
1
Human Probiotics & Functional Foods
A legal perspective
Bianca Herr
Regulatory and Technical Consultancy Services
Leatherhead Food International

2
→
Probiotics were defined by a group of
experts convened by the
Food and
Agriculture Organization of the United
Nations (FAO)
as
"live micro organisms administered in
adequate amounts which confer a
beneficial health effect on the host".
What are Probiotics?
What are Prebiotics?
→
“specific indigestible substances which
selectively support the growth of
bifidobacteria and possibly other micro
organisms in the intestines.".
e.g. inulin, oligofructose
3
What are Synbiotics?
→
“products in which probiotics and
prebiotics are combined to produce a
synergistically beneficial effect.".
What is a Probiotic foodstuff?
→
According to probiotic working group of
German Federal Institute for Consumer
Health Protection and Veterinary medicine
BgVV (now BfR):
‘Probiotic foodstuffs are foods containing
probiotics in an amount sufficient to produce
probiotic effects when such food is ingested.’
4
→
Help to fight bacteria with pathogenic
effects in a natural way and at the same
time,
→
Beneficial for human and animal
digestive system which supports
general well-being
Why add
probiotics
?
Also..
→
The EU market alone for probiotics and
yoghurts was worth £ 8 bn in the first half of
decade.
→
high consumer demand
→
market worth over 10 bn by 2010
→ growth in food market 1 – 2 % per year!
Why add
probiotics
?
5
→ Traditional cultures used:
→
L. acidophilus group – L. acidophilus and L.
johnsonii
→
L. casei group & L. reuteri
→
Bifidobacterium spp. – B. animalis [B.
bifidum], B. longum, B. lactis, B. infantis, B.
breve
Probiotic cultures
Situation in Italia
→
Definitions of pre- and probiotics
→
Widely accepted in food supplements >50 on the market
→
Yogurt - probiotic cultures must not substitute the fermentative
action of Lactobacillus bulgaricus and Streptococcus
thermophilus
→
When fermentation process is just carried out by other cultures→
‘yogurt’ but ‘fermented milk’
•
Guidelines on composition and labelling available on:
www.ministerosalute.it/alimenti/dietetica/dieApprofondimento.jsp
?lang=italiano&label=int&id=388
•
www.ministerosalute.it/alimenti/nutrizione/linee.jsp?lang=italiano
&label=pro&id=398&dad=s
6
Situation in Magyarország
→
Definitions are provided by Codex Alimentarius
Hungaricus 2-51 of Dairy products
→
Use of pro- and prebiotics widely used and
accepted in foods and food supplements in
Hungary
→
ca. 40 food supplement products
Situation in Deutschland
→
Use of pro- and prebiotics widely used and
accepted in Germany
→
ca. 40 food supplement products, whereas
categorization as foodstuff or medicine
depending on concentration
7
Situación en España
→
No existing legislation or guidance for prebiotics and probiotics
→
According to survey (2003), every year 16 % increase in sales of pre-
and probiotic products
→
Prebiotics (Fructooligosaccharides-FOS) are added among others to
dairy products, beverages, biscuits and bread
→
At present time the only probiotic foodstuff in the market are :
→
Yogurt, exclusively with Streptococcus thermophilus and
Lactobacillus bulgaricus
or acidofilus
→
other fermented milks with Bifidobacteria, Lactobacillus casei
inmunitass, etc.
→
Drinks containing fruit juices, fermented milk and bacterial cultures.
EFSA – Qualified Presumption of
Safety
→
Working document (2003) “On a generic approach to the safety
assessment of microorganisms used in feed/food and feed/food
production” proposed QPS – a system to evaluate groups of
microorganisms to use as basis for establishing safety of
individual products
→
Aim to harmonise situation without introducing unnecessary
legislative burden, but allowing for safety concerns to be
addressed
– Address lack of harmonisation, proportionality (is there really
a risk?) and recognition of familiarity of microorganism
→
Intended as similar to USA’s GRAS system
→
Streamline and provide quicker route to market for safe
microorganisms
8
QPS - Principles
→
A group of organisms could be considered safe for use, provided:
– Their
identity
could be established →
taxonomy
– There is sufficient
familiarity
on which to establish safety →
Body of knowledge
– There are no known pathogenic strains (or knowledge allows
to exclude existence of these)
→
Strains given QPS status would still be subject to qualifications –
“safe provided that…” (absence of antibiotic resistance,
restricted use etc.)
