
* Corresponding author. Tel.: 0044 0151 706 5606; fax: 0044 151
706 5803.
Biomaterials 20 (1999) 631 — 637
The corrosion behaviour of Ti—6Al—4V, Ti—6Al—7Nb
and Ti—13Nb—13Zr in protein solutions
M.A. Khan, R.L. Williams*, D.F. Williams
Department of Clinical Engineering, University of Liverpool, Duncan Building, P.O. Box 147, Liverpool L69 3GA, UK
Received 6 March 1996; accepted 13 October 1998
Abstract
Ti alloys are used in orthopaedic applications owing to their appropriate mechanical properties and their excellent corrosion
resistance. The release of titanium and the other alloying elements into the surrounding tissue has been reported due either to passive
corrosion or accelerating processes such as wear. Since the passive layer can be broken down in certain circumstances by wear it is
important to study the ability of these alloys to repassivate in biological environments, in particular in the presence of proteins, and
evaluate how the repassivated surface may vary from the original surface. In this study we investigated the ability of Ti—6Al—4V,
Ti—6Al—7Nb and Ti—13Nb—13Zr to repassivate in phosphate buffered saline (PBS), bovine albumin solutions in PBS and 10% foetal
calf serum in PBS at different pH values and at different albumin concentrations. It was found that an increase in pH had a greater
effect on the corrosion behaviour of Ti—6Al—4V and Ti—6Al—7Nb than on Ti—13Nb—13Zr in PBS and that the addition of protein to
the PBS reduced the influence of pH on the corrosion behaviour of all the alloys. The effect of the corrosion and repassivation was
investigated by measuring changes in the surface hardness of the alloys and it was found that corrosion reduced the hardness of the
surface oxides of all the alloys. In PBS the reduction was smallest for Ti—6Al—4V and largest for Ti—13Nb—13Zr and that corrosion in
protein solutions further reduced the hardness of the surface oxides. This effect was greater for Ti—6Al—4V and Ti—6Al—7Nb than for
Ti—13Nb—13Zr. In conclusion, proteins in the environment appear to interact with the repassivation process at the surface of these
alloys and influence the resulting surface properties.
1999 Elsevier Science Ltd. All rights reserved
Keywords: Mechanical properties; Corrosion
1. Introduction
Ti—6Al—4V alloy has been used extensively for many
years as an implantable material mainly in the applica-
tion of orthopaedic prostheses. It has been shown on
numerous occasions that its corrosion resistance is far
superior to many of the alternatives presently available
such as stainless steel alloys and even better under most
circumstances than alloys based on cobalt-chromium
[1, 2]. Recently, however, much concern has developed
over the issue of biocompatibility with respect to the
dissolution of aluminium and vanadium ions and the
possibility of any toxic effects [3, 4]. Consequently, other
titanium alloys are currently being considered as alterna-
tives to the Ti—6Al—4V alloy. In the present study, two
such alloys (i.e. Ti—6Al—7Nb and Ti—13Nb—13Zr) have
been investigated in terms of their relative corrosion
resistance compared to that of the Ti—6Al—4V alloy.
The Ti—6Al—7Nb was developed using the alloying
element niobium to replace vanadium in the Ti—6Al—4V
alloy, since both vanadium and niobium are beta
amorphous (i.e. have similar phase relations) with body-
centred cubic (b.c.c.) titanium, and thus, can both act as
suitable beta phase stabilizers, suppressing the formation
of the alpha phase which is a close-packed hexagonal
(c.p.h.) structure [5]. Recent studies have also mentioned
the excellent short- and long-term biocompatibility of
niobium [6, 7]. The aluminium continues to fulfil its role
as a useful alpha phase stabilizer, and thus, maintain the
improved mechanical properties over pure titanium.
The other material being considered is Ti—13Nb—13Zr
which once again relies on niobium as a beta phase
stabilizer. The other alloying element, zirconium, is one
that is unique in that it is isomorphous with both the
alpha and beta phases of titanium [5]. A combination
0142-9612/99/$ — see front matter
1999 Elsevier Science Ltd. All rights reserved.
PII: S 0 1 4 2 - 9 6 1 2 ( 9 8 ) 0 0 2 1 7 - 8

of these two alloying elements has made it possible to
develop a structure that is a ‘near’ beta phase supposedly
possessing a superior corrosion resistance over the
alpha—beta phase alloys mentioned earlier, but one that
still has enough alpha phase present in the final structure
to provide the necessary mechanical strength. It has been
proposed that Ti—13Nb—13Zr alloy is more favourable
for orthopaedic implants than Ti—6Al—4V alloy because
of its superior corrosion resistance and biocompatibility.
Reasons for this superiority have included the fact that
less metal ion release is likely to occur during sponta-
neous passivation of Ti—13Nb—13Zr alloy because the
corrosion products of the minor alloying elements,
niobium and zirconium, are less soluble than those of
aluminium and vanadium. Also, that the passive oxide
layer on the surface of the alloy is more inert consisting of
a dense rutile structure providing greater protection to
the underlying alloy [8, 9].
