
Biomaterials 23 (2002) 349–356
Mechanical and in vitro testing of aerosol–gel deposited titania
coatings for biocompatible applications
Miguel Manso
a,1,
*,Samuel Ogueta
b,1
,Predestinaci
!oon Garc!ııa
b
,Jos
!ee P!eerez-Rigueiro
c
,
Carmen Jim
!eenez
d
,J.M. Mart
!ıınez-Duart
a
,Michel Langlet
d
a
Departamento de F
!ıısica Aplicada, Universidad Aut !oonoma de Madrid, 28049 Madrid, Spain
b
Departamento de Biolog
!ııa Molecular, Universidad Aut !oonoma de Madrid, 28049 Madrid, Spain
c
Departamento de Ciencia de Materiales, ETSICaminos, Canales y Puertos, Universidad Polit
!eecnica de Madrid, 28040 Madrid, Spain
d
Laboratoire des Mat
!eeriaux et du G!eenie Physique, Rue de la Houille Blanche-BP 46, 38402 Saint Martin d’H"eeres, France
Received 4 September 2000; accepted 7 March 2001
Abstract
The biocompatible properties of sol–gel titania have increased the interest in the mechanical properties of this material in the form
of functional coatings for prosthetic applications. In the present work,titania coatings with thicknesses of 1 mm have been prepared
using the aerosol–gel process. The main objective has been to evaluate the mechanical properties of the coatings and to prove their
in-vitro biocompatibility. For this purpose,the hardness and Young’s modulus of the coatings were measured by nanoindentation
with loads in the 6–30 mn range. A continuous increase of these magnitudes was observed for the coatings treated at increasing
sintering temperatures (150–8001C). The hardness and the Young’s modulus ranged between 15.8–19.5 GPa and 142–186 GPa,
respectively. This behaviour has been confirmed by measurements of the plastic energy of deformation in 10 mn full loading–
unloading tests and by determination of the mean indentation creep under 30 mn loads. The films were additionally characterised by
XRD,FTIR and ellipsometry to study the chemical and structural changes produced by sintering. Biocompatibility tests are very
conclusive. Cells seeded on aerosol–gel titania coatings grow while adhered onto the surface. These coatings are thus of potential
interest for the enhancement of the properties of prosthetic TiAlV alloys. r 2001 Elsevier Science Ltd. All rights reserved.
Keywords:
Aerosol–gel; Titania; Coatings; Biocompatibility; Mechanical properties; Ultra-microindentation
1. Introduction
Titania films prepared by diverse methods present
promising biocompatible properties. Electrochemically
prepared TiO
2
coatings have proved to be highly stable
in surgical practice [1]. In addition,sol–gel derived
titania coatings have been described to be apatite
nucleation inducers not only in vitro but also in vivo
[2]. It has been proposed that the nucleation of the
calcium phosphate is activated by the presence of
hydroxyl functional groups in the film [3]. Descriptions
concerning thickness and morphology effects on the
nucleation ability of these films have been reported [4].
The formation of TiO
2
coatings on TiAlV alloys was
thus proposed to ameliorate the biocompatibility of
load bearing prostheses. The titania coatings also
present a barrier function which would avoid the
negative effects of Al and V ions released by wear
processes over the TiAlV prostheses [5]. Furthermore,
in vivo studies of the performance of hydroxyapatite
coatings (HAP,Ca
10
(PO
4
)
6
(OH)
2
),a material that
provides faster fixation with bone tissue [6],have
shown that failures of these coatings are mainly
produced at the interface with the metallic substrate
[7]. It has been shown that calcium and phosphate
ions diffuse through the metallic substrate during
sintering treatments applied to enhance the crystal-
linity of the coatings [8]. A preferential diffusion
can dangerously modify the Ca=P ratio at the inter-
face,leading thus to a coating failure [9]. Titania
coatings can play the role of a protective barrier
avoiding the diffusion of these ions into the implant.
Both effects,i.e. apatite nucleation induction and
*Corresponding author. Tel.: +34-913-974-919; fax: +34-913-973-
969.
E-mail address:
miguel.manso@uam.es (M. Manso).
1
Authors with equal contributions.
0142-9612/02/$ - see front matter r 2001 Elsevier Science Ltd. All rights reserved.
PII: S 0 1 4 2 - 9 6 1 2 ( 0 1 ) 0 0 1 1 2 - 0

diffusion
barrier
properties,permit
to
envisage
applications for the improvement of metallic load
bearing prostheses,even more when a high bonding
strength to the commonly used TiAlV alloy is ensured.
In any case,the mechanical properties of titania
coatings remain crucial for any biological application
envisaged.
The sol–gel method has been explored for the
preparation of titania coatings. Its principal advant-
ages are the control of surface morphology and
composition and the possibility of low temperature
processing which allows the use of thermally fragile
substrates [10]. The aerosol–gel method,used in this
work,is based on the ultrasonic nebulisation of an
aerosol from a reactive sol–gel solution. The aerosol,
constituted of microdroplets,is driven onto the
substrate where the droplets spread and coalesce,
leading to a liquid film. After deposition,drying and
sol–gel polymerisation take place,forming a xerogel
film that can then be heat-treated. The method
introduces some extra advantages over the conven-
tional
methods
of
spin-
and
dip-coating.
The
thickness
can
be
precisely
tailored
by
selecting
different deposition times (aerosol flowing) and the
system lends itself to the deposition on large and
three-dimensional substrates [11]. These conditions
are essential for the processing of plates,screws
and elements of complex design such as hip-joint
prostheses. Previous works on aerosol–gel titania
coatings include the study of the changes produced
by doping coatings [12] and the preparation of
high refractive index coatings at low temperature [13].