→
Strains not meeting conditions for QPS assessed case-by-case
QPS - Principles
Closed consultation in March 2007:
→
List of microorganisms already notified to EFSA
→
List of taxonomic units proposed for QPS status
→
Assessment of Bacillus Bacteria with respect to QPS
→
Assessment of gram positive non-sporulating bacteria
with respect to QPS
→
Assessment of yeasts with respect to QPS
→
Assessment of filamentous fungi with respect to QPS
http://www.efsa.europa.eu/EFSA/efsa_locale-
1178620753812_1178620759439.htm
9
Nutrition & Health Claims
Regulation
→
Regulation (EC) No 1924/2006
of the
European Parliament and of the Council of
20 December 2006 on nutrition and health
claims made on foods
– Corrigendum published 18 January 2007, into
force 19 January 2007, applies from 1 July
2007.
Regulation on Nutrition &
Health Claims – key areas
→
Covers commercial communications (labelling, advertising,
presentation, menus, branding)
→
General principles and conditions for all claims
→
Lists authorised nutrition claims (Annex)
→
EC register of well established health claims (Article 13)
→
Pre-market approval system (EFSA) for disease risk
reduction and other health claims (Article 15 - 18)
10
General Conditions – Article 5
→
The substance must be present in the final
product in a quantity that will produce the
effect claimed (compare BgVV definition!)
….’Survival’ issues of the bacteria?
→
The average consumer must be able to
understand the beneficial effect.
Scientific substantiation for
claims -
Article 6
→
Based on and substantiated by generally
accepted scientific evidence.
