
Intelligent thermoresponsive polymeric stationary phases for
aqueous chromatography of biological compounds
Akihiko Kikuchi, Teruo Okano*
Institute of Advanced Biomedical Engineering and Science, Tokyo Women's Medical University, 8-1Kawadacho, Shinjuku,
Tokyo 162-8666, Japan
Received 5 November 2001; revised 21 January 2002; accepted 25 January 2002
Abstract
Sensitive polymers with external physical, chemical, and electrical stimuli are termed as `intelligent materials'
and currently have been used in variety ®elds of engineering, and medicine. Numerous research papers utilizing
stimuli-responsive intelligent materials are found in the literature to date. In this manuscript, the authors described
several applications of surfaces and interfaces modi®ed with stimuli-responsive polymers for stimuli-responsive
surface property alteration and their application for the separation sciences. The special attention is paid to the
temperature responsive polymers, poly(N-isopropylacrylamide) (PIPAAm) and its derivatives as surface modi®ers
for novel `green' chromatography in which only aqueous mobile phase was utilized for separation of bioactive
compounds. Several factors were investigated and discussed the effects on separation of bioactive compounds;
these include the effects of the temperature-responsive hydrophilic/hydrophobic changes, copolymer composition,
graft polymer molecular architecture and the incorporation of charged groups. Furthermore, application of
PIPAAm-grafted surfaces for af®nity separation of proteins will be discussed. The technique has superior char-
acteristics in reducing organic wastes and costs to run chromatographic separation, and thus must be an envi-
ronmentally friendly separation tool. q 2002 Elsevier Science Ltd. All rights reserved.
Keywords: Stimuli responsive polymer; Surface function; Biocompatibility; Hydrophilic/hydrophobic property alteration;
Charge density alteration; Chromatography; Penetration control; Aqueous mobile phase; Bioactive compounds; Separation;
Dye af®nity chromatography; Protein
Contents
1. Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1166
2. Molecular separation and permeation control through the stimuli-responsive pore size regulation . . . .1167
3. Thermoresponsive chromatography using poly(N-isopropylacrylamide) as column matrix . . . . . . . . . .1169
4. Temperature-responsive wettability changes of PIPAAm-modi®ed surfaces . . . . . . . . . . . . . . . . . . . .1172
Prog. Polym. Sci. 27 (2002) 1165±1193
0079-6700/02/$ - see front matter q 2002 Elsevier Science Ltd. All rights reserved.
PII: S0079-6700(02)00013-8
www.elsevier.com/locate/ppolysci
* Corresponding author. Tel.: 181-3-3353-8111x30233; fax: 181-3-3359-6046.
E-mail address: tokano@lab.twmu.ac.jp (T. Okano).
5. Detachment control of cultured cells from PIPAAm-grafted culture dishes . . . . . . . . . . . . . . . . . . . . .1175
6. Novel `Green' chromatography utilizing PIPAAm-modi®ed surface . . . . . . . . . . . . . . . . . . . . . . . . . .1176
6.1. Effects of hydrophilic/hydrophobic property changes of PIPAAm surfaces on elution behavior . .1176
6.2. Effect of surface morphology of PIPAAm modi®ed matrix on the separation . . . . . . . . . . . . . . .1181
6.3. Effect of surface charged groups on analyte separation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1183
7. Selective adsorption/desorption of bioactive proteins . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1185
8. Conclusion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1190
Acknowledgements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1190
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1190
1. Introduction
Considerable researches have been focused on polymeric materials those change their structure and
functions responding to external physical, chemical, and electrical stimuli (light, temperature, pH,
substance concentration, solvent composition, and electric ®elds, etc.). These materials termed `intel-
ligent materials', sense one or more external stimuli (sensor) as signals, judge the magnitude of these
signals (processor), and change their structure and functions in direct response (effecter). The response
of the intelligent materials toward above described stimuli induce several kinds of changes such as
phase, shape, surface energies, permeation rates, reaction rates, and molecule recognition. Introduction
of stimuli-responsive polymeric materials as switching sequences into both arti®cial materials and
bioactive compounds (peptides, proteins, nucleic acids, and others) permit modulation of their structure
induced by corresponding external stimuli. `On±off' switching of their respective functions, thus, can be
achieved at molecular level [1±5]. Intelligent materials embodying these concepts might contribute to
establish fundamental principles for fabrication of novel systems.
In the present review, several approaches to create stimuli-responsive polymer-modi®ed surfaces and
interfaces are described. Special attention will be paid to the temperature-responsive polymer, poly(N-
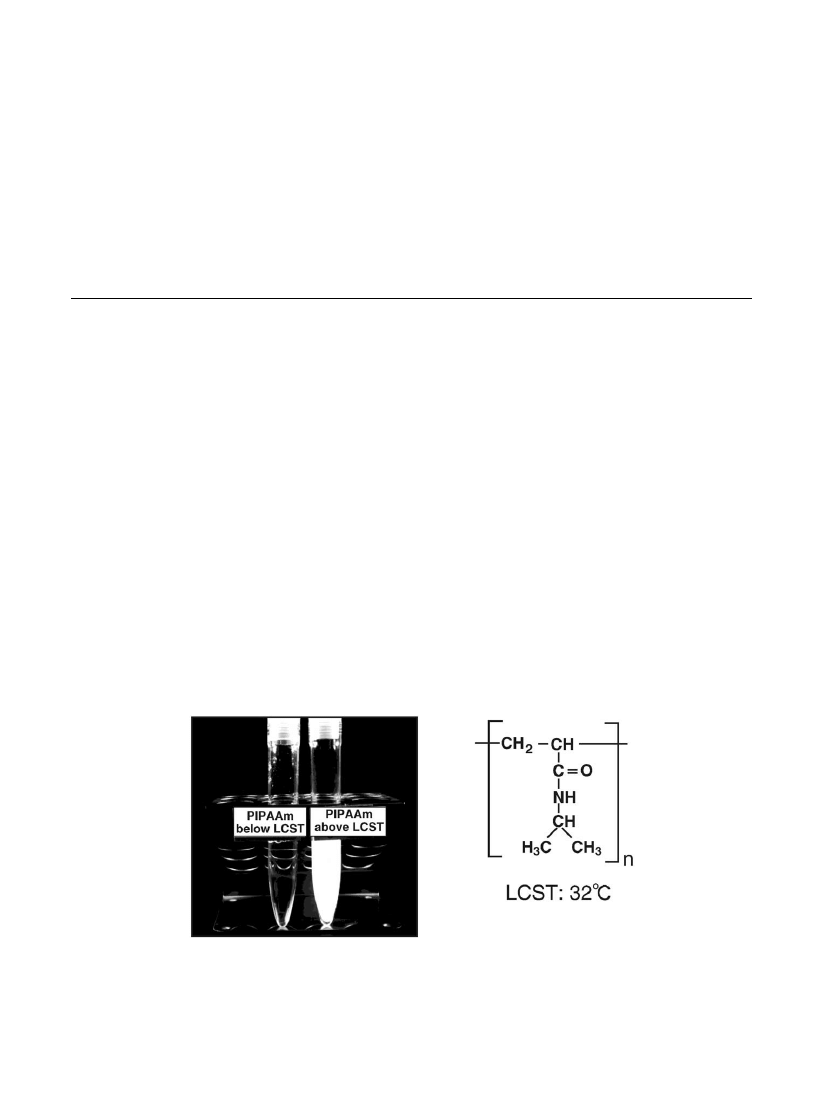
isopropylacrylamide) (PIPAAm), which shows lower critical solution temperature (LCST) around 32 8C
in water [6,7]. As shown in Fig. 1, PIPAAm is soluble in water below the LCST, while precipitation of
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1166
Fig. 1. Structural formula of poly(N-isopropylacrylamide) (PIPAAm) and appearance of its aqueous solution below and above
the LCST.

polymer and solution turbidity are observed above this temperature. Introduction of PIPAAm onto solid
surfaces will produce intelligent surfaces those show temperature-responsive hydrophilic/hydrophobic
changes as described in detail in Section 4. Furthermore, this surface property alteration would be
utilized to modulate solute interaction in the chromatographic separation by changing temperature in
sole aqueous condition, creating novel liquid chromatography systems. These systems might be an
alternative of reversed-phase chromatography in which separation is controlled through the regulation
of the interaction between octadecylsilica stationary phase surfaces and solute molecules by mobile
phase composition and polarity.