It is well known that proteins affect the corrosion
behaviour of some metals, and that their presence
can either inhibit or accelerate the corrosion phenomena.
They are known to behave differently with different
metals, since their role in a corrosive environment
is governed by many factors such as the surface chemistry
of the metal, protein adsorption characteristics, inter-
action of protein molecules with other ions present
in the electrolyte solution to produce organic com-
plexes, and the transport of anionic and cationic
charges around and away from the local environment
[10—12].
In this study we have assessed the corrosion resistance
of the alloys using cyclic anodic polarisation techniques
to break down the passive layer and then allow repas-
sivation to occur. The value of the breakdown potential
for Ti alloys is difficult to assess owing to the instability
of the aqueous electrolytes at the high potentials re-
quired. The ability of the surfaces to repassivate in the
environment, as measured by the hysteresis in the cyclic
polarisation curve, is a better measure of the corrosion
behaviour of these material. For these reasons we have
assessed the corrosion resistance of these alloys by re-
cording the difference between the breakdown potential
(E) and the repassivation potential (E). This study
shows that in different environments (i.e. at different pHs
or in the presence of proteins) the ability of the alloys to
repassivate is affected. We have used hardness measure-
ments to evaluate the surface oxide formed during repas-
sivation and to determine that it is different from the
original surface oxide.
2. Materials and methods
The titanium alloys were obtained from different
sources: The Ti—6Al—4V alloy from IMI Titanium Ltd.,
England, the Ti—6Al—7Nb alloy from Sulzer Medical
Technology Ltd., Switzerland and the Ti—13Nb—13Zr
alloy from Smith and Nephew Richards inc., USA.
All materials were received in the annealed condition
in the form of rods between 10 and 15 mm in diameter.
Disc specimens were cut from the rods with a thickness of
approximately 5 mm. A wire lead was attached to the
back of each disc using a small amount of conducting
paste. Each specimen was mounted into a thermosetting
resin mould to form a 30 mm diameter rod. Each speci-
men was polished down to 1200 grit specification and
then polished using 6, 1 and 0.25
lm diamond paste.
A portion of the surface around the margins between the
alloy and the resin was covered with an insulating laquer
leaving a specimen area of 5 mm
.
The electrolytes used included:
E Phosphate-buffered saline (PBS) solution at three pH
levels 5, 7.4 and 9.
E Phosphate-buffered saline (PBS) solution #1 mg/ml
bovine albumin (Sigma, UK) at three pH levels 5, 7.4
and 9.
E Phosphate-buffered saline (PBS) solution#albumin
at concentrations of 0.1, 1 and 10 mg/ml at pH 7.4.
E Phosphate-buffered saline (PBS) solution#10% foe-
tal calf serum at pH 7.4.
The electrolyte cell was maintained at 37°C through-
out the tests using a suitable water bath. All tests were
carried out under aerated conditions.
Cyclic polarisation data were obtained in the form of
potential vs. current density curves between a range of
0—5000 mV using an ‘AUTOSTAT’ computer controlled
potentiostat. The potential was increased at a rate of
200 mV/min, starting with the rest potential (E0). Tripli-
cate measurements were obtained in all cases. The elec-
trolyte cell was made of glass and had a capacity of
200 ml. The specimen acted as a ‘working’ electrode
(anode) and was placed in the cell facing a platinum
‘auxiliary’ electrode (cathode). A saturated calomel elec-
trode was used as the ‘reference’ electrode. The corrosion
resistance of the alloys was evaluated by measuring the
difference between the breakdown and repassivation po-
tentials, E and E, respectively. E was noted to be the
value at which the potential-current density plot was seen
to show a sudden increase in current density. E was
noted as the value of potential at which the current
density returned to the passive current density on the
reverse scan. Generally, smaller differences between these
two values indicated a better corrosion resistance. Fol-
lowing the corrosion tests the surfaces of the specimens
were examined under a microscope for evidence of pits.
Hardness
measurements
were
undertaken
using
a Vickers hardness indentation machine to obtain values
of the macrohardness of the surface of each alloy both
prior to and following corrosion. Measurements were
undertaken on three specimens in all cases to ensure
reproducibility and the average of four or five indents
was calculated for each surface.