→
Justify the use of the claim.
→
Produce all relevant elements and data
establishing compliance with this Regulation.
11
Other
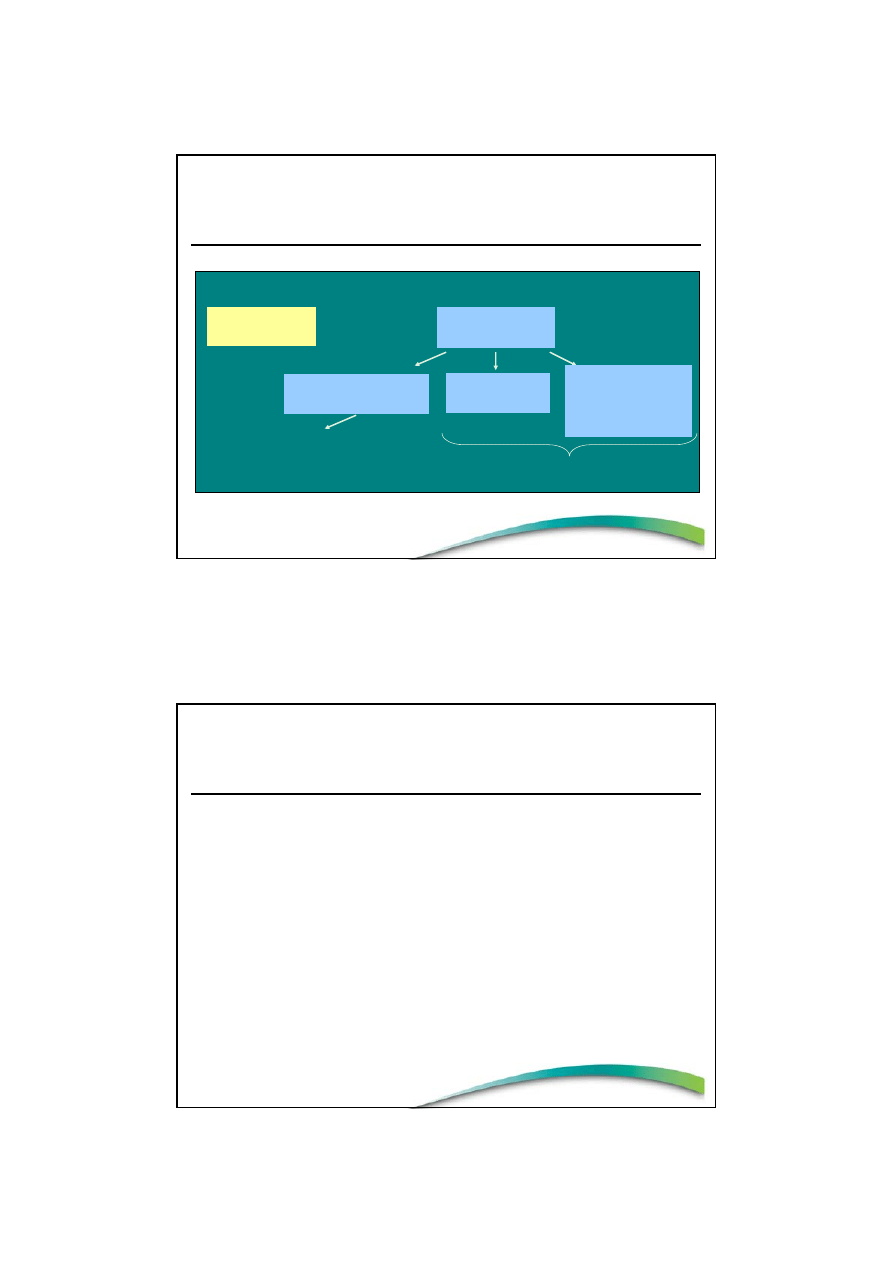
NUTRITION
CLAIMS
Article 13 claims
(well-accepted)
Pre-market approval (EFSA)
(Articles 15–18)
HEALTH
CLAIMS
Disease
risk-reduction
and Children
(Article 14)
Positive list
Claim Categories
Accepted Nutrition Claims
• Low energy
• Energy-reduced
• Energy-free
• Low fat
• Fat-free
• Low saturated fat
• Saturated fat-free
• Low sugar
• Sugar-free
• With no added sugars
• Low sodium/salt
• Very low sodium/salt
• Sodium-free/Salt-free
• Source of fibre/High fibre
• Source of protein
• High protein
• Source of vitamins/minerals
• High vitamins/minerals
• Contains name of nutrient
• Increased (name of nutrient)
• Reduced (name of nutrient)
• Light/lite
• Naturally/Natural
And any claim likely to have the
same meaning..

12
Criteria for Nutrition Claims
‘CONTAINS’ (name of nutrient or other substance)
May only be used where it complies with the general
principles:
→
Must not be false or misleading
→
Must not give rise to doubt about the
safety/nutritional adequacy of other foods
→
Must not
state or imply that a balanced and varied
diet cannot provide appropriate quantities of nutrients
in general
→ Consensus that “contains probiotics” not a nutrition
claim
→ Contains a specific strain - uncertain
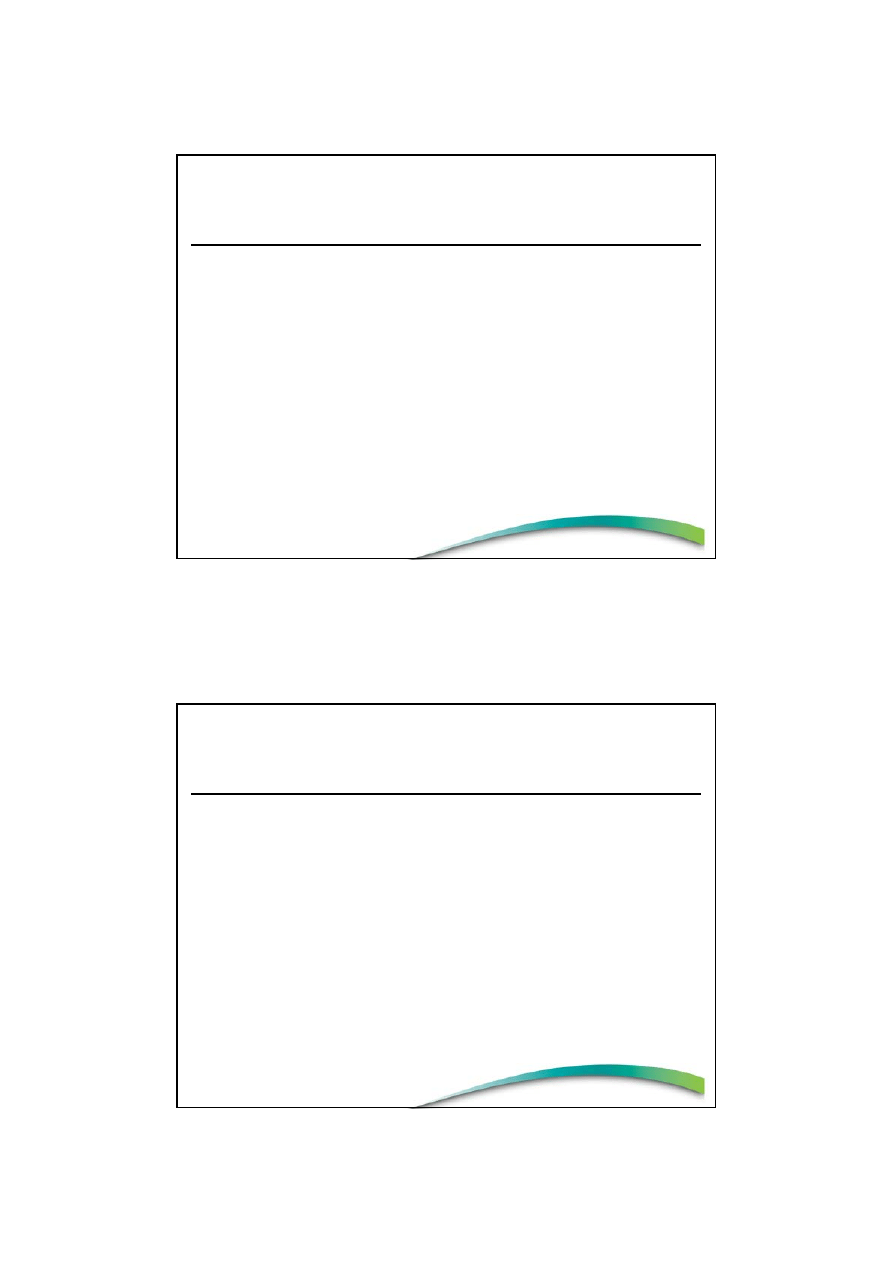
Other
NUTRITION
CLAIMS
Article 13 claims
(well-accepted)
Pre-market approval (EFSA)
(Articles 15–18)
HEALTH
CLAIMS
Disease
risk-reduction
and Children
(Article 14)