Studies related with the precursor solution prepara-
tion,condensation and densification have also been
published [14,15].
The aim of this work is to demonstrate the
biocompatibility of aerosol–gel derived titania films
and to describe their mechanical behaviour taking
account of their physico-chemical properties. For
that purpose,we have not only studied the changes of
the Young’s modulus and hardness for increasing
sintering temperatures,but also the singular differences
in the plastic energy of deformation (PED) and the
behaviour under constant loads reflected in the mean
indentation creep (MIC). To achieve these characterisa-
tions,we used the nanoindentation technique. This
technique is a powerful tool that has already been used
by several groups to establish the mechanical differences
between bulk materials and their corresponding thin
film structures. It has also proved to be useful to
evidence different orientations of single-crystals and to
measure the stresses in thin films grown by different
techniques or over different substrates [16]. In addition,
it has been successfully applied to biological systems to
characterise human cortical and trabecular lamellar
bone [17].
2. Experimental procedure
2.1. Sample preparation
Silicon wafers (1 0 0) were used as substrates for the
deposition of titania coatings. TiAlV plates were used
for biocompatibility tests. Before deposition,the Si
wafers were treated in air at 5001C for 2 h to eliminate
adsorbed impurities and to form a thermally stable SiO
2
surface film.
The precursor solution was optimised considering
that highly viscous solutions (for instance,due to a high
precursor concentration or reactivity) are not easily
transformed into an aerosol. Therefore,a 0.4 m tetra-
isopropyl-orthotitanate (TIPT,Fluka chemicals) solu-
tion diluted in ethanol with a TIPT/water ratio
r
w
¼ 0:82 and a pH ¼ 1:27 was prepared as described
previously [14]. This solution exhibited a moderate
reactivity and low viscosity (2 cP). The ultrasonically
nebulised aerosol was conducted during 20 s by an
ethanol saturated air flux into the thermally regulated
deposition chamber (201C). The derived liquid films
were then allowed to dry and to polymerise in air. The
so-formed xerogel films were sintered in air at 1501C,
3001C,5001C,and 8001C for 10 min in a conventional
furnace. In order to prepare thicker TiO
2
films,a
multilayer deposition procedure was followed,i.e. the
deposition/heat-treatment cycle was repeated ten times.
The final coating was then sintered for 1 h at the
temperature used for the intermediate treatment. In
these pages,the film nomenclature refers to the sintering
temperature (i.e. TiO150,TiO300,TiO500 and TiO800).
2.2. Physico-chemical characterisation
The coatings characterisation was carried out by the
use of the following techniques. X-ray diffraction
(XRD) was performed in a y=2y Siemens diffractometer
using a 0.041 scanstep and 6 s integration time. Fourier
transform infrared (FTIR) spectra were recorded in
transmission using a Bio-Rad FTS165 spectrometer (100
scans at 20 Hz,4 cm
@1
resolution). The Si substrate
contribution was subtracted from the spectra for the
analysis of the different coatings. The thickness and
refractive index of the coatings were measured by
ellipsometry (Gaertner L116B) with a 632.8 nm wave-
length and 701 beam incidence. The values of thickness
and refractive index of transparent coatings grown on Si
can be obtained in these conditions from the values of
the phase ellipsometric parameter D,and the amplitude
parameter c.
2.3. Mechanical characterisation
The Young’s modulus and hardness have been
obtained using the nanoindentation technique. In the
M. Manso et al. / Biomaterials 23 (2002) 349–356
350
present work,the mechanical model described by Pharr
and Oliver has been followed in order to determine the
values of hardness and Young’s modulus [18]. The basis
of this method is the introduction of geometrical and
experimental corrections to the analytic expression used
for the calculation of the projected area of contact,
which is obtained from the estimation of the depth of
contact.
Indentation tests were performed in a Shimadzu
DUH-200 dynamic ultra microhardness tester with
resolutions of 0.02 mn in load and 0.005 mm in depth.
Six full loading–unloading tests (10 mn maximal load)
were applied to each sample to obtain the PED values.
The coatings behaviour under constant load was
analysed by measuring the MIC values. Each sample
was probed 10 times at 30 mn during 60 s. For the
hardness and Young’s modulus calculation,maximal
loads of 6,10,18 and 30 mn were applied. For a statistic
purpose,the samples surface was probed at six different
places with a Berkowich diamond tip indentor. Five
loading cycles were monitored,allowing a 50% unload
after 1 s. The tip area had been previously calibrated
using quartz and aluminium references.