632
M.A. Khan et al. / Biomaterials 20 (1999) 631—637
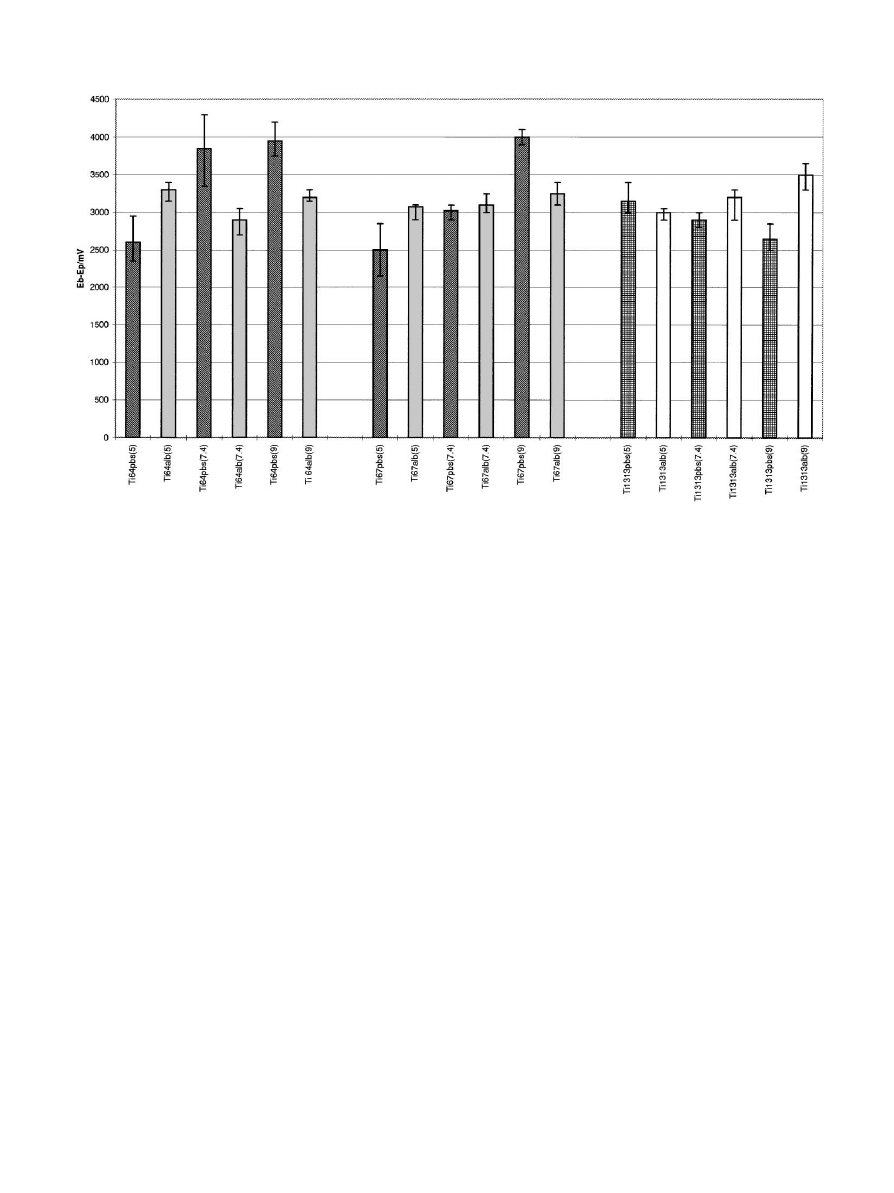
Fig. 1. E—E values for all three Ti alloys in PBS and 1 mg/ml bovine albumin solution at different pH values, presented as the mean and the range of
three measurements. Ti64"Ti—6Al—4V, Ti67"Ti—6Al—7Nb, Ti1313"Ti—13Nb— 13Zr, PBS"phosphate-buffered saline, Alb"bovine albumin,
the value in parenthesis is the pH of the electrolyte.
3. Results
Figures 1 and 2 present the corrosion resistance data
for the three alloys in different environments. From
Fig. 1 the corrosion resistance of the alloys (i.e. E—E
values) at three different pH levels (i.e. 5, 7.4 and 9) can be
evaluated. Generally, it was noticed that as the pH level
increased in PBS, the corrosion resistance of Ti—6Al—4V
and
Ti—6Al—7Nb
decreased,
whereas
that
of
Ti—13Nb—13Zr was seen to increase but only slightly.
When albumin, at a concentration of 1 mg/ml, was added
the effect of pH on the corrosion resistance of Ti—
6Al—4V and Ti—6Al—7Nb was reduced whereas for
Ti—13Nb—13Zr the addition of albumin caused a de-
crease in corrosion resistance as the pH increased,
although again the changes were small.
From Fig. 2 the corrosion resistance of the alloys,
in environments containing progressively increasing
concentrations of albumin, can be evaluated. It was ob-
served that by adding albumin (0.1 mg/ml) to PBS, the
corrosion resistance increased for Ti—6Al—4V. For
Ti—6Al—7Nb and Ti—13Nb—13Zr the changes in cor-
rosion resistance were smaller showing a slight increase
for Ti—6Al—7Nb and a slight decrease for Ti—13Nb—
13Zr. As the concentration of albumin increased
the corrosion resistance of Ti—13Nb—13Zr appeared
to remain at the same level, whereas, that for the other
two alloys tended to decrease. The Ti—6Al—4V alloy
was more corrosion resistant in all the albumin solu-
tions than in PBS, whereas the Ti—6Al—7Nb and
Ti—13Nb—13Zr alloys were generally less corrosion
resistant in protein solutions than in PBS. The addition
of foetal calf serum produced a similar effect with each
alloy as had the presence of higher concentrations of
albumin.