Positive list
Claim Categories
13
Health Claims
Definition ad Reg. 1924/2006:
“any claim that states,
suggests or implies
that a
relationship exists between a food category, a food
or one or its constituents and health”
“Health” means a state of complete physical,
mental and social well-being and not merely the
absence of disease or infirmity.
(World Health Organisation Constitution)
Conditions for Use of Nutrition
and Health Claims –
Article 4
9
Nutrient profiles - 19 January 2009
Taking into account:
→
Quantities of certain nutrients e.g. fat, saturated fatty
acids, trans-fatty acids, sugars, salt/sodium
→
Role and importance of the food in the diet
→
Overall nutritional composition and presence of nutrients
with recognised effect on health
14
Generally Permitted Health
Claims -
Article 13
Future EC register of permitted health claims describing
or referring to:
→
the role of a nutrient or other substance in growth,
development and the functions of the body; or
→
psychological and behavioural functions; or
→
slimming or weight-control or a reduction in the sense
of hunger or an increase in the sense of satiety or to
the reduction of the available energy from the diet,
ARTICLE 13 Claims
Member states to provide the Commission with
lists of claims (diet and health relationships) 12
months after entry into force of the law with
references to the relevant scientific justification
and conditions applying to them.
31
st
January 2008
Commission (based on opinion from EFSA) shall
compile a “COMMUNITY LIST OF PERMITTED
CLAIMS” 3 years after the law enters into force.