2.4. Biocompatibility tests
Human chondrosarcome cells were used to test the
biocompatibility of TiO
2
coatings in comparison with
the TiAlV alloy surface. The experimental procedure
followed for the observation of the cell on opaque
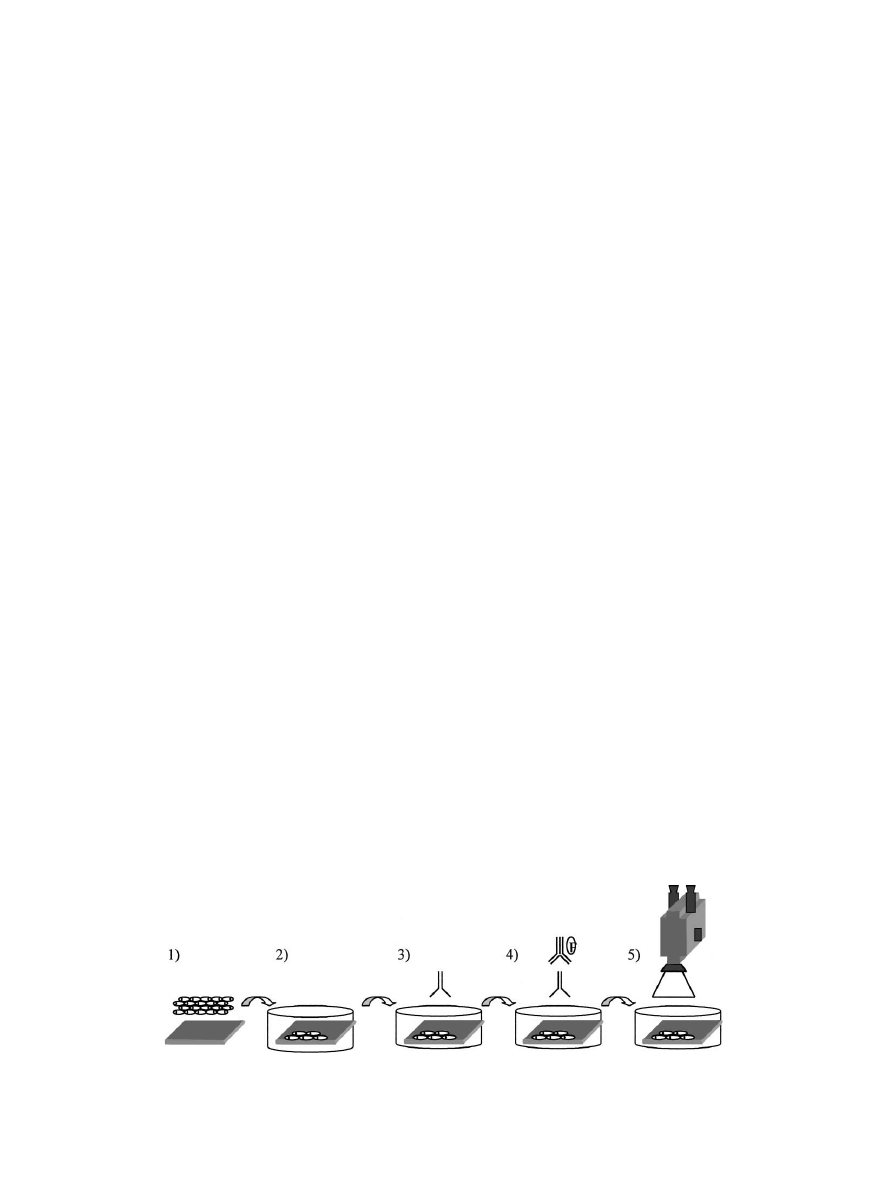
substrates is described in Fig. 1. The cells were cultured
and harvested as described previously [19] prior to
seeding onto TiO
2
surfaces. To perform the cell culture
onto the samples,one square cm of a titania coating
(TiO500) and a bare TiAlV substrate were washed with
PBS (phosphate buffer saline),placed individually on
tissue culture dishes and sterilised by over-night
exposure to UV in a tissue culture cabin. 200,000 cells
were seeded on each surface and incubated during 48 h
at 371C in an O
2
/CO
2
(96/4 v/v) atmosphere. Then,the
cells were washed with PBS,fixed with methanol and
kept in PBS at 41C until observation.
In order to visualise the cells on the samples,we
developed an easy immune technique overcoming the
material opacity. The above mentioned cultured sur-
faces were blocked with 5% milk protein in TBST
(10 mm Tris–HCl pH 7.5,150 mm NaCl and 0.05 Tween
20) during 2 h. The surfaces were washed three times
with 1 ml of 1% milk proteins in TBST and incubated
for 2 h at room temperature with 200 ml of sera from
transgenic mice at 1/400 dilution in TBST. These mice
develop an auto-immune disease and present antibodies
against nuclear and cytoskeleton proteins [19]. These
antibodies recognise human homologous proteins. After
incubation period,surfaces were washed three times
with 1% milk protein in TBST and incubated in dark
conditions for 30 min with a secondary antibody. An
anti-mouse labelled with fluorescein (Santa Cruz,CA) at
1/4000 dilution was used. After incubation,the surfaces
were washed and the cells were visualised by illumina-
tion at 495 nm in a fluorescence microscope (Axiovert
35). Three different experiments were carried out with
triplicate samples from TiO500 and TiAlV.
3. Results
3.1. Structure and composition
The XRD diagrams corresponding to TiO150 and
TiO300 samples (Fig. 2a and b) present no peaks arising
from the coating indicating that the phase formed is
amorphous. Sintering at higher temperatures drastically
activated the film crystallisation. Multiple peaks appear
in the XRD diagrams (Fig. 2c and d) proving that a
polycrystalline coating formed the TiO500 and TiO800
samples. The position of the peaks is in agreement with
the formation of a pure anatase phase corresponding to
the marked Miller indexes (JCPDS 84-1286). Two
residual peaks,issued from the Si substrate,are also
present in the diagrams. Sintering at 8001C led to
Fig. 1. General procedure followed to observe the cells overcoming substrate opacity. (1) Substrate pretreatment and cell culture. (2) Cell incubation
48 h. Methanol fixation. Albumin blockage. (3) Auto-immune serum incubation 1 h. (4) Secondary antibody reaction. 30 min. (5) Observation.
495 nm.