Figure 3 outlines the surface hardness of the three
alloys following corrosion in PBS with and without al-
bumin at varying pH levels and generally demonstrates
that corrosion of all the alloys in all the environments
reduced the surface hardness. For all three alloys the
surface hardness was least affected by corrosion in
PBS at pH 7.4. For Ti—6Al—4V the surface hardness was
only slightly reduced following corrosion in PBS at pH
7.4 but it was significantly reduced following corrosion
in PBS at pH 5 and more so at pH 9. For the other two
alloys the surface hardness was significantly reduced
following corrosion in PBS at pH 7.4 but also reduced
further following corrosion in PBS at both higher and
lower pH.
Following corrosion in albumin at pH 7.4 the surface
hardness of Ti—6Al—4V and Ti—6Al—7Nb was signifi-
cantly less than that following corrosion in PBS at pH
7.4, whereas, that for Ti—13Nb—13Zr was not signifi-
cantly affected by the addition of albumin at pH 7.4. At
M.A. Khan et al. / Biomaterials 20 (1999) 631—637
633
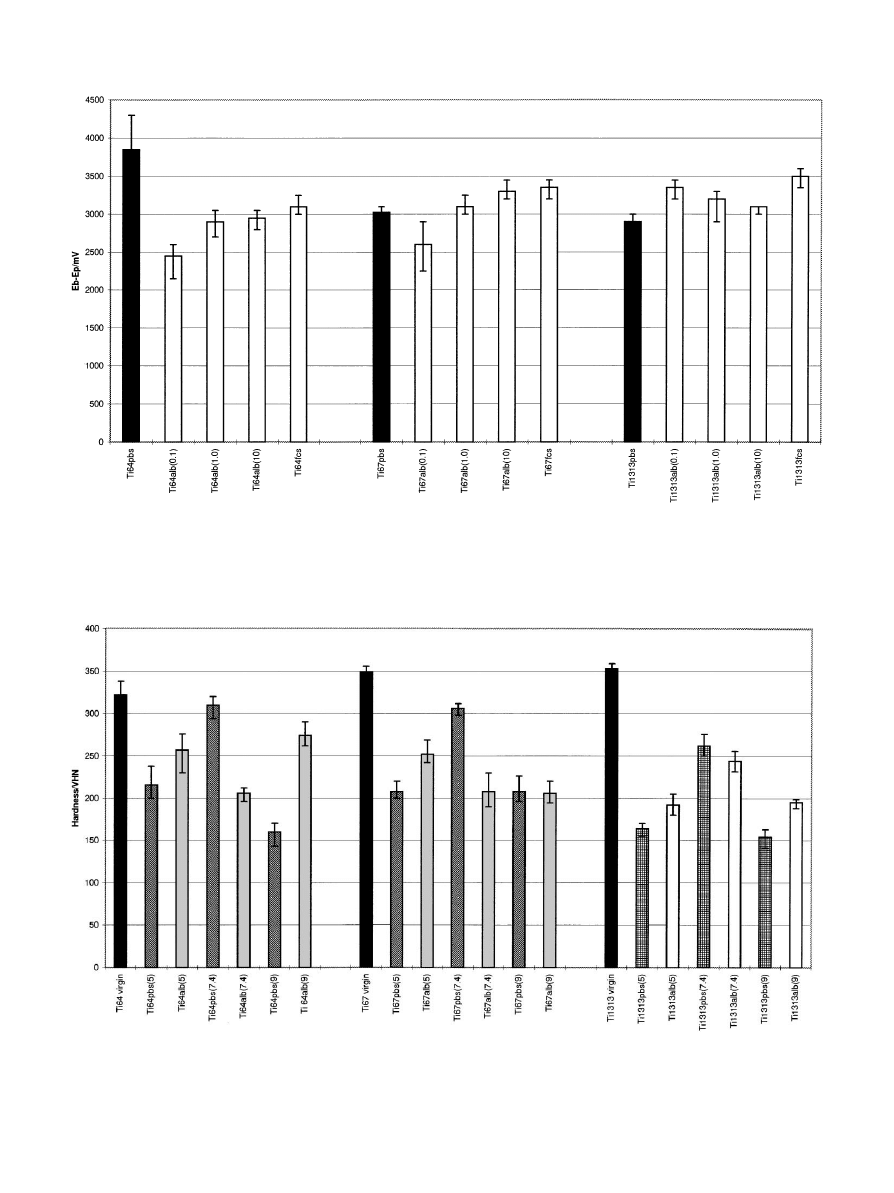
Fig. 2. E—E values for all three Ti alloys in PBS, bovine albumin at different concentrations and 10% foetal calf serum, presented as the mean and the
range of three measurements. Ti64"Ti—6Al—4V, Ti67"Ti— 6Al— 7Nb, Ti1313"Ti—13Nb—13Zr, PBS"phosphate-buffered saline, Alb"bovine
albumin, fcs"10% foetal calf serum and the value in parenthesis is the concentration of albumin in mg/ml.
