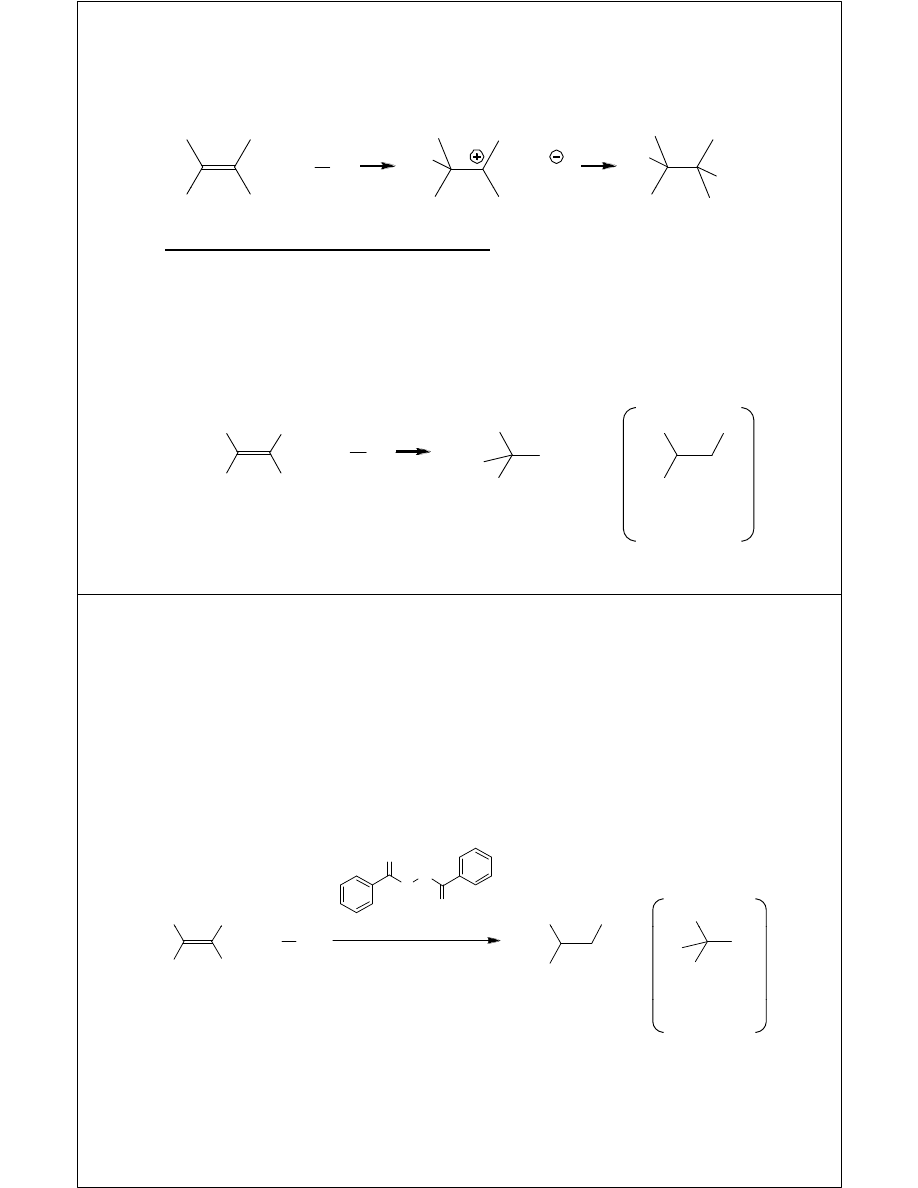
Electrophilic Addition to Alkenes
E
X
+
E
X
+
E
X
Hydrohalogenation (addition of HX)
Markovnikov’s Rule of Electrophilic Addition:
Electrophile adds in such a way as to generate the
most stable cation intermediate. (Usually, such that
electrophile is bound to less substituted carbon.)
H
3
C
H C
H
H
H
Br
+
H
3
C
Br
CH
3
H
3
C
Br
+
H
3
C
H
H
3
C
selectively
not
observed
H
3
C
We say reaction is regioselective.
Anti-Markovnikov Addition w/
HBr + peroxide
HBr + peroxide
For most Markovnikov-rule electrophilic additions, there are
alternative conditions that generate anti-Markovnikov preference.