M. Manso et al. / Biomaterials 23 (2002) 349–356
351
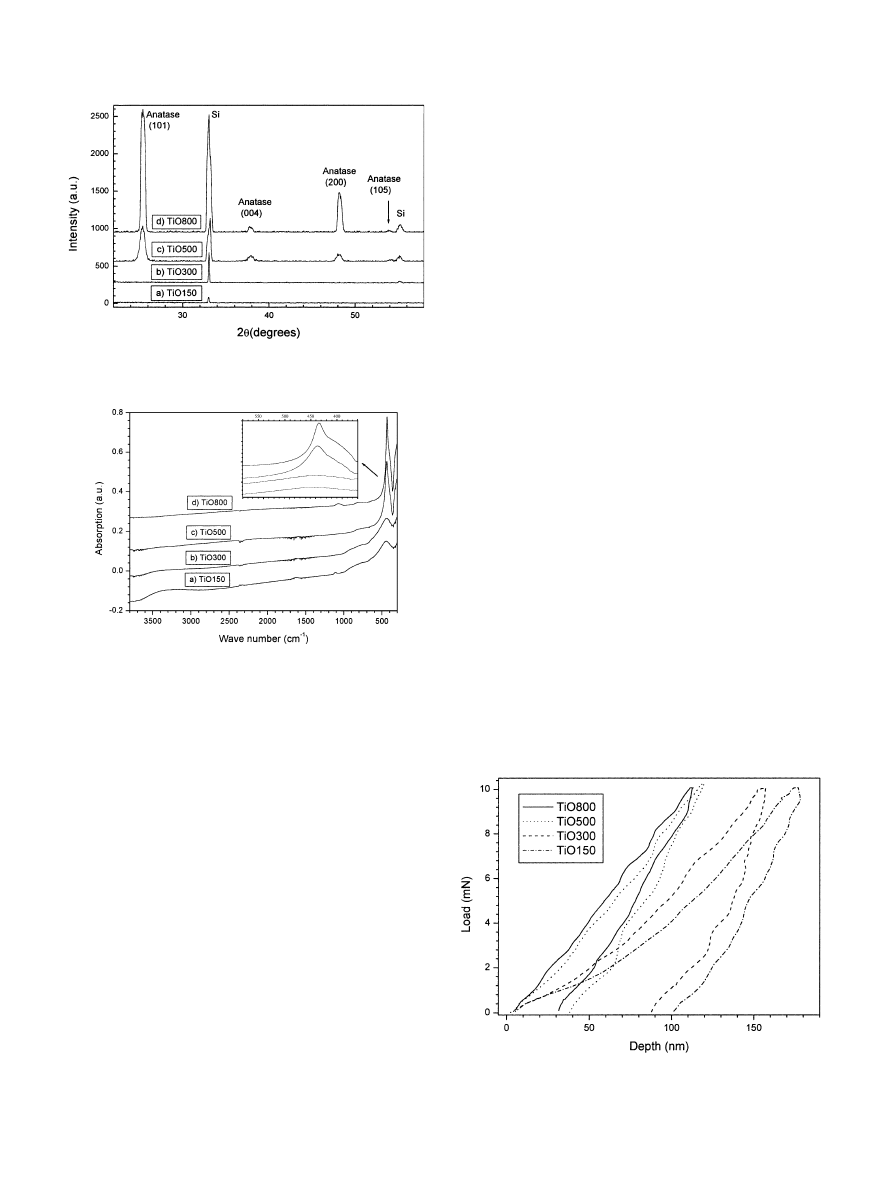
narrower and more intense peaks that prove an increase
in the crystallite size of the titania coating. The relative
intensities of the diffraction peaks are in agreement with
those obtained from anatase standards showing that the
substrate induces no preferential growth directions in
the films.
FTIR spectra are presented in Fig. 3. Several bands
associated with Ti-alkoxi bonds [15] are detected at 930,
1040,1070,1125 and 1375 cm
@1
in the TiO150
coatings. Their intensity is drastically reduced or they
even disappear after sintering at 3001C. An interesting
feature concerning the Ti–O–Ti bonds is the related
band shift from 450 cm
@1
for the TiO150 coating to
435 cm
@1
for the coatings sintered at higher tempera-
tures (inset of Fig. 3). This evolution is in agreement
with structural and chemical changes of the titanium
environment,from the nonhydrolysed alkoxide to the
solid oxide obtained after hydrolysis/polyconensation.
Sintering at 5001C causes a notable intensity increase of
the Ti–O–Ti band that is produced by the amorphous to
crystalline transition already depicted by XRD. An
extra band appears at 1070 cm
@1
in the spectra of the
TiO800 coating due to the increasing absorption from
the SiO
2
film growing on the nonpolished backside of
the substrate. The band at 1625 cm
@1
associated with
molecular water is also discerned with decreasing
intensity for the samples prepared with increasing
sintering temperature. The wide band observed at
3600–3100 cm
@1
is assigned to hydroxyl groups which
are gradually eliminated for increasing sintering tem-
peratures.
The ellipsometric measurements performed on films
fired at 5001C showed that the thickness of a single layer
was 0.1 mm and that the film thickness increased linearly
with the number of deposition/heat-treatment cycles.
The thickness of the final coating after 10 cycles was
thus estimated to be 1 mm. Such a thickness was
necessary to perform nanoindentation measurements
reasonably insensitive to the substrate response. The
surface homogeneity was proved by the low dispersion
(
70.5%) in the values of the refractive index measured
throughout the film surface. For films fired at 5001C,the
averaged value of the refractive index was 2.22.
Ellipsometric measurements carried out on the rest of
the coatings showed an increase of the refractive index
for an increasing annealing temperature. Values of 1.93
and 2.34 were obtained for TiO150 and TiO800 coat-
ings,respectively. The refractive index increase with
increasing temperature reflects a thermally activated
densification mechanism.