Kanazawa et al. [8] recently reviewed results for temperature-responsive hydrophobic chromatogra-
phy in aqueous mobile phase from the standpoint of analytical science. In this review, the point is
focused on the intelligent interface preparation using stimuli-responsive polymeric materials and the
prepared surface application in the separation sciences.
2. Molecular separation and permeation control through the stimuli-responsive pore size
regulation
Polymer chain coil/globule transition with chemical and/or physical stimuli are utilized to control
pore size and applied for the stimuli-responsive molecular valve of substance diffusion and permeation
through the pores.
Iwata and Matsuda [9] reported the modi®cation of porous membranes with either poly(acrylic acid)
(PAAc) or polyacrylamide (PAAm) through air plasma-induced polymerization of corresponding mono-
mers. In case of PAAc as the membrane modi®er, the polymer molecules on the surfaces acted as the
environmental pH-sensing device, at the same time, as the molecular valve to regulate permeation
through the pores. Filtration rate at pH 5.2 was approximately one-tenth (1/10) of that at pH 1.4.
With decreasing the ®ltration rate modi®ed membrane showed ultra®ltration character for high mole-
cular weight substances, such as albumin and dextran. They further investigated the PAAc-modi®ed
membranes by means of atomic force microscope (AFM) to visualize pH-dependent pore size control
[10]. At lower pH than pK
a
of carboxyls in PAAc, pores on the modi®ed membrane are clearly observed
under AFM, while at higher pH carboxyl groups were deprotonated to become carboxylate anions and
polymer chains extended out of pores to make hill-and-valley structure in the AFM views. Thus, pH-
dependent polymer chain conformational changes caused open/close changes of the membrane pores,
regulating substrate permeation as schematically shown in Fig. 2a. The pH-dependent membrane
characteristic changes are reversible and reproducible, PAAc-modi®ed membrane could be utilized in
various membrane technologies.
Ito et al. [11,12] utilized same concept for pH-controlled drug delivery systems. They immobilized
PAAc onto the cellulose membrane. Using side chain carboxyl groups, glucose oxidase was co-immo-
bilized onto surface grafted PAAc chains via amide bond formation. Inside the membrane, insulin as
bioactive drug was reserved. At physiological pH of 7.4, where PAAc grafts are ionized through
deprotonation and existed in expanded conformation, drug permeation was almost stopped because
membrane pores were in closed state with expanded polymer chains. When glucose is presented in
the medium, glucose oxidase mediates the glucose conversion to gluconic acid, which reduces the
environmental pH inducing protonation of PAAc. With the protonation, PAAc chains form compact
conformation, which open the membrane pores. Thus, membrane permeation rate changes and
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1167
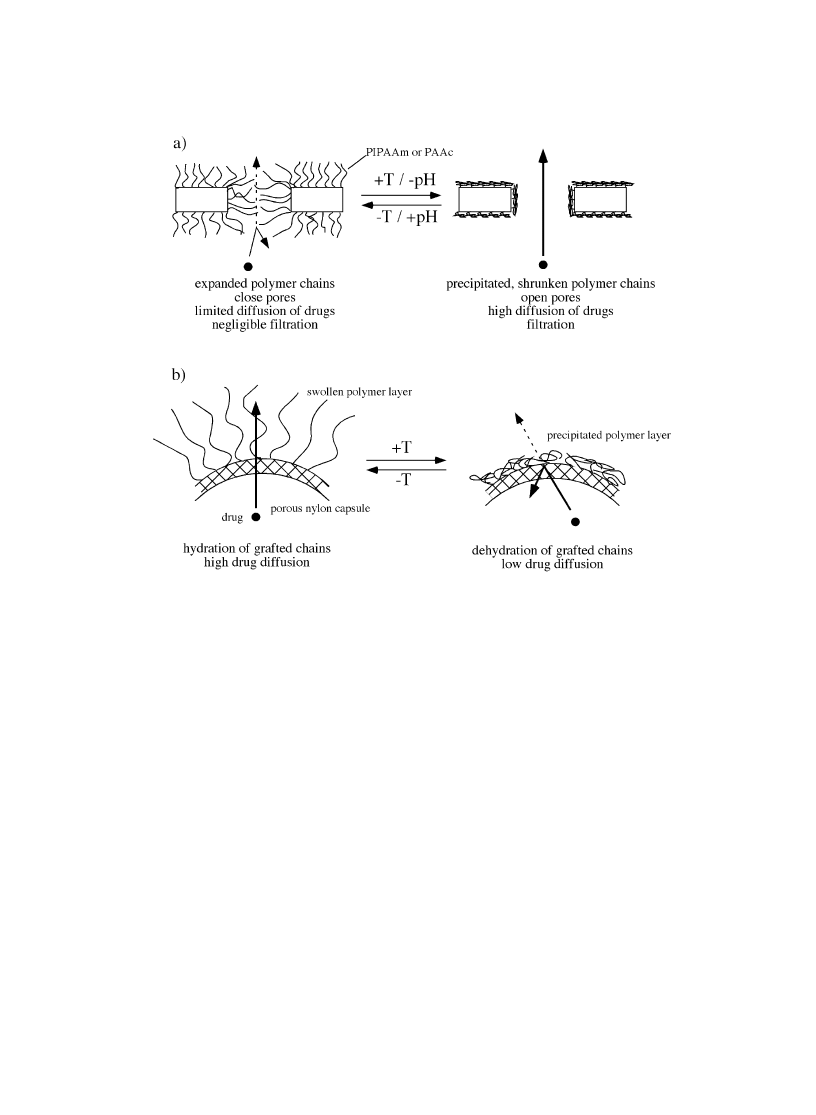
eventually insulin inside the modi®ed membrane was released out of the membrane. There are many
points to be solved before utilizing this material to in vivo application, such as in vivo response toward
glucose concentration, and prevention of denaturation and immune response toward conjugated glucose
oxidase. Further optimization of this system to in vivo application will provide blood glucose level for
diabetes mellitus patients.
By utilizing temperature as a stimulus, similar permeation control is achieved.
Okahata et al. [13] reported thermoresponsive drug release capsules (Fig. 2b). The capsule was made
from porous nylon membrane on which PIPAAm chains were grafted covalently. Sodium dinaphthalene
sulfonic acid was used as model drug molecules. Release of drug was controlled by temperature; release
enhanced at lower temperature where surface grafted PIPAAm was hydrated and extended form, while
signi®cant drug release suppression was achieved by raising temperature more than PIPAAm's LCST.
This was due to the dehydration of PIPAAm chains above its LCST. PIPAAm molecules precipitated out
on the nylon capsule surfaces those prevented drug molecule permeation through the pore. Thus,
temperature responsive open/close changes of membrane pores were achieved.
Iwata et al. [14] reported PIPAAm-grafted porous membranes for water ¯ow control through the pore
by temperature changes. They treated porous poly(vinylidene di¯uoride) membrane with Ar plasma
followed by IPAAm polymerization onto plasma-treated membrane surfaces. Above the PIPAAm's
transition temperature, 10 times higher ®ltration rate for water was observed while negligible ®ltration
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1168
Fig. 2. (a) Stimuli-responsive porous membrane to control pore size. (b) Diffusion control using temperature-responsive
PIPAAm-grafted nylon membrane capsules.

was occurred at lower temperature. In their case, shrunken polymer molecules on the membranes open
channels of pore for water to pass through. Thus, Fig. 2a represents this model.
These two examples showed sharp contrast in terms of thermoresponsive polymer conformation,
although both membranes were grafted with PIPAAm. The difference is probably the density of
PIPAAm chains on the membrane surfaces and/or the modi®cation sites, inside or outside the pores
as schematically shown in Fig. 2a and b.
In all cases described here, however, complete `on±off' control of molecule diffusion or penetration
was not achieved. Instead, background diffusion was apparent (1±2 type control). To achieve `0±1 type
on±off' control, more precise design of membranes should be necessary in terms of graft chain density,
length (molecular weight), and con®guration on the substrate surfaces.
Recognition site of substrate in enzymes could also be regulated with a similar concept [15±17] but
not discussed in detail here.
3. Thermoresponsive chromatography using poly(N-isopropylacrylamide)as column matrix
Above described concept of pore size control with stimuli-responsive polymers can be utilized for
chromatography systems, especially in size exclusion chromatography mode [18±21].