Fig. 3. Vickers hardness values for all three Ti alloys before corrosion and following corrosion in PBS and 1 mg/ml bovine albumin solution at
different pH values, presented as the mean and standard deviation bars. Ti64"Ti— 6Al—4V, Ti67"Ti—6Al—7Nb, Ti1313"Ti —13Nb—
13Zr, PBS"phosphate-buffered saline, Alb"bovine albumin, the value in parenthesis is the pH of the electrolyte.
634
M.A. Khan et al. / Biomaterials 20 (1999) 631—637
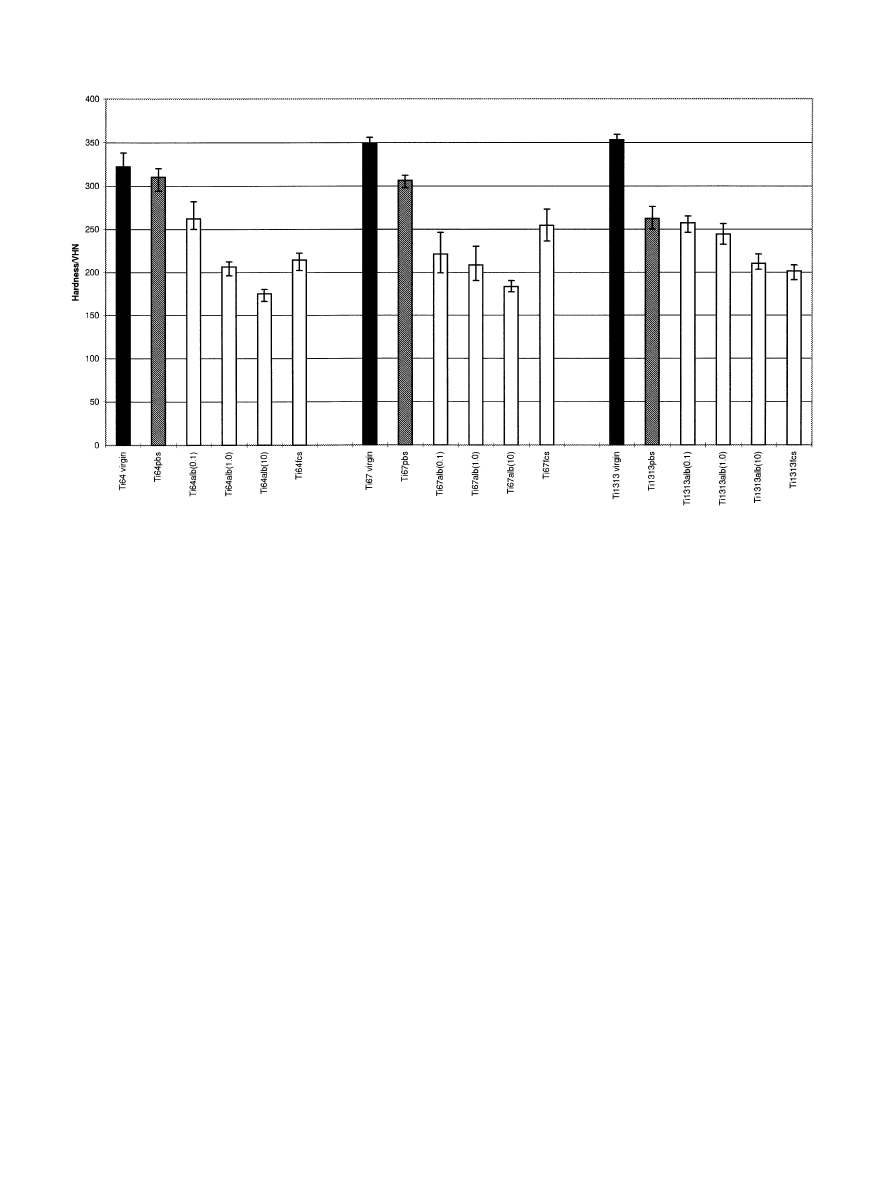
Fig. 4. Vickers hardness values for all three Ti alloys before corrosion and following corrosion in PBS, bovine albumin at different concentrations and
10% foetal calf serum, presented as the mean and standard deviation bars. Ti64"Ti —6Al—4V, Ti67"Ti—6Al—7Nb,
Ti1313"
Ti—13Nb—13Zr, PBS"phosphate-buffered saline, Alb"bovine albumin, fcs"10% foetal calf serum and the value in parenthesis is the concentra-
tion of albumin in mg/ml.
higher pH the surface hardness of Ti—6Al—7Nb was
unaffected by the presence of albumin and that of
Ti—13Nb—13Zr was only slightly increased. At low pH
the surface hardness of Ti—6Al—7Nb was influenced to
a greater extent by the presence of albumin than that of
Ti—13Nb—13Zr, resulting a slight increase in hardness.
At low pH the surface hardness of Ti—6Al—4V was not
significantly changed, however at high pH the presence of
albumin greatly increased the surface hardness following
corrosion.