3.2. Mechanical properties
Typical 10 mn loading–unloading versus displacement
curves are presented in Fig. 4 for the different coatings.
All the parameters presented in this work are derived
from the differences existing between these curves after
Fig. 2. XRD diagrams of a TiO150 (a),a TiO300 (b),a TiO500 (c) and
a TiO800 (d) coating.
Fig. 3. FTIR spectra of a TiO150 (a),a TiO300 (b),a TiO500 (c) and a
TiO800 (d) coating.
Fig. 4. Full loading–unloading versus depth curves in 10 mn tests for a
TiO150 (dash–dot),a TiO300 (dash),a TiO500 (dots) and a TiO800
(straight) coating.
M. Manso et al. / Biomaterials 23 (2002) 349–356
352
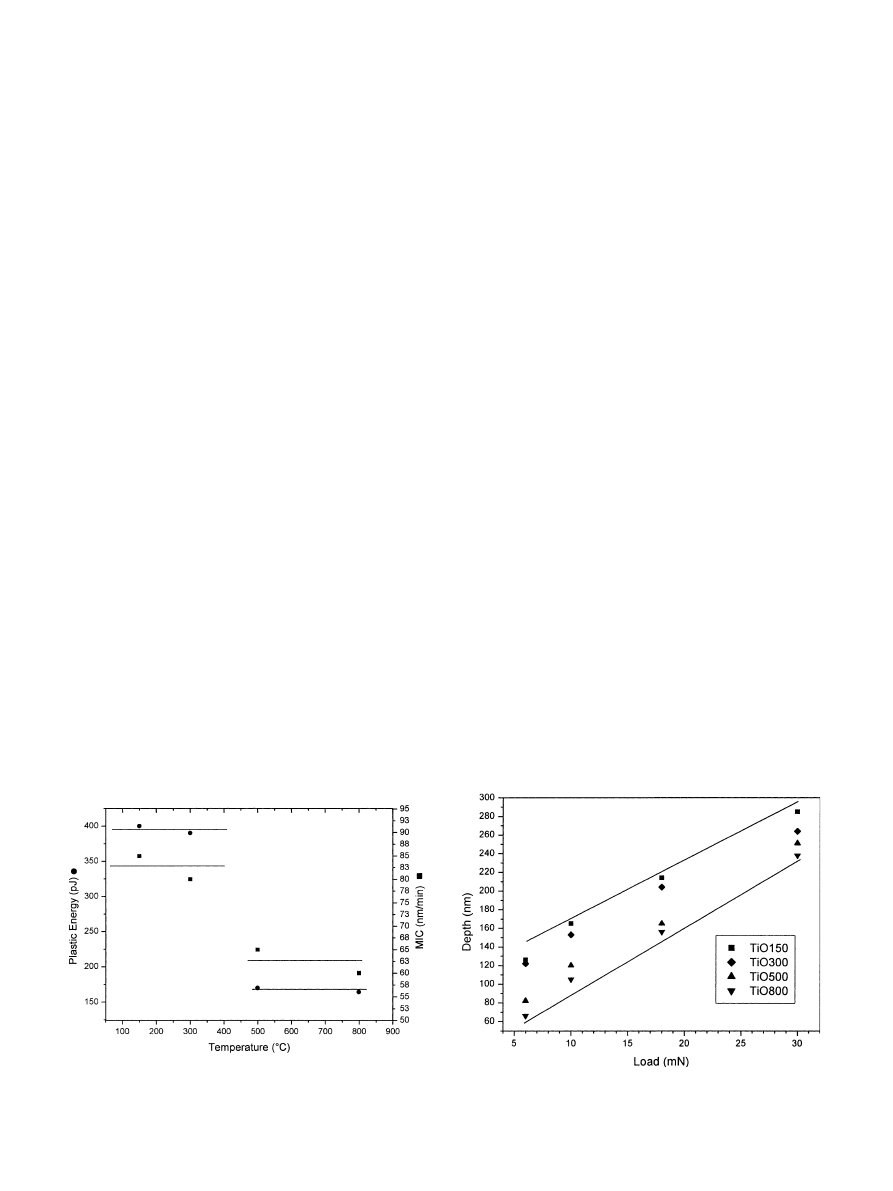
an averaging process. The subtraction of the area
enclosed by the unloading curve from that defined by
the loading curve determines that coatings sintered at
1501C and 3001C suffered from considerably higher
plastic deformation than the coatings prepared with
5001C and 8001C sintering treatments (Fig. 5,left axis).
This difference is linked to the structural evolutions
depicted by FTIR,XRD and ellipsometry. The MIC
values measured at 30 mn (Fig. 5,right axis) are also
correlated with the structural evolution of the coatings.
Polycrystalline TiO500 and TiO800 coatings present
lower deformations with a significant gap compared to
TiO150 and TiO300 coatings. The mechanical differ-
ences are also evidenced by the values of maximal
indentation depth and by the unloading slope. The
maximal indentation depths,obtained from the root
mean square of six measurements after five loading–
unloading cycles,are presented in Fig. 6. It is clearly
seen that the coatings sintered at lower temperatures
generate a weaker opposite force to the loads and suffer
from deeper indentations. On the plot of Fig. 6,two
groups of samples can be distinguished in terms of
resistance to the indentation load (TiO150 and TiO300
vs the stiffer TiO500 and TiO800). These results are still
correlated with XRD,FTIR and ellipsometry data.