Gewehr et al. [18] prepared PIPAAm-grafted porous glass beads as column packing materials for gel
permeation chromatography. PIPAAm was end-functionalized through telomerization polymerization
of IPAAm in the presence of mercaptopropionic acid (MPA) used as chain transfer agent. By this
method, each chain end of PIPAAm molecules possessed carboxyl group. Porous glass beads were
®rstly aminated with 3-aminopropyltriethoxysilane followed by conjugation of PIPAAm with active
ester chain ends through amide bond formation. Bare porous glass beads with 156, 171, 237, and 408 AÊ
pores were used as base materials. The PIPAAm-modi®ed glass beads were packed into column
(200 mm long with 7 mm inner diameter) and elution of dextran having a variety of molecular weights
was examined changing column temperature. In GPC mode, smaller molecular weight substances show
longer retention time due to the permeation through the matrix. In case of PIPAAm-grafted porous glass
bead-packed columns, similar effects were seen; i.e. the smaller the molecular weight of dextran used as
model samples, the longer the retention was observed. Temperature change induced PIPAAm
chain conformational changes; hydrated, and extended conformation at low temperature below the
LCST to dehydrated, and shrunken aggregates above the LCST. Due to this conformational
change on the PIPAAm-grafted porous glass bead surfaces, effective pore size changed depending
on the original pore sizes. With smaller pore beads, large pore size change by temperature
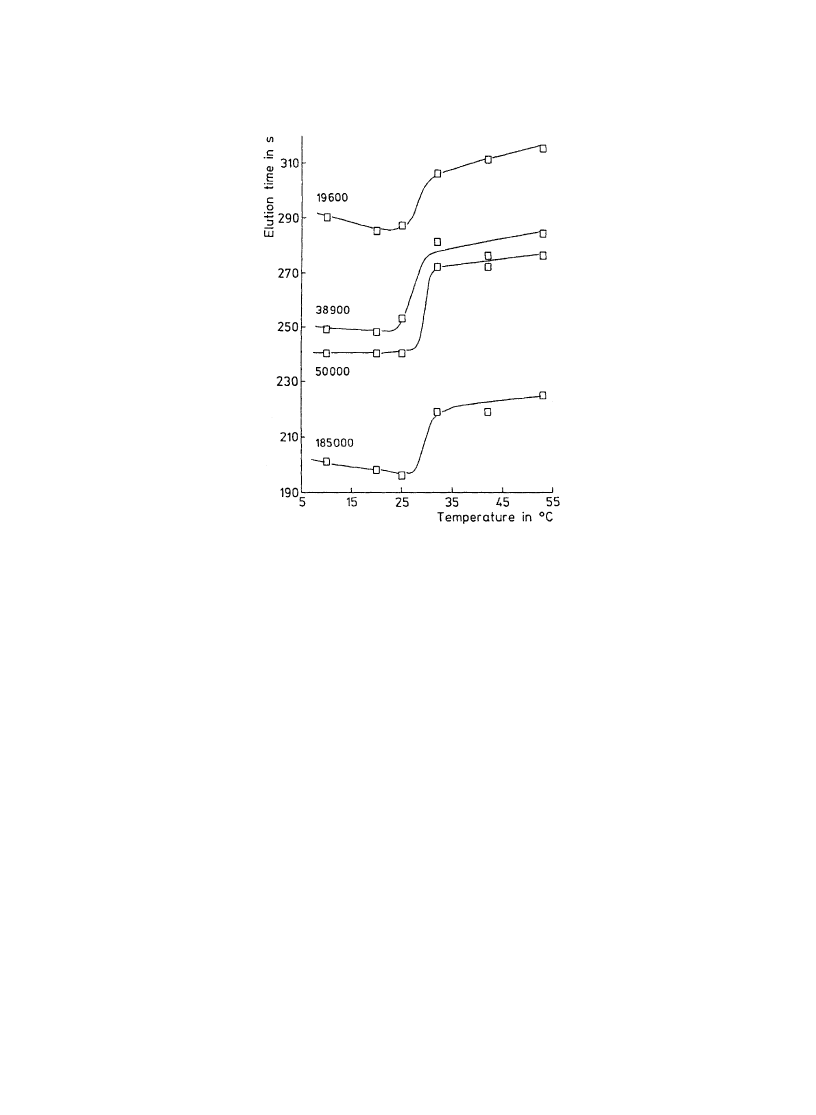
change was occurred. In case of beads with 237 AÊ pores, discontinuous elution time shift was
observed for all dextran samples in the temperature range of 25±35 8C as shown in Fig. 3. The
results strongly suggested the temperature-modulated pore size changes, and thus, successful resolution
of wide molecular weight range of dextran in GPC mode. As was pointed out, balance of PIPAAm
molecular lengths and pore size of base materials are important to control permeation of substances to be
separated.
Hosoya et al. [19,20] developed an in situ surface-selective modi®cation to introduce PIPAAm into
porous polymer beads. They used porous polystyrene beads (1 mm diameter) as seed particles and
IPAAm polymerization was then carried out using either cyclohexanol or toluene as porogen agent in
water. In case of cyclohexanol used as porogen, propagating PIPAAm radical is soluble in cyclohexanol
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1169
and thus entire surfaces (both inside and outside) of the porous beads were covered with PIPAAm. On
the other hand, PIPAAm was grafted only on the external bead surfaces with toluene used as porogen
since PIPAAm cannot penetrate into toluene-®lled pores of the beads due to the existence of non-
solvent, toluene for PIPAAm. In this way, PIPAAm was selectively grafted on either entire surfaces
or only external surfaces of the beads. Microscopic appearance of the beads was relatively homogeneous
for modi®ed beads with cyclohexanol as porogen, and heterogeneous, rough surface morphology was
observed for modi®ed beads with toluene as porogen.
These surface structures in¯uenced on the elution behavior of dextran in aqueous systems. They
carried out size exclusion chromatography using these two sets of modi®ed beads as column matrixes.
When cyclohexanol was used as porogen agent, temperature increase affects to longer elution time for
higher molecular weight dextran. At temperature below PIPAAm's LCST, PIPAAm chains exist in
expanded conformation due to hydration of PIPAAm chains, restricting dextran molecules to penetrate
into deep inside of the pores. At higher temperature, PIPAAm dehydrated, and shrunken at the interface,
opening pores wide, which permit dextran to penetrate into the pores. Thus, the elution times were
retarded. Contrary to the case for beads modi®ed with cyclohexanol, temperature-dependent dextran
elution was opposite for beads modi®ed in the presence of toluene as porogen agent. The result indicates
that at higher temperature than LCST, faster elution was achieved than that at lower temperature due to
the reduction of pore size through the shrinkage of surface grafted PIPAAm chains. These results suggest
that the in situ surface selective modi®cation methods were achieved. It is indicated that separation mode
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1170
Fig. 3. Temperature dependence of the elution time of various molecular weight dextran on the PIPAAm-modi®ed porous silica
beads. Pore size of original silica beads is 237 AÊ. (Reprinted with permission from Ref. [18]. Copyright (1992) Wiley±VCH
GmbH.)
can be controlled through the modi®cation route even the modi®er is the same PIPAAm. Obtained beads
could be applied for a number of chromatographic applications.