Figure 4 shows the influence of protein concentration
on the hardness values following corrosion. It demon-
strates that the addition of protein reduces the hardness
of all surfaces following corrosion. Increasing the concen-
tration generally caused further reduction in the
hardness. The surface hardness of Ti—13Nb—13Zr was
reduced less than that of the other two alloys. When FCS
was added at a concentration of 10% the surface hard-
ness of Ti—6Al—4V and Ti—6Al—7Nb was not reduced as
much as in the albumin solutions whereas the result for
Ti—13Nb—13Zr was very similar to that for the surface
hardness of the repassivated surface following corrosion
in the albumin solutions.
Microscopic examination of all alloy surfaces showed
evidence of pits following cyclic polarisation.
4. Discussion
Titanium alloys are used in orthopaedic applications
and there is concern about the release and subsequent
build up of material in the tissues. It is unlikely that
the passive layers on these alloys will be broken
down electrochemically in the physiological environ-
ment. It is known, however, that the integrity of
the passive layer can be influence by wear. It is impor-
tant therefore to investigate the repassivation processes
of these alloys in a biological environment. In this
study we used electrochemical techniques to breakdown
the passive layer so that we could investigate the re-
passivation and the interaction of proteins with this
process.
To understand comprehensively how corrosion takes
place in terms of charge transfer at the metal/electrolyte
interface, it is necessary to consider an accepted model
for the charge distribution in terms of a charge double-
layer structure (Fig. 5). In this case, a double-layer is
present due to hydroxylated ions at the metal surface and
charge transfer can occur within the layer depending on
whether the metal oxide is stable (i.e. acting as a cathode)
or whether it is passivating up to and beyond removal of
its protective oxide layer (i.e. as an anode).
M.A. Khan et al. / Biomaterials 20 (1999) 631—637
635
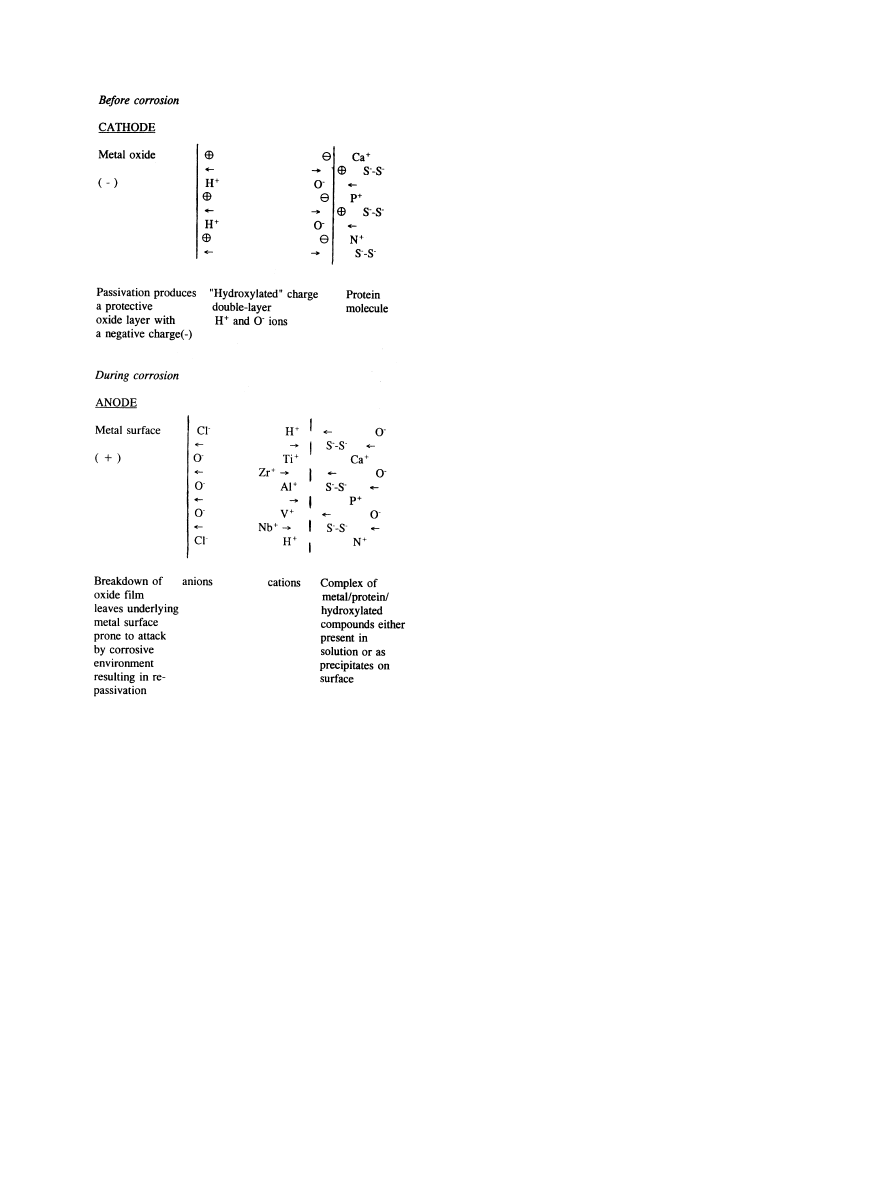
Fig. 5. Theoretical model of charged double layer showing the transfer
of charge at the metal/oxide/protein interfaces before and during the
corrosion process.