However,both groups could no longer be distinguished
for the highest load,presumably due to an increasing
interaction with the substrate. Thus,the overall results
confirmed that the condensation,crystallisation and
densification degrees reached after sintering at 5001C
drastically influence the film mechanical behaviour. The
values of hardness are presented in Table 1 with the
references of the load and the maximal depth reached
for every coating. The Young’s moduli were 142
77,
150
79,170720,190720 GPa for coatings sintered at
1501C,3001C,5001C and 8001C,respectively. Both
hardness values and Young’s moduli confirm that a
clear correlation exist between sintering temperature
and the mechanical properties.
3.3. Biocompatibility tests
To determine whether aerosol–gel deposited titania
coatings could present a suitable biocompatibility,
we compared the adherence and growth of human
cells on our films with the behaviour on bare TiAlV
surfaces. The immuno assay performed to visualise
adhered
cells
on
opaque
surfaces
was
achieved
through a two step process. In the first immune reac-
tion,nuclear and cytoskeleton proteins were recogn-
ised by an auto-immune serum from mouse. Secondly,
the immune complexes formed were reacted with
an anti-mouse antibody labelled with fluorescein,
which
excites
the
fluorescence
reactions
in
cell
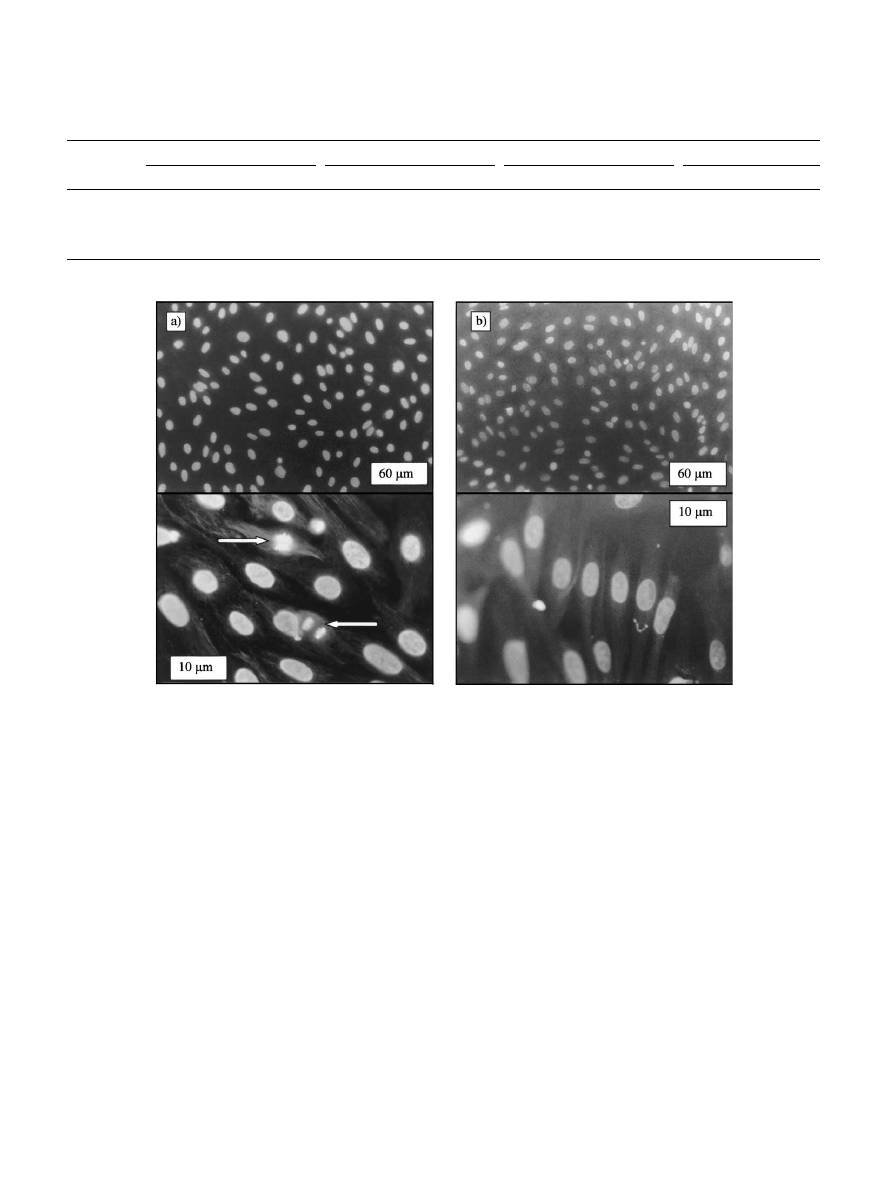
nuclei and cytoskeleton (Fig. 7). It was stated that
80% of seeded cells were adhered to both material
surfaces. However,some differences were observed.
For the titania surface (Fig. 7a),the distance between
cells was larger than that observed for the bare TiAlV
surface (Fig. 7b). This can be interpreted as a conse-
quence
of
an
increase
in
extra
cellular
matrix
components of cells adhered to titania. At higher
magnification,cytoskeleton of cells on titania showed
a polygonal array,while cells on TiAlV presented a
more
parallel
appearance.
In
addition,cells
on
titania surface showed growth signals since cells
were detected at different stages of the cellular cycle.
The arrows of Fig. 7 show these evolving nuclei,
which were not observed on TiAlV substrates. These
results support the notion of improved biocompatible
properties of aerosol–gel deposited titania in compar-
ison with the TiAlV surface.
Fig. 5. Values of the plastic energy of deformation (PED, K left axis)
and mean indentation creep (MIC, ’ right axis) versus the sintering
temperature applied to the titania coating.
Fig. 6. Maximal depth versus maximal load curves for a TiO150 ’, a
TiO300 ~,a TiO500 m and a TiO800 . coating.