Hosoya et al. [20] further investigated prepared PIPAAm-modi®ed particles as chromatographic
matrix for separation of several drug molecules in reversed-phase chromatography mode using aqueous
acetonitrile with different composition. They compared elution of several drug molecules with different
functionalities, possessing either hydrophobic or hydrophilic groups. They considered that as amide
groups in PIPAAm chains might be exposed to outward below the LCST, speci®c interaction should
be occurred with drug molecules with hydrophilic, polar functional groups (amine, amide, carbonyl,
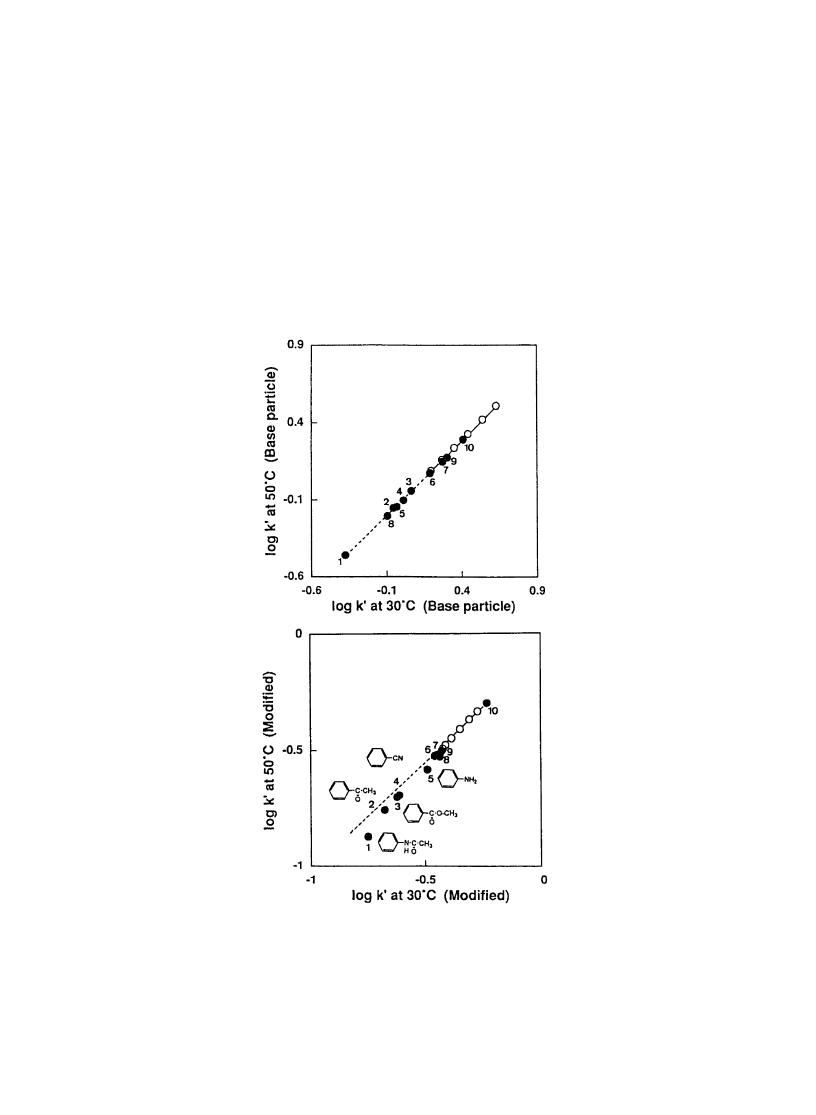
hydroxyl, nitrile, and other functions). In fact, as shown in Fig. 4, when separation selectivity was
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1171
Fig. 4. Comparison of separation selectivity in 60% aqueous acetonitrile on base and PIPAAm-grafted packing materials.
Solute: 1, acetanilide; 2, acetophenone; 3, methyl benzoate; 4, cyanobenzene; 5, aniline; 6, nitrobenzene; 7, m-dinitrobenzene;
8, phenol; 9, p-dinitrobenzene; 10, bromobenzene (open circle, alkylbenzenes). (Reprinted with permission from Ref. [20].
Copyright (1995) American Chemical Society.)

compared, stronger interaction of drugs with more polar functional groups was observed at lower
temperature, at which PIPAAm exists in expanded conformation for packing matrix with PIPAAm
modi®ed on internal surfaces of pores. No selectivity was found on base packing materials. Furthermore,
they found the co-existing polypeptide, albumin, could be eluted out before exclusion volume for the
column packed with PIPAAm modi®ed on external bead surfaces and baseline separation of drugs is
achieved in reversed-phase chromatographic mode. Thus, with the surface-selective modi®cation with
PIPAAm, temperature-dependent separation of drug molecules is performed. These packing materials
could be selectively utilized for medical diagnosis; drug monitoring in the blood samples of the patients
without interference from serum proteins.
Go et al. [22] used silane coupler with methacryloylpropyl groups to introduce polymerizable groups
onto silica beads. Then, they carried out IPAAm polymerization to produce PIPAAm-grafted porous
silica beads for reversed-phase chromatography in GPC mode. Introducing PIPAAm onto silica beads
could shorten elution times, and show separation of dextrans with different molecular weight in aqueous
mobile phase. There is only a small difference in elution time change of dextran at lower temperature;
however, relatively larger elution time was observed above the polymer transition temperature. They
further analyzed lower molecular weight benzene derivatives with different polar functions in reversed-
phase chromatography using aqueous methanol solution. They showed the preferential retention of
hydrogen bond acceptors at low temperatures in low amount of methanol containing aqueous solution,
while hydrophobic interaction is dominant factor for solutes at higher temperature and in the mobile
phase with higher content of methanol. These results suggest that, at low temperature where PIPAAm
exists in expanded conformation, polar groups in the sample analyte exhibit relatively strong interaction
with amide side chains in PIPAAm and at higher temperature than PIPAAm LCST the hydrophobic
interaction regulates the solute retention.
The problem arose in their methods may be the use of organic solvent in the mobile phase preparation,
which may be disadvantageous from the environmental reason. Thus, separation of drug substances
should be carried out in the column system utilizing sole aqueous mobile phase.
Lakhiari et al. [21] introduced diethylaminoethyl (DEAE)-dextran onto silica beads through electro-
static interaction followed by cross-linking reaction of dextran hydroxyls with 1,4-butanediol diglyci-
dylether (BDGE). On this matrix surface, carboxyl terminated PIPAAm was further grafted using
BDGE. The PIPAAm-modi®ed bead packed column was characterized with high performance size
exclusion chromatography using aqueous mobile phase. Although the modi®ed column showed
temperature-responsive elution changes for lower molecular weight substances, proteins, and polysac-
charides, elution was almost same for both sample types with more than 10 000 molecular weights
regardless of temperature. For some proteins, elution time was retarded at higher temperature, probably
due to the hydrophobic nature of proteins those lead the hydrophobic interaction of proteins with
dehydrated, hydrophobized matrix surfaces. Although the base silica bead matrix has pore of 1000±
1250 AÊ, after the modi®cation with cross-linked DEAE-dextran and PIPAAm grafts the pore size and its
distribution might change dramatically. Therefore, surface characteristics of modi®ed beads were signif-
icantly altered as size exclusion chromatography matrix. Pore size control should be an important factor
to design thermoresponsive size exclusion chromatography matrix.
4. Temperature-responsive wettability changes of PIPAAm-modi®ed surfaces
As mentioned in Section 1, PIPAAm molecules in aqueous solution show temperature-responsive
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1172
soluble/insoluble changes due to the hydration/dehydration of side groups. Hydrophobic hydration
around isopropyl groups plays a role in this phenomenon. Above described reports are mainly focused
on PIPAAm's thermoresponsive characteristics; expanded coils at lower temperature than the LCST to
dehydrate globules above the LCST, to control matrix pore sizes. Other than such temperature-respon-
sive conformational changes to control pore sizes, the PIPAAm-modi®ed surfaces should show hydro-
philic/hydrophobic changes in response to temperature change in aqueous environment. In fact,
PIPAAm-grafted surfaces show temperature-dependent water contact angle changes.
Takei et al. [23] investigated the effect of graft chain conformations on the temperature-responsive
wettability changes of PIPAAm-grafted surfaces. They conjugated end-carboxyl PIPAAm and poly(N-
isopropylacrylamide-co-acrylic acid) (P(IPAAm-co-AAc)) to aminated glass cover slips with poly(styr-
ene-co-aminomethylstyrene) coatings independently to form free end linear PIPAAm-grafted surfaces
and multipoint attached PIPAAm surfaces, respectively, at 4 8C in water. They then investigated surface
wettability using Wilhelmy plate technique changing temperature. A large contact angle change was
obtained for PIPAAm-end grafted surfaces around 24 8C while smaller contact angle changes was
observed for P(IPAAm-co-AAc)-grafted surfaces with a wide temperature range. The transition
temperature was lower than PIPAAm's LCST for both surface types, probably due to the in¯uence of
base coating with polystyrene derivative as well as density of PIPAAm chains. Small contact angle
changes of the PIPAAm-multi-point attached surfaces might be due to the restricted chain conformation
of the grafted polymers.