by
31
st
January 2010

15
Reduction of Disease Risk Claims and
Claims referring to Children’s
Development and Health -
Article 14
→
May be made, subject to pre-market
authorisation
→
Disease risk reduction claims – must
include labelling/advertising statement
indicating that the disease has multiple
risk factors and altering one of these risk
factors may or may not have a beneficial
effect
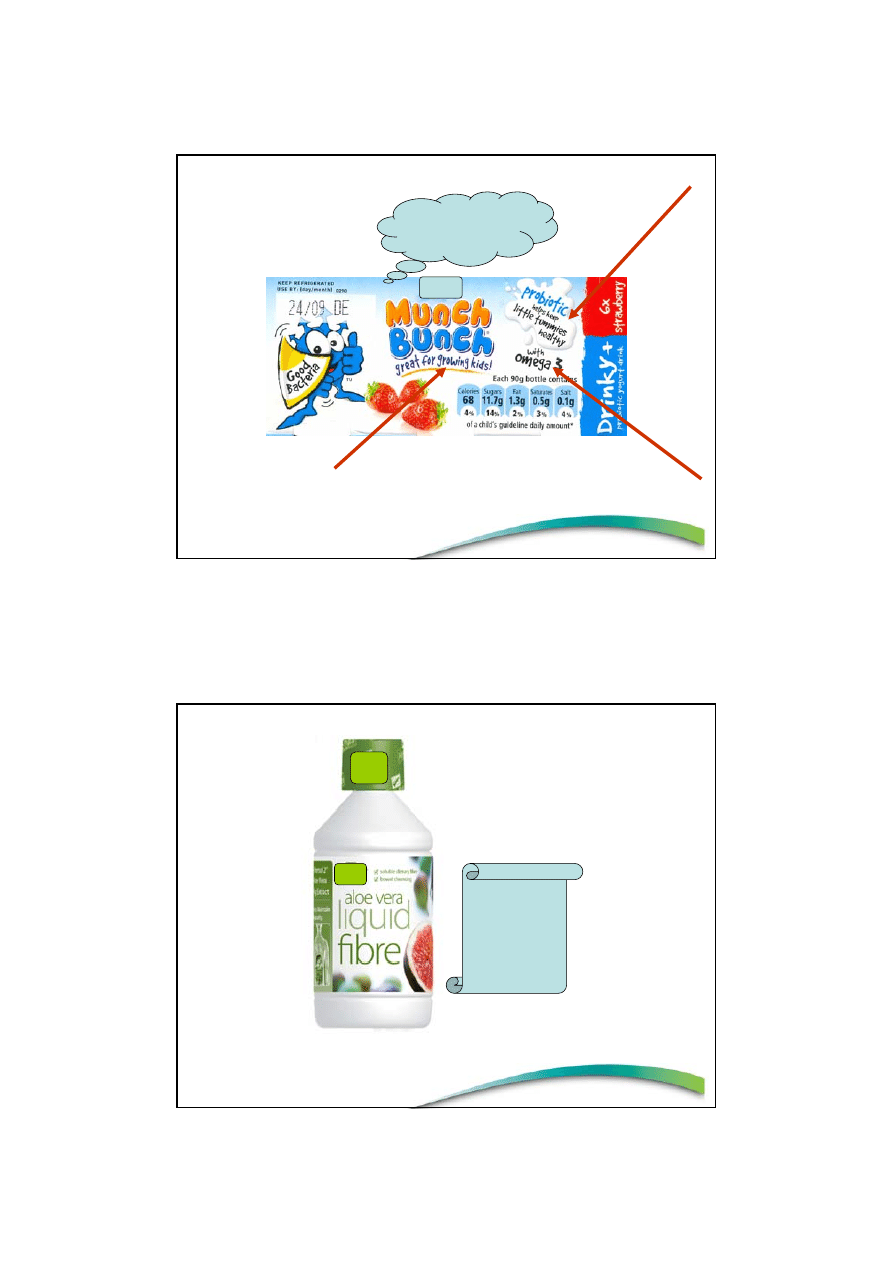
Contains a unique natural
culture, Bifidus Essensis,
specially selected by xx
researchers for its proven
benefits to your digestion.
Help keep your body in
balance with Probiotics
Understandable?
Understandable?
16
Article 14 claim?
Bowel
cleaning?
17
Submitted claims to EFSA by
CIAA
• For approx.
75!
individual probiotic
bacteria claims have been submitted:
→‘enhances/supports natural defences’
→‘helps balance/maintain the intestinal
flora
→‘improves your intestinal transit’
Prohibited Claims
x
No claims on alcoholic beverages more than 1.2%
alcohol (other than those which refer to low alcohol or a
reduction in alcohol or energy content).
x
Suggestions
health affected by not consuming a food
x
References
to rate or amount of weight loss
x
References to
recommendations of individual doctors or
health professionals
x
X% Fat free
18
Check list
• Nutrient profiles
• “Contains” claims
• Interpretation of implied claims
• Standard of scientific evidence
• Understanding of the average
consumer
Health Claims
– Additional Labelling
→
A statement indicating importance of a
varied diet
→
The quantity to obtain claimed effect
→
Pattern of consumption to obtain claimed
effect
→
If anyone should avoid the food
→
Warnings
→
name of probiotic bacteria
19
Comparison to Japan
→
Since 1991 specific rules for functional foods
→
FOSHU – Foods for Specified Health Use
→
Every food needs approval from Japanese
Health Nutrition Food Association and
authorisation from the Japanese Ministry for
Health and Welfare.