O
H
3
C
H
H
3
C
Br
O
O
O
O
ben o l per o ide
H
3
C
not
H
3
C
H
H
Br
+
H
3
C
benzoy l per oxide
H
3
C
Br
CH
3
selectively
not
observed
Markovnikov Addition of H
2
O
Hydration
H
2
SO
4
/H
2
O
Harsh and reversible
H C
H
H
3
C
H
3
C
OH
t
H
3
C
H
3
C
H
H
H
3
C
H
3
C
HO
CH
3
H
3
C
H
3
C
OH
selectively
not
observed
Hydroxymercuration-
D
ti
1. Hg(OAc)
2
, H
2
O
2. NaBH
4
Demercuration
Milder, higher yielding
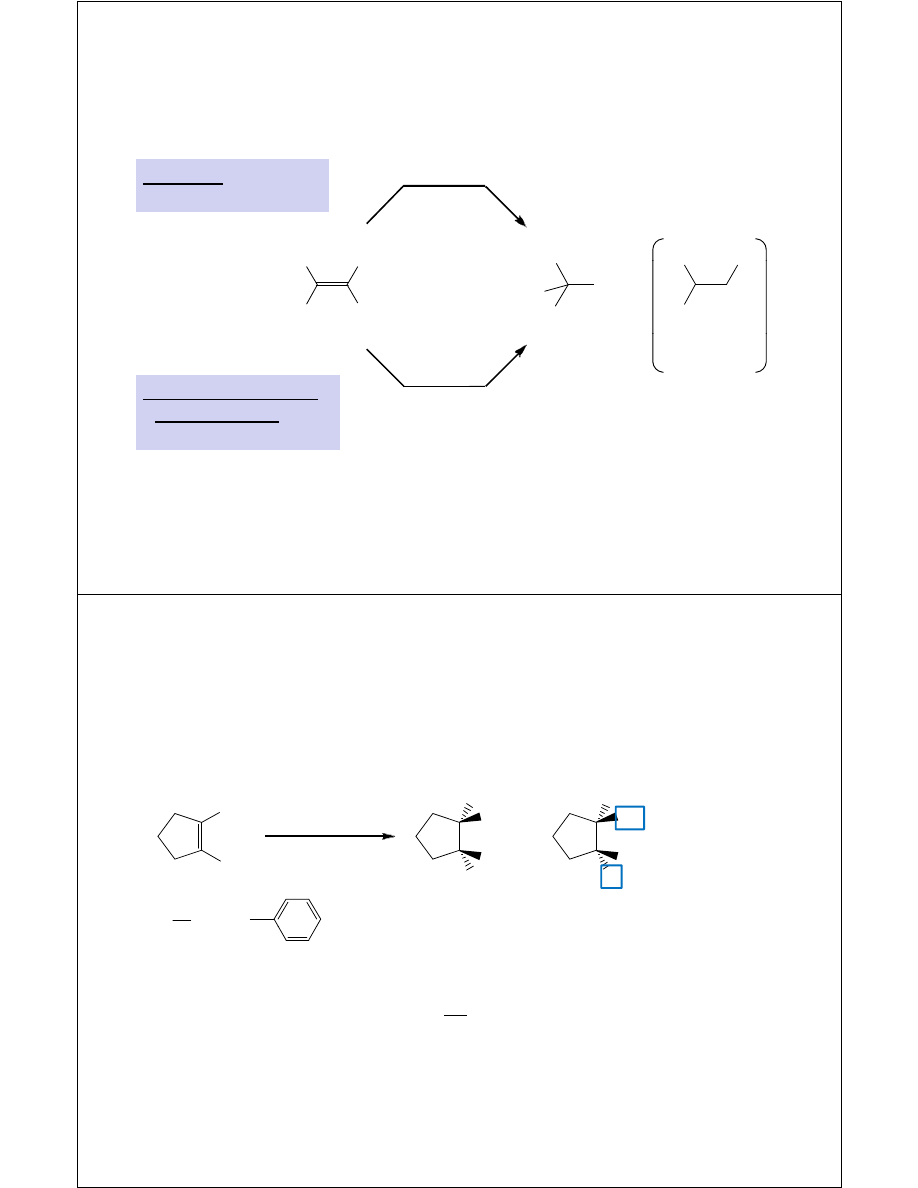
Diastereoselectivity of
Hydroxymercuration-Demercuration
Ph
Ph
OH
1. Hg(OAc)
2
, H
2
O
2. NaBH
4
OH
Ph
+
(S)
(R)
H & OH add to
opposite faces
CH
3
H
CH
3
CH
3
H
(R)
(S)
of alkene
enantiomers
Ph
=
Reaction is diastereoselective: (R,R) or (S,S) diastereomers are
not produced
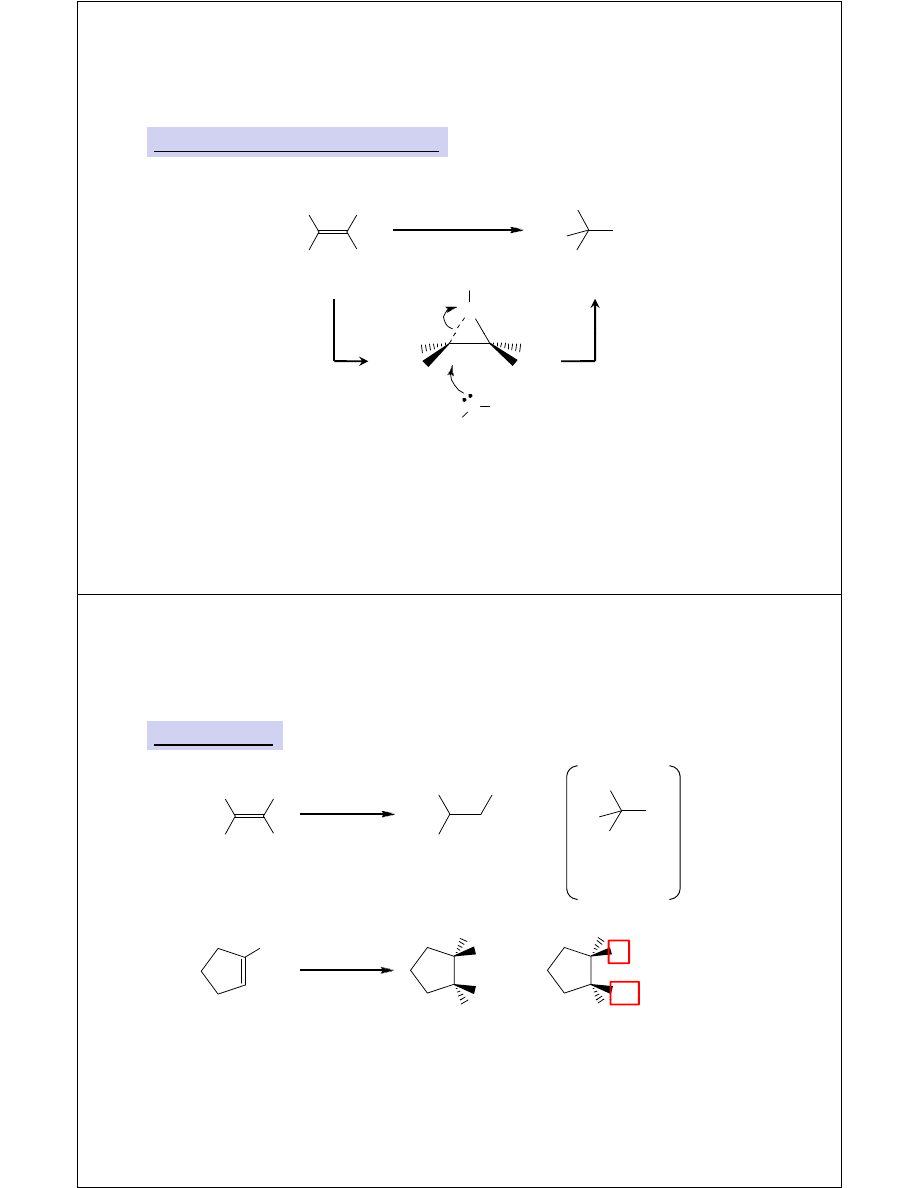
Markovnikov Addition of ROH
Alkoxymercuration-Demercuration
l
i
l
H
3
C
H
3
C
H
H
1. Hg(OAc)
2
, ROH
2. NaBH
4
H
3
C
H
3
C
RO
CH
3
selectively
H
3
C
H
H
3
C
Hg
OAc
δ+
M
i i
i
A
ith H O
H
3
C
H
H
3
C
H
δ+
O
H
Mercurinium ion
trapped by ROH
instead of H
2
O
As with H
2
O,
reaction is
stereospecific
O
R
H
Anti-Markovnikov Addition of H
2
O
Hydroboration
1 BH
3
·THF
H
3
C
H
3
C
OH
H
3
C
H
3