The effects of the graft polymer chain conformation were further investigated on temperature-respon-
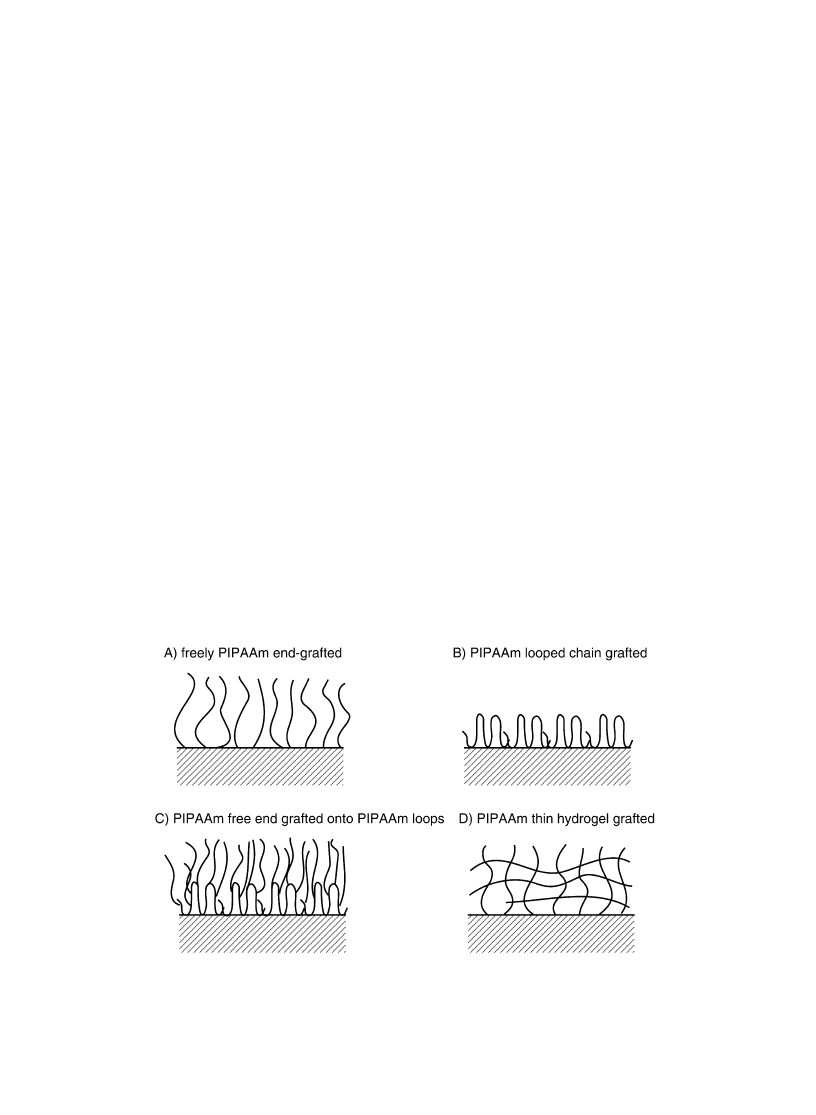
sive wettability changes of PIPAAm-grafted surfaces [24,25]. Four model surfaces were prepared and
these are schematically shown in Fig. 5; (a) free end linear PIPAAm-grafted surface, (b) multi-point
grafted looped PIPAAm surface, (c) free end PIPAAm grafted onto PIPAAm looped chain modi®ed
surface, and (d) PIPAAm thin hydrogel layer modi®ed surface. When PIPAAm was immobilized onto
the surfaces with multi-point fashion (type b) in Fig. 5) in good solvent for PIPAAm, PIPAAm
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1173
Fig. 5. Schematic representations of four types of PIPAAm-modi®ed surfaces with different molecular architecture.
molecules were attached with extended chain conformation, resulting in linear and small increase in
contact angle with temperature increase. On the contrary, immobilization medium contained poor
solvent to PIPAAm (two volume fractions of toluene to eight volume fractions of good solvent,
dioxane), PIPAAm chains were grafted onto the surface with looped chain con®guration with relatively
mobile nature. Thus, graft chain density of the looped PIPAAm should be increased than the previous
report [23]. For modi®cation of PIPAAm-grafted surfaces, amination of the base glass cover slips was
carried out. Considering from the surface density of amine functions and reaction with polymer mole-
cules, all prepared surfaces have higher PIPAAm density than the surfaces reported by Takei et al. [23].
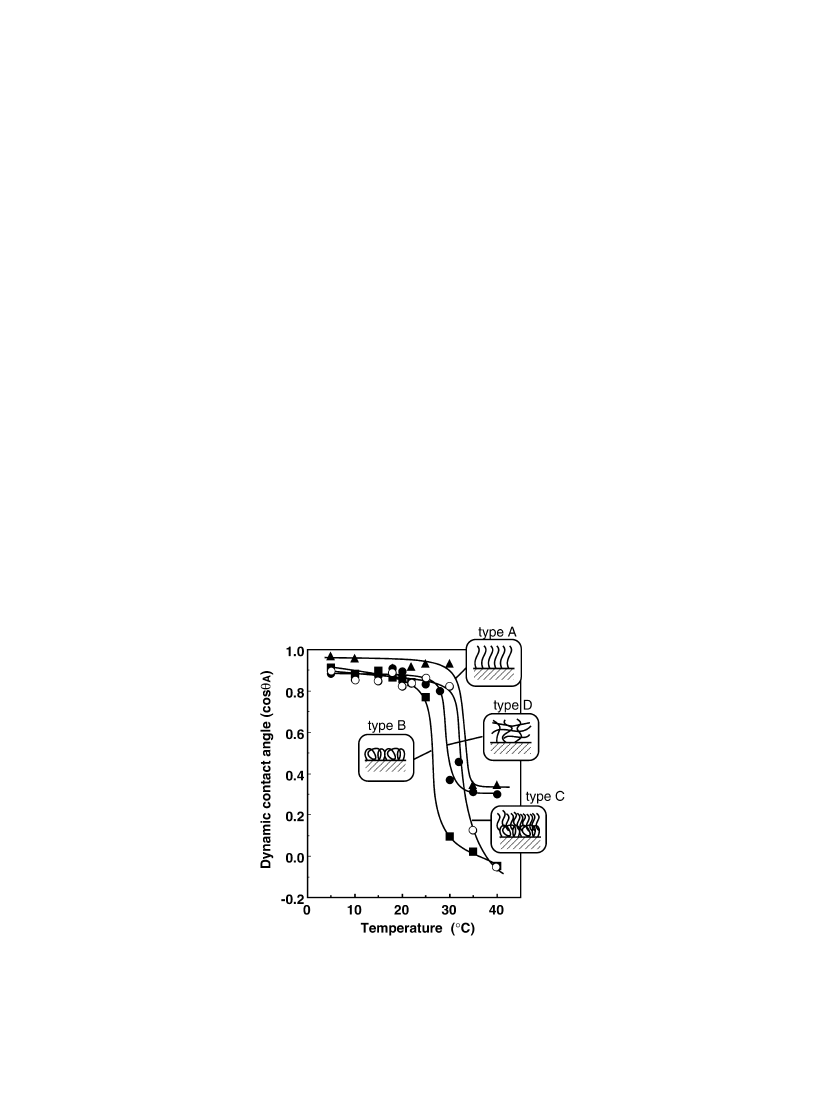
The temperature-responsive contact angle changes measured using Wilhelmy plate technique are
indicated in Fig. 6. All the surfaces were ¯at enough to measure contact angles as con®rmed by
AFM mean roughness analysis. All surfaces showed temperature responsive contact angle changes,
low contact angles (high cos
u
A
) at lower temperature and higher contact angle at higher temperature.
Thus, the PIPAAm-grafted surfaces show temperature-responsive hydrophilic/hydrophobic changes.
With a multi-point graft chain conformation, transition temperature decreased slightly than PIPAAm
LCST in water. Thus, only a small restriction of the chain freedom was obtained. With increasing
polymer density on the surface by grafting free end PIPAAm on PIPAAm loops, large change in contact
angle was observed around polymer transition temperature. For PIPAAm hydrogel grafted surfaces,
however, smaller contact angle change was seen, probably due to the restricted chain mobility arising
from the cross-linking. Since the graft chain conformation has great in¯uence on the surface property
alteration in terms of wettability to water, the property modulation should affect on the interaction with
biomolecules and cells.
Morra and Cassinelli [26] utilized UV irradiation in the presence of benzophenone as photosensitizer
to polymerize and graft PIPAAm molecules onto the surfaces. They dissolved IPAAm monomer in the
presence of 0.1% benzophenone into 2-propanol at concentration ranging 5±40 wt%. UV light irradiation
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1174
Fig. 6. Temperature-dependent water contact angle changes for four types of PIPAAm-grafted surfaces with different molecular
architecture.