→
Statement ‘ This is a food for specified health
use’
Food Safety
→
Regulation (EC) No. 178/2002
→
Food must be safe
→
Potential issues:
- Antibiotic resistant strains
- Side effects e.g. systemic infections
→
Regulation (EC) No. 1925/2006
→
Allows substances to be prohibited or restricted in
use
→
EFSA – qualified presumption of safety
20
The
Novel
Foods Regulation (EC)
258/97
Art 1(2): Definition
“Foods or food ingredients which
has not been available on the EU market
for
human consumption to a significant degree
before 15 May 1997”
→
Mandatory pre-market safety assessment
Protective Cultures in meat
and dairy products
Safe & clean?
21
History
• Traditional ‘biotechnology’
used since 1000’s of years –
preservation of food with
lactic acid fermentation
• Kopenhagen and Kiel 1890 -
first starter cultures to
produce thick sour cream
What are protective cultures?
According to Danisco:
Protective cultures are bacteria
especially selected and developed for
their ability to control the growth of
pathogenic and/or spoilage
microorganisms in fermented foods
.
22
What are protective cultures?
Examples of protective cultures:
→
Lactobacillus plantarum
,
→
Lactobacillus rhamnosus
,
→
Lactobacillus sakei
,
→
Lactobacillus paracasei
and
→
Propionibacterium freundenreichii
subsp. shermanii
.
Why add protective cultures?
→
Substitution of additives –
Clean labelling
→
Extending shelf life
→
Influence taste
→
help to meet food safety
→
microbiological requirements
23
Meat products
→
Lactic acid bacteria Lactobacillus sakei used
in ham products
→
39 days fermentation achieved:
→
Double shelf life
→
Excellent sensory results
Dairy Applications
Growth control of yeasts and
moulds
Fresh fermented dairy products
(yoghurt, sour cream, quark,
cottage cheese)
Growth control of undesired
bacteria such as Leuconostoc,
enterococci and
heterofermentative lactobacilli
Semi-hard and hard cheese
Growth control of Listeria
Soft and smear cheese
Functionality
Dairy applications
24
Meat Applications
Growth control of Listeria
Cooked meats, fresh ground
meats, etc.
Growth control of Listeria
Dry and semi-dry cured meats
Functionality
Meat applications
Applications
→
Used alone or in association with
ripening starters, protective cultures can
also bring:
- Texturising,
- Colouring or
- Flavouring
25
Regulatory status
→
long history of safe use in food where
they can be used as ingredients.
!!
Local regulations should always be
consulted concerning the status of
these products as legislation regarding
their use in food may vary from country
to country.
Food Safety
→
Regulation (EC) No. 178/2002
→
Article 14 - Food must be safe
→
Potential issues:
- Antibiotic resistant strains
- strains producing metabolites not safe to
human
→EFSA – qualified presumption of safety!
26
Food Safety
→
problems with individual Lactobacillus
species – clinical isolates
→
found in people with impaired immune
function
→
not identical with strains used in food
Importance lies within the
specific
genetic makeup
of the strain
!!
Microbiological Requirements
→
Hygiene Regulations No. 852/2004 and No.
853/2004
→
Regulation No. 2073/2005 on microbiological
criteria for foodstuffs, Listeria
monocytogenes, Salmonella, E. coli
- Provisions on meat and meat products
- Provisions on dairy products
27
Clean labelling
Clean labels – not regulated as such
Concept causes confusion
Focus on…
→
Labelling requirements
→
Allergens
→
Additive versus ingredient
→
Definition of ‘natural’ and labelling claims
Food Additives
→
Definition laid down in framework
Directive No. 89/107/EC
→
Additives legislation harmonised on EU
level via EC Directive No. 95/2/EC
→
Authorisation procedure laid down in No.
89/107/EC
28
Food Additives
Definition:
‘any substance
not normally consumed as a food
in
itself and not normally used as a characteristic
ingredient of food whether or not it has nutritive
value, the
intentional addition
of which to food for a
technological purpose
in the manufacture,
processing, preparation, treatment, packaging,
transport or storage of such food results, or may be
reasonably expected to result, in it or its by-products
becoming
directly or indirectly a component
of
such foods’
Preservatives
Clean labelling?