M. Manso et al. / Biomaterials 23 (2002) 349–356
353
4. Discussion
The structure of titania coatings deposited by the
aerosol–gel process has been found to depend closely on
the applied sintering temperature. FTIR and XRD
analysis help to understand the sequence of processes
taking place during sintering. It was shown that an
important activation of the Ti–O–Ti network condensa-
tion and crystallisation is produced when a temperature
of 5001C is reached. Ellipsometric measurements of the
refractive index show that the processes are accompa-
nied by a significant film densification.
Indentation tests showed that physicochemical differ-
ences between the samples can be correlated with a
characteristic mechanical behaviour. It was stated that
the coatings can be classified into two groups. Coatings
sintered at 5001C or higher temperatures present lower
MIC and suffer from lower plastic deformations. This
behaviour can be related with the main mechanism
governing the indentation process,which is charac-
terised by the plastic deformation suffered for a fixed
applied load. Coatings sintered at 1501C and 3001C
suffer a dominant cutting mechanism (higher plastic
deformations),while coatings sintered at 5001C and
8001C undergo a compression strain mechanism (lower
plastic deformations) [20]. FTIR,ellipsometry and XRD
measurements show that this feature is closely related to
the Ti–O–Ti network polycondensation,densification
and crystallisation. Coatings sintered at higher tempera-
tures are also harder and present higher Young’s
moduli. It must be stated at this point that,taking into
account the properties of the TiO150 and TiO300
samples,these coatings are more prone to suffer from
substrate influence during indentation. Furthermore,
these coatings suffer from higher plastic deformations so
that,the measurement at the same time is actuating as a
driving force for the condensation and strengthening of
the coating. These two factors may explain the high
Table 1
Hardness values (H,
71 GPa) and maximal depth (h
max
,
75 nm) vs load (L) for the different coatings
L
(mn)
TiO150
TiO300
TiO500
TiO800
h
max
(nm)
H
(GPa)
h
max
(nm)
H
(GPa)
h
max
(nm)
H
(GPa)
h
max
(nm)
H
(GPa)
6
125
9
120
9
80
16
65
18
10
155
11
155
12
120
19
105
19
18
200
15
195
18
165
19
160
20
30
285
16
265
18
250
19
240
20
Fig. 7. Fluorescence micrograph showing the evolution of human chondrocite cells seeded on a TiO500 coating (a) and on the surface of TiAlV
alloy (b).
M. Manso et al. / Biomaterials 23 (2002) 349–356
354

values of Young’s moduli obtained for the not
completely densified TiO150 and TiO300 films.
A comparison of these coatings with other titania
films produced by alternative methods reveal interesting
features. The hardness and Young’s modulus values of
ion bombarded spin-coated films measured by ultra-
microindentation are considerably lower than the values
obtained for any of our coatings [21]. The results for arc
deposited titania coatings are nearer to the values
obtained in our study [22]. When amorphous arc
coatings
are
produced,the
Young’s
modulus
(140 GPa) is quite close to the value of our TiO150
coatings (142 GPa). When rutile crystalline coatings are
produced,the Young’s modulus (180 GPa) shows a
good level of coincidence with the value of our TiO800
coating (190 GPa). However,it should be mentioned
that the hardness values of aerosol–gel titania coatings
are higher than those obtained for arc TiO
2
coatings. In
any case,our hardness values appeared systematically
lower than the values measured for r.f. sputtered titania
films (23 GPa) [23].
Comparing titania coatings prepared by the afore-
mentioned techniques,it is worth mentioning that the
aerosol–gel titania coatings introduce a noticeable
advantage from the point of view of their mechanical
properties. Depending on the sintering temperature,
they present a range of mechanical properties that
makes it possible to tailor their response under stress.
Consequently,a multilayer deposition procedure can
produce coatings with gradual mechanical properties.
This configuration is considered of primary interest
since it has been shown that the drastic differences in
Young’s modulus between bone (30 GPa) and the
prostheses alloy (up to 200 GPa) can produce severe
damage. This effect is dramatic in the case of femur
heads sustaining hip-joint prostheses [5].
The development of an immune process has allowed
the in-situ observation of human cells seeded on titania
coatings. The adherence,growth and proliferation of the
cells prove that aerosol–gel titania coatings present
improved biocompatible properties with respect to the
traditional TiAlV alloys. This improved biocompatibil-
ity demonstrates that our coatings can be safely used for
prosthetic applications.
5. Conclusion
The aerosol–gel process has demonstrated to be a
suitable tool for the deposition of titania coatings
with tailored properties. The coatings mechanical
properties have been studied with respect to the sintering
temperature. The mechanical properties appear to be
strongly correlated to the polycondensation,densifica-
tion and crystallisation behaviour. The combination
of mechanical results,which prove the possibility to
tailor the mechanical properties of aerosol–gel derived
titania coatings,and of biological results,which show
enhanced biocompatibility of these coatings when
compared with a TiAlV alloy surface,are considered
of primary interest since they open the view to new
generation implants with a more suitable bone-implant
mechanical matching.
Acknowledgements
M. Manso thanks the Regional Government of
Madrid for his Research Grant and the financial
support during the stay at the ‘‘Laboratoire des
Mat
!eeriaux et du G!eenie Physique’’ in Grenoble.
References
[1] Szabo G,Kovacs L,Vargha K,Barabas J,Nemeth Z. A new
advanced surface modification technique- titanium oxide ceramic
surface implants: the background and long term results. J Long
Term Effects Med Impl 1999;3:247–59.