at 365 nm wavelength was carried out for 5±30 min. Surface analyses were done using ESCA with take-
off angle of 458. ESCA analysis revealed the existence of repeating units of PIPAAm on the modi®ed
surfaces. They measured surface wettability of the modi®ed surfaces. Volpe et al. [27] investigated the
contact angle changes of PIPAAm modi®ed surfaces using Wilhelmy plate measurements. They ®rstly
applied air plasma treatment of polystyrene sample surfaces followed by the polymerization of IPAAm
(10 wt%) in water at 25 8C in the presence of 0.2% ammonium cerium nitrate as initiator. ESCA
measurements revealed that after polymerization atomic ratio of the carbon, nitrogen and oxygen was
close to the theoretical ones, suggesting complete modi®cation with PIPAAm on the surfaces. They then
investigated the surface wettability toward water by means of Wilhelmy plate technique in detail,
changing immersion speed and temperature. Immersion speed was critical factor to change surface
wettability, though, it could be concluded that the PIPAAm-grafted surfaces show temperature-respon-
sive wettability changes; at 20 8C the surface is hydrophilic with low water contact angle, and at 37 8C
the surface become hydrophobic.
Liang et al. [28] prepared glass surface modi®ed with silane coupling agent with dithiocarbamate
groups and photopolymerization was carried out in the presence of IPAAm to form PIPAAm layer on the
glass surfaces, glass plate, and glass capillary tubing. These surfaces showed temperature responsive
wettability changes due to the hydration/dehydration changes of PIPAAm graft chains. Furthermore,
when PIPAAm was photopolymerized on the capillary tube surfaces, temperature responsive meniscus
height changes was observed; higher meniscus at lower temperature below the LCST and lower menis-
cus at higher temperature; 7 mm difference was seen for meniscus between 20 and 40 8C for capillary
with 2 mm diameter. Modi®cation was simple and easily controlled by UV irradiation times for poly-
merization. Therefore, the authors concluded the usefulness of the modi®cation methods to apply for
anti-fouling surface for biomolecules, and micro-channel for separation membranes. Their system
should be useful to modify UV transparent base materials, however, it would be dif®cult to modify
the sample with UV opaque materials thus, further modi®cation should be necessary. Pan et al. [29]
recently utilized plasma polymerization of IPAAm monomer gas in the radio-frequency plasma poly-
merization reactor. IPAAm monomer ¯ask was heated to 72 8C to evaporate monomer gas and intro-
duced into reactor vessel that contained base samples; Si wafers, and glass capillary tubing. Both FTIR
spectra and ESCA analysis of the modi®ed samples con®rmed successful modi®cation of samples with
PIPAAm. Thermoresponsive wettability change was observed by the meniscus height changes. A
difference of the meniscus is 0.7 cm between 5 and 55 8C.
5. Detachment control of cultured cells from PIPAAm-grafted culture dishes
Above-mentioned thermoresponsive wettability changes could be applied for cultured cell detach-
ment control. Takezawa et al. [30,31] utilized thermoresponsive soluble polymers for surface property
changes to recover cultured ®broblasts. They failed to culture cells on PIPAAm coating on tissue culture
polystyrene dishes because of dif®culty to get enough hydrophobicity at 37 8C. They actually mixed cell
adhesive protein, collagen, to achieve cell adhesion. As PIPAAm is solubilized by decrease in tempera-
ture, adhered cells are detached from the surface. However, detached cell suspension contained
solubilized PIPAAm and collagen as contaminants, those may interfere further use of detached cells.
Furthermore, detached cells form cell agglomerates and never succeeded to recover as a cell monolayer
sheet.
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1175

It is reported that the PIPAAm-grafted cell culture surfaces modi®ed by means of electron beam
irradiation to polymerize and graft PIPAAm on the surfaces covalently [32±34]. Recovery of cultured
cells was succeeded for various cell types from PIPAAm-grafted culture dishes by only lowering
temperature treatment without any use of harmful enzymes [33,35,36]. PIPAAm-grafted culture dishes
were utilized to construct novel tissue constructs in vitro [35±42]. Details are not discussed in this
manuscript and thus refer to above-mentioned references. Following these reports, several researchers
presented similar PIPAAm modi®cation of the surfaces to control and manipulate cultured cells
[26,27,43,44].
Thermoresponsive hydrophilic/hydrophobic changes of PIPAAm-modi®ed surfaces should also be
applied for novel hydrophobic chromatography as described in Section 6.
6. Novel `Green' chromatography utilizing PIPAAm-modi®ed surface
6.1. Effects of hydrophilic/hydrophobic property changes of PIPAAm surfaces on elution behavior
In Section 5, PIPAAm-modi®ed matrix was introduced to show thermoresponsive pore size control to
separate high molecular weight substances in GPC manner [18±20]. The authors group proposed that
thermoresponsive surface wettability changes of PIPAAm-grafted surfaces could be utilized to separate
hydrophobic bioactive compounds in aqueous mobile phase.
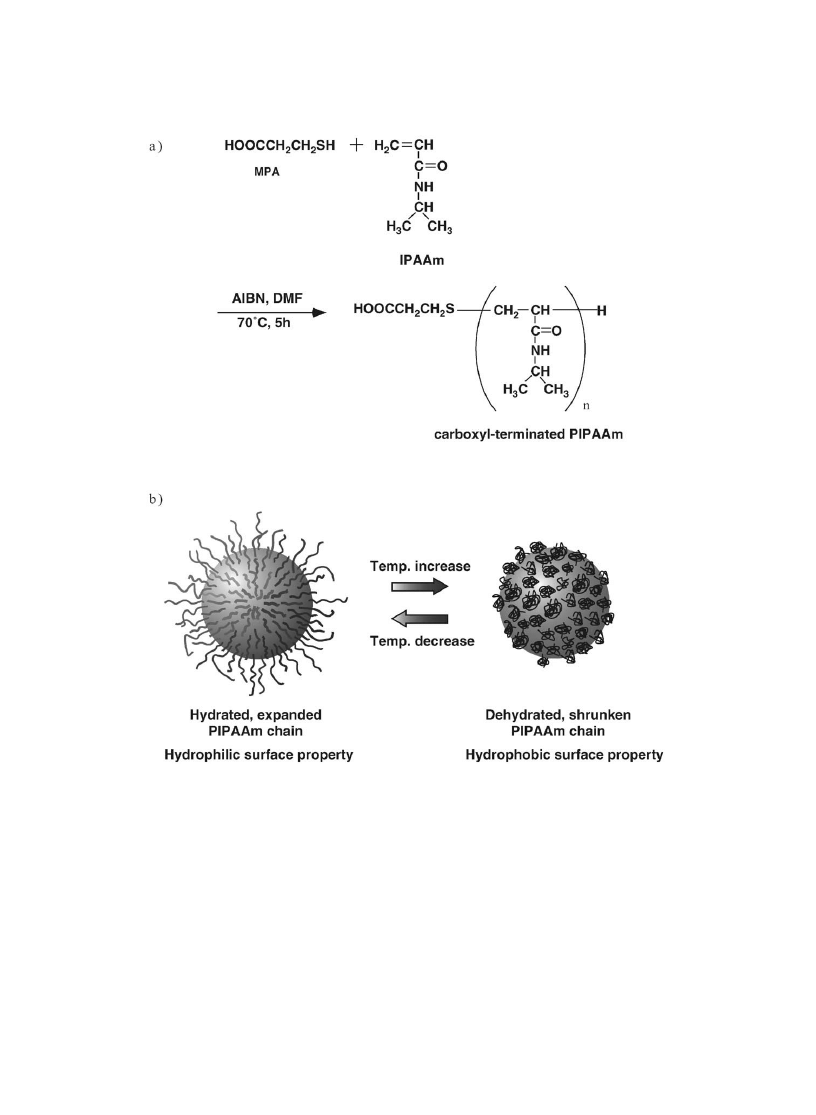
Kanazawa et al. [45] grafted end-carboxyl PIPAAm onto aminopropylsilica beads with 5 mm
diameter and used as packing materials for high performance liquid chromatography column. End
carboxyl PIPAAm was prepared according to the reports by Takei et al. [23,46] and utilizing radical
telomerization reaction in the presence of MPA as telogen. Initiator radicals attack thiol groups of MPA,
which initiated IPAAm polymerization. Synthetic route of the end carboxyl PIPAAm is shown in Fig. 7a.
