovery from a thermal energy storage based on the
Ca(OH)
2
/CaO cycle. Applied Thermal Engineering
2003;
23:733–741.
26. Kyaw K, Shibata T, Watanabe F, Matsuda H,
Hasatani M. Applicability of zeolite for CO
2
storage
in a CaO
–CO
2
high temperature energy storage
system. Energy Conversion and Management 1997;
38:1025–1033.
27. Chen KS, Hwang WC. On the chemical heat pump
system and its second-law ef
ficiency. Energy Conver-
sion and Management 1988;
28:123–127.
28. Chung Y, Kim B-J, Yeo Y-K, Song HK. Optimal
design of a chemical heat pump using the 2-
propanol/acetone/hydrogen system. Energy 1997;
22:525–536.
29. Ogura H, Yamamoto T, Kage H. Ef
ficiencies of CaO/
H
2
O/Ca(OH)
2
chemical heat pump for heat storing and
heating/cooling. Energy 2003;
28:1479–1493.
30. Meng N, Shinoda S, Saito Y. Improvements on
thermal ef
ficiency of chemical heat pump involving
the reaction couple of 2-propanol dehydrogenation
and acetone hydrogenation. International Journal of
Hydrogen Energy 1997;
22:361–367.
31. Fujimoto S, Bilgen E, Ogura H. Dynamic simulation
of CaO/Ca(OH)
2
chemical heat pump systems.
Exergy, An International Journal 2002;
2:6–14.
32. Kato Y, Honda T, Kanzawa A. Kinetic measurement
on the isobutene/water/tert-butanol chemical heat
pump; dehydration of tert-butanol. International
Journal of Energy Research 1996;
20:681–692.
33. Chadda D, Ford JD, Fahim MA. Chemical Energy
Storage by the Reaction Cycle CuO/Cu
2
O. Inter-
national
Journal
of
Energy
Research 1989;
13:63–73.
34. Kato Y, Harada N, Yoshizawa Y. Kinetic feasibility of
a chemical heat pump for heat utilization of high-
temperature processes. Applied Thermal Engineering
1999;
19:239–254.
35. Izquierdo M, Aroca S. Lithium bromide high-temperature
absorption heat pump: Coef
ficient of performance and
exergetic ef
ficiency. International Journal of Energy
Research 1990;
14:281–291.
36. Arjmand M. Thermochemical Recovery of Heat:
Conceptual Integration of Chemically Recuperative
Cycle and Chemcial Heat Pump. LAP LAMBERT
Academic Publishing GmbH &Co. KG: Saarbrücken,
2011; 1
–84.
37. Olszewski M, Zaltash A. High-Lift Chemical Heat
Pump Technologies for Industrial Processes. ASME
International Congress and Exposition, 1995. US
Department of Energy. Available from: www.osti.
gov/bridge/servlets/purl/100092-7tELqq/webviewable/,
Accessed 2010-10-26.
38. Kato Y, O-shima T, Yoshizawa Y. Thermal perfor-
mance of a packed bed reactor for a high-temperature
chemical heat pump. International Journal of Energy
Research 2001;
25:577–589.
39. Kato Y, Yoshizawa Y. Application of a chemical heat
pump to a cogeneration system. International Journal
of Energy Research 2001;
25:129–140.
40. Zam
firescu C, Naterer GF, Dincer I. Upgrading of
Waste Heat for Combined Power and Hydrogen
Production
With
Nuclear
Reactors.
Journal
of
Engineering for Gas Turbines and Power 2010;
132:102911–102919.
41. Smith JM, Van Ness HC, Abbott MM. Introduction To
Chemical Engineering Thermodynamics. Mc-Graw
Hill: New York, 2001; 169
–176.
42. Raldow WM, Wentworth WE. Chemical heat pumps--
A basic thermodynamic analysis. Solar Energy
1979;
23:75–79.
43. Raldow W. Thermal ef
ficiencies of chemical heat
pump con
figurations. Solar Energy 1981; 27:307–311.
Exergetic efficiency of high-temperature high-lift chemical heat pump
M. Arjmand, L. Liu and I. Neretnieks
Int. J. Energy Res. (2012) © 2012 John Wiley & Sons, Ltd.
DOI: 10.1002/er

44. Moran MJ, Shapiro HN. Fundamentals of Engineering
Thermodynamics. John Wiley & Sons Inc.: West
Sussex, 2006; 303
–306.
45. Ahern JE. The Exergy Method of Energy Systems Anal-
ysis. John Wiley & Sons Inc.: New York, 1980; 45
–52.
46. Jones JB, Hawkins GA. Engineering Thermodynamics:
An Introductory Textbook. John Wiley & Sons Inc.:
New York, 1986.
47. Ishida M, Zheng D, Akehata T. Evaluation of a chemi-
cal-looping-combustion power-generation system by
graphic exergy analysis. Energy 1987;
12:147–154.
48. Lee J-S, Kim J-H, Kim J-T, Suh J-K, Lee J-M, Lee
C-H. Adsorption Equilibria of CO
2
on Zeolite 13X
and Zeolite X/Activated Carbon Composite. Jour-
nal
of
Chemical
&
Engineering
Data
2002;
47:1237–1242.
49. Lander JJ. Experimental Heat Contents of SrO,
BaO, CaO, BaCO
3
and SrCO
3
at High Tempera-
tures. Dissociation Pressures of BaCO3 and SrCO3.
Journal of the American Chemical Society 1951;
73:5794–5797.
50. Perry RH, Green DW. Perry
’s Chemical Engineering
Handbook. McGraw-Hill: New York, 1997.
51. Jacobs GK, Kerrick DM, Krupka KM. The high-
temperature heat capacity of natural calcite (CaCO
3
).
Physics and Chemistry of Minerals 1981;
7:55–59.
Exergetic efficiency of high-temperature high-lift chemical heat pump
M. Arjmand, L. Liu and I. Neretnieks
Int. J. Energy Res. (2012) © 2012 John Wiley & Sons, Ltd.
DOI: 10.1002/er
Wyszukiwarka
Podobne podstrony:
Electronic phase diagram of high temperature copper oxide superconductors
Roman, Małgorzata; Niska, Monika Development of Kubłowo Palaeolake in Central Poland During Eemian
51 721 736 Evaluation of the Cyclic Behaviour During High Temperature Fatique of Hot Works
Prediction Of High Weight Polymers Glass Transition Temperature Using Rbf Neural Networks Qsar Qspr
Castles & Crusades Wilderlands of High Adventure
Capability of high pressure cooling in the turning of surface hardened piston rods
Monitoring the Risk of High Frequency Returns on Foreign Exchange
DESIGN, SIMULATION, AND TEST RESULTS OF A HEAT ASSISTED THREE CYLINDER STIRLING HEAT PUMP (C 3)(1)
W Borek Mechanical properties of high manganese austenitic TWIP type steel
3 27 37 Influence of the Temperature on Toughness of DIEVAR
Modeling of high
Study of the temperature?pendence of the?initic transformation rate in a multiphase TRIP assi
Comparison of theoretical and experimental free vibrations of high industrial chimney interacting
80 1125 1146 Spray Forming of High Alloyed Tool Steels to Billets of Medium Size Dimension
10 Grandes Etudes for Clarinet of High Virtuosity (clarinet) Fernand Carion
collimated flash test and in sun measurements of high concentration photovoltaic modules
Castles & Crusades Wilderlands of High Adventure
więcej podobnych podstron