When the passive layer is broken the titanium alloy is
able to release ions into solution until the passive layer is
rebuilt. The action of rebuilding this layer results from
the chemical interaction of anions in the environment
reacting with the surface. It is hypothesised that any
anions in the environment may have a bearing on the
final composition of the repassivated layer and its ability
to form. It is this hypothesis that we have investigated in
this study. We have used cyclic polarisation studies to
assess the ability of the surface to repassivate and hard-
ness measurements to investigate the properties of the
repassivated surface.
Throughout this study we examined the surface under
a microscope following cyclic polarisation and found
evidence of pit formation. This evidence and the presence
of hysteresis during cyclic polarisation suggests that the
passive layer is being broken down and repassivated
under these experimental conditions. The difference be-
tween the breakdown potential and the repassivation
potential is related to the reactions that are taking place
at the surface of the material as repassivation occurs. In
this study we are using this measurement to elucidate
how proteins in the environment might be interacting
with the repassivating process and thus influencing the
properties of the passive layer. When the pH of the
environment changes the proteins will have a different
charge owing to their zwitterion character and therefore
it is reasonable to suspect that they may interact with the
repassivation process in a different way.
In an environment without the presence of proteins it
was observed that as the pH increased the corrosion
resistance of Ti—6Al—4V and Ti—6Al—7Nb was reduced.
In other words, the repassivation process became more
difficult and therefore there would be the possibility of
greater release of metal ions into solution. This could
occur with these two alloys owing to the solubility of Al
and V ions and their increased solubility in the presence
of increased hydroxyl concentration. It was observed
that, under the same conditions the corrosion resistance
of Ti—13Nb—13Zr increased slightly although the cha-
nges were very small. This could be explained by the
lower solubility of Zr and Nb ions, in comparison with Al
and V, thus repassivation occurs in preference to dissolu-
tion.
When albumin is added to the PBS at the three pH
levels it appears to reduce the effect of pH on the cor-
rosion resistance of Ti—6Al—4V and Ti—6Al—7Nb. Pro-
teins can interact with the corrosion reactions in several
ways and thus shift the position of equilibrium. For
example, proteins can bind to metal ions and transport
them away from the interface thus encouraging further
dissolution or proteins may adsorb onto the metal sur-
face thus restricting the diffusion of oxygen to the surface
thus make it harder for the surface to repassivate. Both
these mechanisms might be expected to decrease the
corrosion resistance of the alloy. Our data suggest that
the proteins are increasing the corrosion resistance of the
alloy, in particular, at the higher pH level. At these pH
levels albumin will have a negative charge since its iso-
electric point is 4.9. It may be possible that under these
conditions a metal/protein/hydroxide complex forms
and becomes adsorbed to the surface restricting metal
dissolution.
The properties of the repassivated surface may be
reflected in the hardness of that surface. For all the alloys
the virgin surface was the hardest and it is assumed that
this is a dense rutile Ti oxide surface following the polish-
ing and cleaning procedures. Following corrosion in PBS
at pH 7.4 the hardness of all the surfaces was reduced but
this reduction was small for Ti—6Al—4V and greatest for
Ti—13Nb—13Zr. As the pH of the PBS was increased or
decreased the hardness of the repassivated surface was
636
M.A. Khan et al. / Biomaterials 20 (1999) 631—637

significantly reduced for all the alloys suggesting that
the repassivated surface had a different character to
the original Ti oxide surface. When albumin was added
to the PBS at pH 7.4, following corrosion the hardness
of the
Ti—6Al—4V
and Ti—6Al—7Nb alloys
was
further reduced whereas that of the Ti—13Nb—13Zr
surface was only marginally reduced. This would
appear to suggest that at this pH the proteins were
interacting with the repassivation process to a greater
extent for Ti—6Al—4V and Ti—6Al—7Nb than for
Ti—13Nb—13Zr.
Increasing the protein concentration from 0.1 to
1 mg/ml was measured to have a small influence on the
corrosion resistance of the alloys. Further increases in
protein concentration did not appear to have a signifi-
cant effect. This can be explained from the knowledge
that such proteins are only sparingly soluble and that
once saturation has been reached in terms of corrosion,
there should be no further influence of additional protein
on corrosion [12].
The results showed that in the presence of whole serum
(10%) the corrosion resistance of Ti—6Al—4V improved
in comparison to that in PBS (in agreement with other
workers [1, 13]) and
the
corrosion resistance of
Ti—13Nb—13Zr and Ti—6Al—7Nb reduced. These results
are similar to those in albumin solutions which may
reflect the high proportion of albumin in whole serum.
The surface hardness of Ti—6Al—4V and Ti—6Al—7Nb
following corrosion in FCS, however, was higher
than those surfaces following corrosion in solutions
with higher concentrations of albumin. This result
is difficult to explain, but may reflect the complexity
of different protein interactions with the alloy sur-
faces.