Number average molecular weight of the PIPAAm was 6800 for surface modi®cation. End carboxyl
group of PIPAAm was then active esteri®ed with hydroxysuccinimide followed by reaction with
aminopropylsilica beads. Thus, PIPAAm-grafted silica beads with thermoresponsive property alteration
were obtained. Temperature-responsive surface property changes for PIPAAm-modi®ed beads are
shown in Fig. 7b. PIPAAm-modi®ed silica beads were packed into stainless steel column
(150 mm £ 4.6 mm F) by a slurry method. Column was then connected to HPLC system with a column
jacket connected to thermostated water bath with temperature control within 0.1 8C. Hydrophobic
steroids with different hydrophobicities were selected as the ®rst examples for separation. These steroids
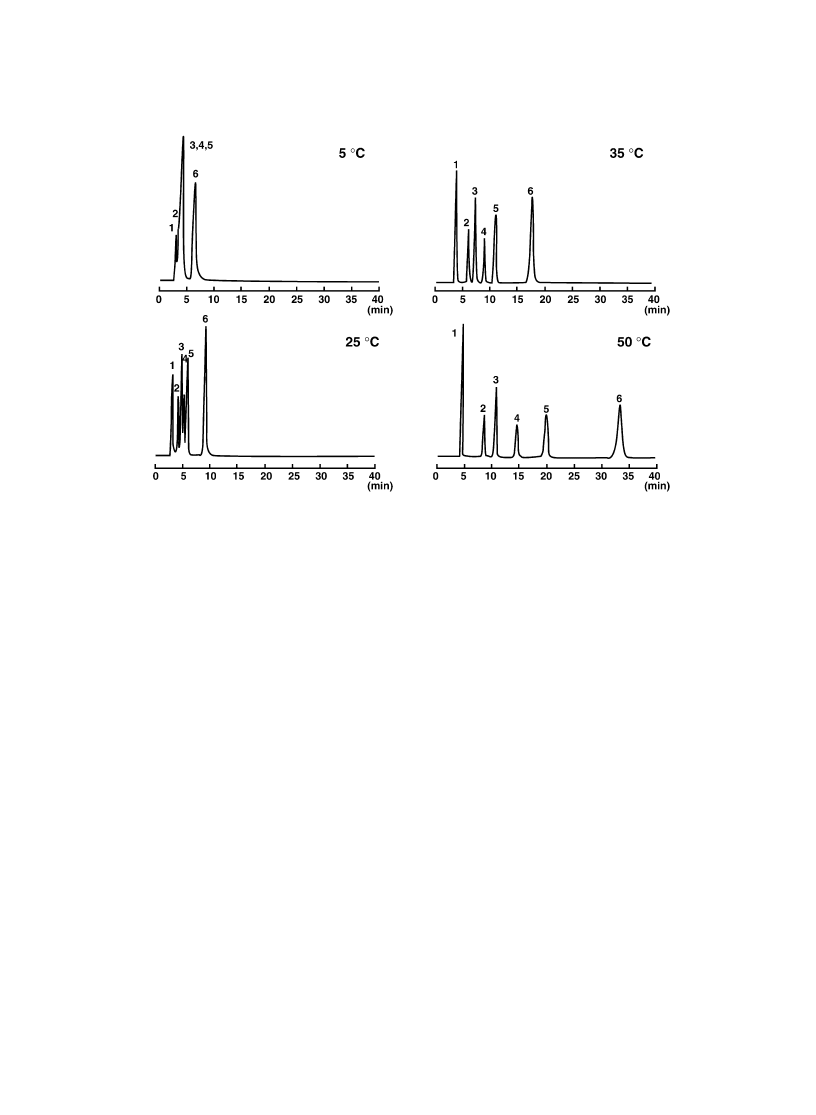
were dissolved in Milli-Q water and injected into the PIPAAm-modi®ed column. As shown in Fig. 8, at
lower temperature than PIPAAm's LCST steroids cannot be separated nicely. With increasing tempera-
ture above the LCST of PIPAAm, retard retention times of the steroid molecules were observed. The
elution order was in accordance with the increasing order of the hydrophobicities of steroids as judged
from the log P values of the steroids where, P is the partition coef®cient of the sample material in 1-
octanol/water system [47]. Since no ef®cient separation was observed for unmodi®ed silica bead-packed
column in aqueous mobile phase, separation mechanism is totally different from the conventional
reversed-phase chromatography. At higher temperature above the PIPAAm's LCST, the surface of
the column packing materials becomes hydrophobic due to the dehydration of surface bound PIPAAm
molecules and precipitated on the interface, it would be plausible that the driving force of the steroid
interaction with the PIPAAm-modi®ed surfaces is the hydrophobic interaction. The discussion was
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1176
further con®rmed by evaluating the effect of hydrophobic moieties into the surface grafted PIPAAm
molecules through copolymerization. Kanazawa et al. [48] investigated the effect of surface hydropho-
bicity on the separation behavior of steroid molecules. Hydrophobic butyl methacrylate (BMA) was
introduced into the end-functionalized PIPAAm during the copolymerization with changing composi-
tion. The resultant copolymers showed lower LCSTs than PIPAAm homopolymer with increasing BMA
content in the copolymer. These modi®ers were then introduced to aminopropylsilica beads and modi-
®ed beads were packed into stainless steel column. With increasing hydrophobic BMA content in the
modi®er polymer, retention time of steroids with higher log P values retarded even at 5 8C. On silica
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1177
Fig. 7. (a) Synthesis of an end-carboxyl PIPAAm. (b) Schematic representation of PIPAAm-grafted silica beads (surface type
A).
beads modi®ed with P(IPPAm-co-BMA) of 3.2 mol% BMA content, sharp peaks of each steroid were
observed at 5 8C. With increasing temperature, longer retention times of steroids on each copolymer-
modi®ed surfaces were seen and baseline separation of steroids was achieved for BMA containing
copolymer-modi®ed surfaces at 30 8C where insuf®cient separation was observed for PIPAAm column.
As BMA is hydrophobic regardless of temperature, hydrophobic steroids have stronger interaction with
BMA moiety within the surface modi®ed copolymer, even though the copolymers were hydrated, and
expanded conformation at lower temperature than LCST. The thermoresponsive polymer-modi®ed
columns were then compared with conventional reversed-phase chromatography column, octadecylsi-
lane (ODS) column for steroid separation. Capacity factors of steroids on each column were evaluated
experimentally. The capacity factors for steroids on BMA-containing copolymer-modi®ed columns
were higher than those observed on PIPAAm-modi®ed column. As BMA content increases from 0.6
to 3.2 mol%, capacity factors for steroids dramatically increased at a given temperature; capacity factor
of testosterone changed from 2.45 for P(IPAAm-co-BMA) (BMA 0.6 mol%) to 7.81 for P(IPAAm-
co-BMA) (BMA 3.2 mol%) at 5 8C and increased from 18.5 to 33.8 at 50 8C, respectively. On the
contrary, when ODS column was used to elute hydrophobic steroids in water no elution of steroids
was achieved. Thus, the elution was performed with 50% methanol/water mixed solvent system, and
signi®cant decrease in capacity factors was apparent with temperature increase from 5 to 30 8C, indicat-
ing decreased interaction with temperature. This is sharp contrast with the case of column packed with
thermoresponsive polymer modi®ed silica.