[2] Li P,Kangasniemi I,De Groot K. In vitro and in vivo evaluation
of bioactivity of gel titania. Bioceramics 1993;6:41–5.
[3] Haddow DB,James PF,Van Noort R. Characterisation of
sol–gel surfaces for biomedical applications. J Mater Sci: Mater
Med 1996;7:255–60.
[4] Haddow DB,Kothari S,James PF,Short RD,Hatton PV,Van
Noort R. Synthetic implant surfaces. 1. The formation and
characterisation of sol–gel titania films. Biomaterials 1996;17:
501–7.
[5] Long M,Rack HJ. Titanium alloys in total joint replacement
F
a materials science perspective. Biomaterials 1998;19:1621–39.
[6] Moroni A,Caja VL,Egger EL,Trinchese L,Chao EYS.
Histomorphometry of hydroxyapatite coated and uncoated
porous titanium bone implants. Biomaterials 1994;15:926–30.
[7] Hayashi K,Inadome T,Tsumura H,Nakashima Y,Sugioka Y.
Effect of surface roughness of hydroxyapatite coated titanium on
the
bone
implant
interface
shear
strength.
Biomaterials
1994;15:1187–91.
[8] Ducheyne P,Van Raemdonck W,Heughbaert KC,Heughbaert
JC. Structural analysis of hydroxyapatite coatings on titanium.
Biomaterials 1986;7:97–103.
[9] Ramselaar MMA,Driessens FCM,Kalk W,De Wijn JR.
Biodegradation of four calcium phosphate ceramics; in vivo
rates and tissue interactions. J Mater Sci: Mater Med 1991;2:
63–70.
[10] Scherrer GW,Brinker CJ. Sol–Gel Science: The Physics and
Chemistry of Sol–Gel. Boston MA: Academic Press,1990.
[11] Langlet M,Joubert JC. French Patent 9014312,1990; European
Patent 0486393 A1,1991.
[12] Viitala RI,Langlet M,Simola J,Lind
!een M,Rosenholm JB.
Aerosol–gel deposition of doped titania thin films. Thin Solid
Films 2000;368:35–40.
[13] Langlet M,Burgos M,Langlet L,Jimenez C,Morant C,Manso
M. Low temperature preparation of high-refractive index
and mechanically resistant sol–gel TiO
2
layers for multi-
player antireflective coating applications. J Sol Gel Sci Technol,
in press.
[14] Burgos M,Langlet M. Condensation and densification mechan-
ism of sol gel TiO
2
layers at low temperature. J Sol–Gel Sci
Technol 1999;17:267–76.
M. Manso et al. / Biomaterials 23 (2002) 349–356
355

[15] Burgos M,Langlet M. The sol gel transformation of TIPT
coatings: a FTIR study. Thin Solid Films 1999;349:19–23.
[16] Mante FK,Baran GR,Lucas B. Nanoindentation study of
titanium single crystals. Biomaterials 1999;20:1051–5.
[17] Jae-Young Rho,Tsui TY,George M. Pharr. Elastic properties of
human cortical and trabecular lamellar bone measured by
nanoindentation. Biomaterials 1997;18:1325–1330.
[18] Oliver WC,Pharr GM. An improved technique for determining
hardness and elastic modulus using load and displacement sensing
indentation experiments. J Mater Res 1992;7:1564–83.
[19] Ogueta S,Olaizabal I,Santos I,Delgado-Baeza E,Garc
!ııa
Ruiz JP. Transgenic mice expressing bovine GH develop arthritic
disorder and self-antibodies. J Endocrinology 2000;165: 329–36.
[20] Samuels LE. Microindentations in Metals. In: Microindentation
techniques in materials science and engineering. American Society
for testing materials,Philadelphia,1986;5–25.
[21] Jamting AK,Bell JM,Swain MV,Wielunski LS,Clissold R.
Measurement of the micromechanical properties of sol–gel TiO
2
films. Thin Solid Films 1998;332:189–94.
[22] Bendavid A,Martin PJ,Takikawa H. Deposition and modifica-
tion of titanium dioxide thin films by filtered arc deposition. Thin
Solid Films 2000;360:241–9.
[23] Bally AR,Hones P,Sanjines R,Schmid PE,Levy F. Mechanical
and electrical properties of fcc TiO
1+x
thin films prepared by r.f.
reactive sputtering. Surf Coat Technol 1998;1–3:166–70.
M. Manso et al. / Biomaterials 23 (2002) 349–356
356
Wyszukiwarka
Podobne podstrony:
Chemical Composition and in Vitro Antifungal Activity Screening
Apoptosis Induction, Cell Cycle Arrest and in Vitro Anticancer Activity
2001 In vitro fermentation characteristics of native and processed cereal grains and potato
Wheat bread enriched with green coffee – In vitro bioaccessibility and
The pathogenesis of Sh flexneri infection lessons from in vitro and in vivo studies
2000 Glucose Based Oligosaccharides Exhibit Different In Vitro Fermentation Patterns and Affect In V
in vitro, studia rolnictwo, rok IV
Kultury in vitro roslin rozmnazanie klonalne
In vitro antitumor actions of extracts
In vitro truskawka id 212540 Nieznany
1 1 Podstawowe definicje; główne kierunki przemian rozwojowych roślinnych tkanek in vitro(1)
53 755 765 Effect of Microstructural Homogenity on Mechanical and Thermal Fatique
Życie ludzkie świętość czy zabawka nt in vitro
Functional improvements desired by patients before and in the first year after total hip arthroplast
Screw mechanisms and joints
In vitro, Sem 1, TMR3
6 Hodowle komórek skóry w warunkach in vitro
więcej podobnych podstron