It is difficult to relate the changes in hardness to the
corrosion resistance data except that changes in surface
hardness may relate to incorporation of species from
the electrolyte into the passive layer and the reactions
occurring to allow this incorporation may influence the
repassivation process and thus the position of the re-
passivation potential. The
hardness measurements
are useful in defining the extent to which the mechanical
integrity of the alloys has been affected following break-
down and repassivation. The reduction in hardness
of these alloy surface may be important in terms of
long-term wear resistance. The retention of the sur-
face hardness of Ti—13Nb—13Zr following repassiva-
tion in an environment containing proteins could point
to enhanced performance of this alloy, in comparison
with the other two, in situations where continual
removal of the passive layer and repassivation are occur-
ring.
5. Conclusion
(1) An increase in pH had a greater effect on the cor-
rosion behaviour of Ti—6Al—4V and Ti—6Al—7Nb
than on Ti—13Nb—13Zr.
(2) The addition of protein to the PBS reduced the influ-
ence of pH on the corrosion behaviour of all the
alloys.
(3) Corrosion reduced the hardness of the surface oxides
of all the alloys. In PBS the reduction was smallest of
Ti—6Al—4V and largest of Ti—13Nb—13Zr.
(4) Corrosion in protein solutions further reduced the
hardness of the surface oxides. This effect was greater
for Ti—6Al—4V and Ti—6Al—7Nb than for Ti—
13Nb—13Zr.
Acknowledgements
This work was supported by a postgraduate student-
ship from the University of Liverpool for M.A. Khan
which is gratefully acknowledged.
References
[1] Aragon PJ, Hulbert SF, Corrosion of Ti— 6Al—4V in simulated
body fluids and bovine plasma. J Biomed Mater Res 1972;
6:155—164.
[2] Mueller HJ, Greener EH, Polarization studies of surgical mate-
rials in Ringers solution. J Biomed Mater Res 1970;4:29— 41.
[3] Black J. Does corrosion matter? J Bone and Jt Surg 1988;
70B:517—20.
[4] Wapner KL. Implications of metallic corrosion in total knee
arthroplasty. Clin Orthop 1991;271:12—20.
[5] Donachie MJ, Titanium—a technical guide. OH, USA: ASM
International, 1988.
[6] Semlitsch MF, Weber H, Streicher RM, Schon R. Joint replace-
ment components made of hot-forged and surface-treated
Ti—6Al—7Nb alloy. Biomaterials 1992;13:781—8.
[7] Plenk H, Schider S. Tantalum and Niobium. In: Williams DF,
editor. Concise encylopedia of medical and dental materials. Ox-
ford: Pergamon Press, 1990:355—60.
[8] Kovacs P, Davidson JA. The electrochemical behaviour of a new
titanium alloy, Ti— 13Nb—13Zr. Presented at the 19th Annual
meeting of the Society for Biomaterials, 1993:88.
[9] Davidson JA, Mishra AK, Kovacs P, Poggie RA. New surface-
hardened, low modulus, corrosion resistant Ti—13Nb— 13Zr alloy
for total hip arthroplasty. Biomed Mater Eng 1994;4:231—43.
[10] Sousa SR, Barbosa MA. Corrosion resistance of titanium CP in
saline physiological solutions with calcium phosphate and pro-
teins. Clin Mater 1993;12:1—4.
[11] Pourbaix M. Electrochemical corrosion of metallic biomaterials.
Biomaterials 1984;5:122—34.
[12] Williams DF. Physiological and microbiological corrosion. Crit
Rev Biocompat 1985;1:1—24.
[13] Brown SA, Merritt K. Electrochemical corrosion in saline and
serum. J Biomed Mater Res 1980;14:173—5.
M.A. Khan et al. / Biomaterials 20 (1999) 631—637
637
Wyszukiwarka
Podobne podstrony:
The corrosion behaviour of Ti
51 721 736 Evaluation of the Cyclic Behaviour During High Temperature Fatique of Hot Works
40 549 563 On the Precipitation Behaviour in Maraging Steels
On the transformation behavior
[Elsevier] Corrosion Behaviuor Of Stressed Magnesium Alloys
Corrosion behaviour of commercially pure titanium shot blast
Corrosion behavior and surface characterization of titanium
Postmodernism In Sociology International Encyclopedia Of The Social & Behavioral Sciences
Report on the Sexual Behavior o Robert F Young(1)
The corrosion resistance of pure titanium
Burnat, Barbara; Blaszczyk, Tadeusz; Leniart, Andrzej Effects of serum proteins on corrosion behavi
Contacts and contracts dyadic embeddedness and the contractual behavior of firms
Corrosion behavior
Corrosion behavior of titanium nitride
A Behavioral Genetic Study of the Overlap Between Personality and Parenting
Post feeding larval behaviour in the blowfle Calliphora vicinaEffects on post mortem interval estima
DYNAMIC BEHAVIOUR OF THE SOUTH Nieznany
więcej podobnych podstron