C
H
H
1. BH
3
THF
2. H
2
O
2
, OH
-
l
ti
l
H
3
C
H
3
C
HO
CH
3
selectively
not
observed
CH
3
CH
3
H
H
H
OH
CH
3
+
1. BH
3
·THF
2. H
2
O
2
, OH
-
H & OH add to
same face of
alkene
OH
H
Wyszukiwarka
Podobne podstrony:
Electrophilic Addition to Alkenes
electrophilic addition of hydrogen halides to alkenes lecture
Electrophilic Addition to Alkynes
Electrophilic addition of hydrogen halides (HX) to alkenes
1 Intro to lg LECTURE2014
Introduction to Algotrade lecture1
Reactions of alkenes lecture1
2000 Evaluation of oligosaccharide addition to dog diets influences on nutrient digestion and microb
(eBook Imray Cruising Guide) Isles of Scilly Iles Scilly additions Robin Brandson & J & F Garey
[Ebook Electronics] How To Make Printed Circuit Boards
Shi ah Additions to the Koran
ENERGY POWER WATER Electricity How to Build a Waterwheel Generator (ebook Home Power Diy 185336
electrophilic additions initiated by protonation
8 Intro to lg socio1 LECTURE2014
4 Intro to lg morph LECTURE2014
12 Intro to origins of lg LECTURE2014
Kto,blokuje tą wiedzę Antenna To Replace?tteries And Provide Unlimited Free Energy For Electric?rs
więcej podobnych podstron