→
Nisin is a bacteriocin,
→
produced by certain strains of the bacterium
Lactococcus (Streptococcus) lactis ssp. Lactis
.
→
Permitted via Directive No. 95/2 as preservative
for ripened cheese and processed cheese,
clotted cream and mascarpone.
→
Maximum levels apply.
29
Starter cultures
→
traditionally used starter cultures are not
classified as additives.
→
recommendation for research on
traditionally used starter cultures having
effects on preservation, via acid or
bacteriocin – if regarding clean labelling
Preservatives & Colours
Clean labelling? Possible example for a starter
culture as a replacement for nitrites in
sausages
→Staphylococcus carnosus
and
staphylococcus
carnosus
combined with
staphylococcus
carnosus vitulinus
depending on production
process
→
Current re-evaluation of food colours by
EFSA
30
Proposals for Additives, Enzymes and
Flavourings
Regulations – July 2006
Current legislative procedure for:
→
Proposal establishing a common authorisation procedure for food additives, food
enzymes and food flavourings 2006/0143 (COD), (amended proposal on
24/10/2007)
→
Food Additives (consolidated Regulation) 2006/0145 (COD), (amended proposal on
24/10/2007)
→
Enzymes 2006/0144 (COD), (amended proposal on 24/10/2007),
→
Flavourings Regulation and certain food ingredients with flavouring properties for use
in and on foods 2006/0147 (COD), (amended proposal on 24/10/2007)
http://ec.europa.eu/food/food/chemicalsafety/additives/prop_leg_en.htm
What other legislation needs
to be considered?
31
GMO
→
GM food and feed Regulation (EC) 1829/2003
scientific assessment, authorisations and labelling of
GMOs and GM food and feed
→
Traceability and labelling of GMOs Regulation
(EC) 1830/2003
identification of GM products throughout supply chain
and accurate labelling in accordance with (EC)
1829/2003
Regulation (EC) 1829/2003
Applies to:
→
Food produced ‘from’ GMO or GMM
→
Food additives
→
Flavourings
→
Enzymes (not microbial)
→
animal feed / feed additives
E.g.flour, oils and glucose syrups will have to labelled as GM if
they are from a GM source
32
The
Novel
Foods Regulation (EC)
258/97
Art 1(2): Definition
“Foods or food ingredients which
has not been available on the EU market
for
human consumption to a significant degree
before 15 May 1997”
→
Mandatory pre-market safety assessment
The
Novel Foods Regulation (EC)
258/97
Art 1(2): Four current novel foods categories
a. Foods and food ingredients containing or consisting of GMOs **
b. Foods and food ingredients produced from, but not containing
GMOs **
c. Foods with a new/modified primary molecular structure
d. Foods consisting of or from micro-organisms/fungi/ algae
e. Foods from plants/animals obtained by traditional practices
but with no history of safe food use
f. Foods produced using a novel process
** removed from scope of (EC) 258/97 in April 2004 due to
1829/2003 on GM foods and feeds

33
Out of the scope of (EC) 258/97:
- Art 2 (1)) : Food additives,
Flavourings,
Extraction solvents
- ANY food on sale in the EU prior to May 1997
- Processing aids
- Whole animals
- Products with medicinal function***
*** UK Medicine Healthcare Products Regulatory Agency
Borderline unit http://www.mhra.gov.uk
The Novel Foods Regulation (EC)
258/97
Thank you for your attention!
bherr@leatherheadfood.com
Wyszukiwarka
Podobne podstrony:
Functional Foods Presentation
Verb form and function
Comparison of Human Language and Animal Communication
Bezhanshivili Lattices and Topology (Lecture Presentation)
Complex Numbers and Functions
Plant Structure and Function F05
Operation And Function Light Anti Armor Weapons M72 And M136 (2)
The Heart Its Diseases and Functions
Human Papillomavirus and Cervical Cancer Knowledge health beliefs and preventive practicies
Human Relations and Social Responsibility
Map Sensor Purpose and Function
HUMAN RIGHTS AND THEIR IMPORTANCE goood
30 Szczupska Maciejczak Jarzebowski Development and Functio
Chater N , Oaksford M Human rationality and the psychology of
Detection and Function of Opioid Receptors on Cells from the Immune System
Verb form and function
SSP 406 DCC Adaptive Chassis Control Design and Function
więcej podobnych podstron