With increasing hydrophobicity of the column modi®er, successful separation of hydrophobic
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1178
Fig. 8. Chromatograms of ®ve steroids and benzene at different column temperatures with water as a mobile phase from
PIPAAm-modi®ed column (surface type A). Peaks: 1, benzene; 2, hydrocortisone; 3, prednisolone; 4, dexamethasone; 5,
hydrocortisone acetate; 6, testosterone. (Reprinted with permission from Ref. [45]. Copyright (1996) American Chemical
Society.)
compounds in aqueous mobile phase was achieved. However, it also increases total elution times. As the
surface property alteration with temperature is reversible phenomenon, step temperature gradient might
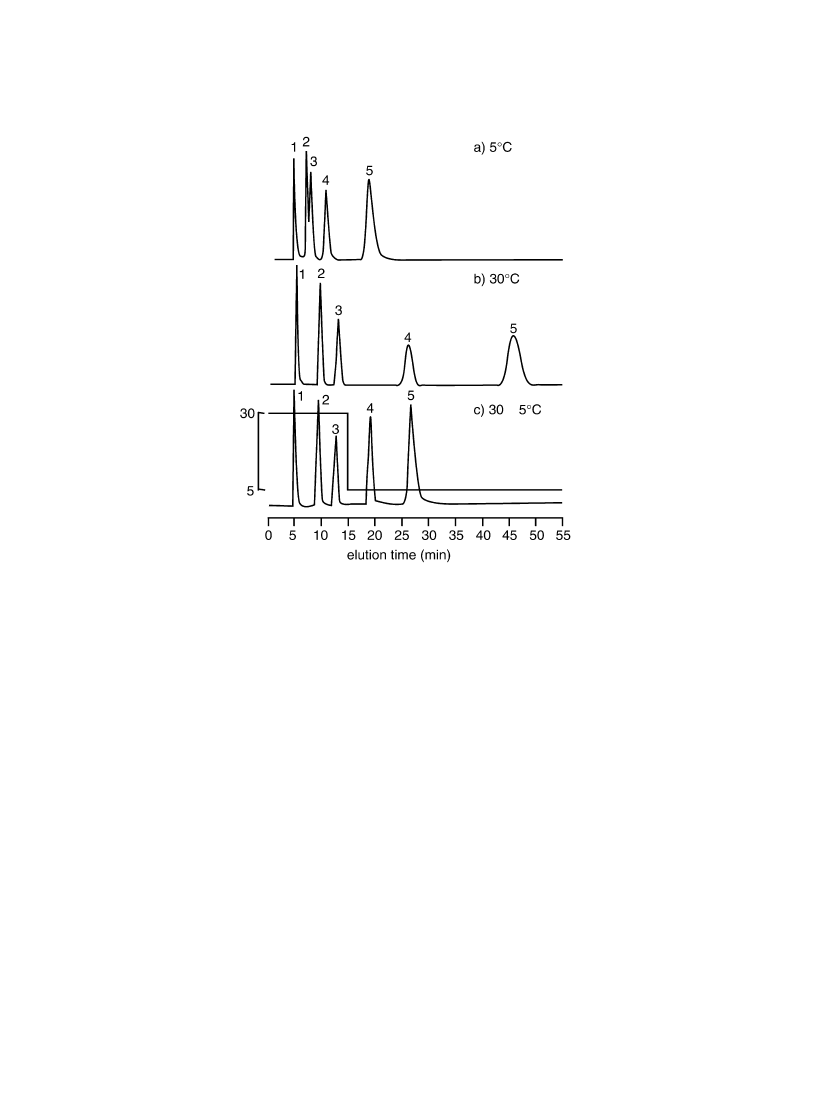
effect to shorten analyzing times. Fig. 9 shows the effect of step temperature gradient on P(IPAAm-co-
BMA) (BMA 3.2 mol%). For steroids with lower partition coef®cients were insuf®ciently separated at
5 8C on P(IPAAm-co-BMA) (BMA 3.2 mol%) (Fig. 9a), while baseline separation was achieved at
30 8C with relatively longer analysis time (Fig. 9b). Thus, after the separation of ®rst three molecules
with lower hydrophobicities at 30 8C, column temperature was decreased to 5 8C by changing thermo-
stated waterbath set at 5 8C through three-way stopcocks. Retention times of steroids with higher
hydrophobicities were shortened with sharper peaks (Fig. 9c). This result indicated the effectiveness
of `thermoresponsive', and `thermoreversible' property alterations of PIPAAm-modi®ed surfaces,
in¯uencing hydrophobic interaction with sample molecules.
Proposed temperature-responsive liquid chromatography can be used with an aqueous mobile phase
without mixing any organic solvents, like acetonitrile and methanol in conventional reversed-phase
chromatography. For protein separation as well as puri®cation, the use of sole aqueous mobile
phase should be an advantage because denaturation of protein and following decrement of bioactivity
is likely caused by the use of organic solvent in the mobile phase. To explore the possibility for
separation of proteins in thermoresponsive chromatography using aqueous mobile phase, mixture
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1179
Fig. 9. Effect of step-temperature gradient on steroid elution from P(IPAAm-co-BMA) (BMA 3.2 mol%)-modi®ed column.
Isocratic elutions using water as mobile phase at (a) 5 8C and (b) 30 8C were indicated to compare with (c) step-temperature
gradient.
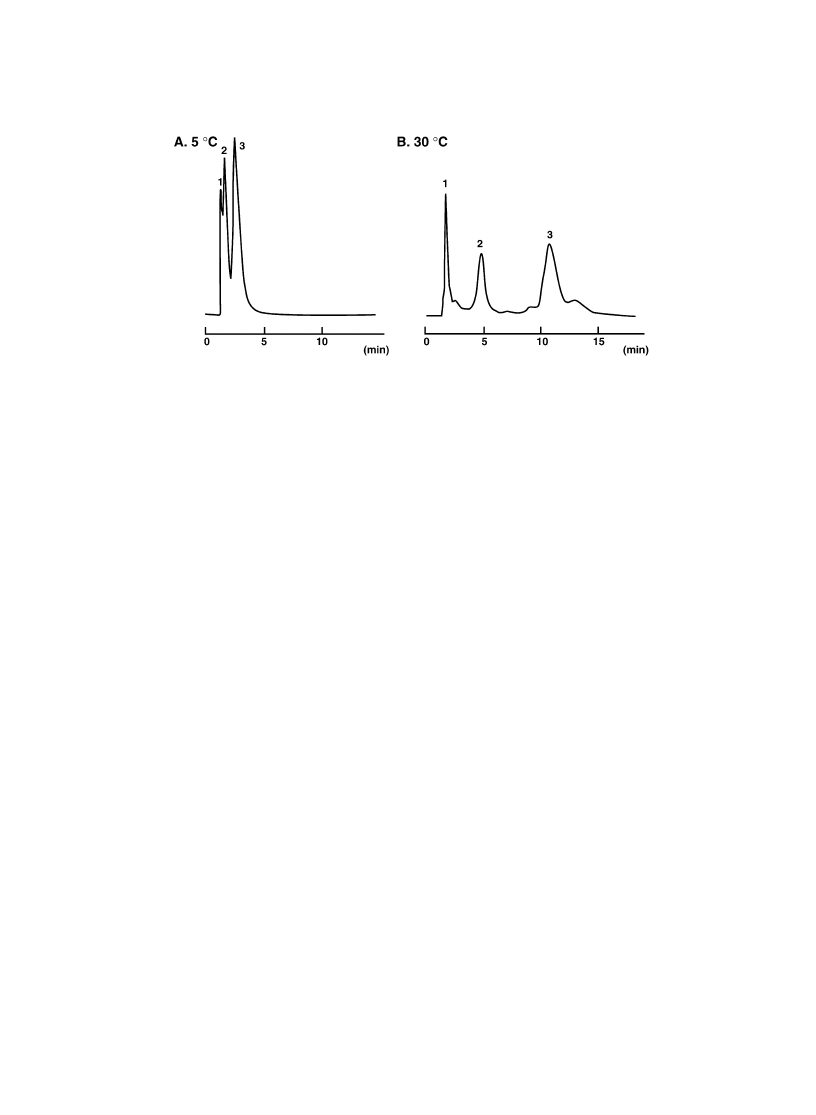
solution of three peptides were applied to P(IPAAm-co-BMA) (BMA 3.2 mol%) column varying
column temperature [48,49]. Used peptide samples were bovine insulin chain A, human b-endorphin
fragment (1±27aa), and bovine insulin chain B. Those samples have approximately same molecular
weight (around 3000) with different amount of hydrophobic amino acid residues. Fig. 10 shows the
elution pro®les of three peptides. At 5 8C, insuf®cient elution of three peptides was obtained. However,
as shown in this ®gure, baseline separation of three peptides were obtained with elution order of insulin
chain A , b-endorphin fragment , insulin chain B. When amino acid sequence of these peptides was
compared, the number of relatively hydrophobic amino acids (leucine, isoleucine, phenylalanine, tryp-
tophan, tyrosine and valine) was increased with the order of insulin chain A , b-endorphin fragment ,
insulin chain B, which directly corresponds to their respective retention times. Thus, it could be
concluded that at least peptides with molecular weight of approximately 3000 are separated through
the hydrophobic interaction with thermoresponsive polymer modi®ed stationary phase and eluted with
the order of their hydrophobicity.
Thermoresponsive P(IPAAm-co-BMA)-modi®ed column is also applicable to the separation and
analysis of phenylthiohydantoin-amino acids (PTH-amino acids), those are formed during amino acid
sequence analysis of the proteins and peptides by applying proteins to automated system for Edman-
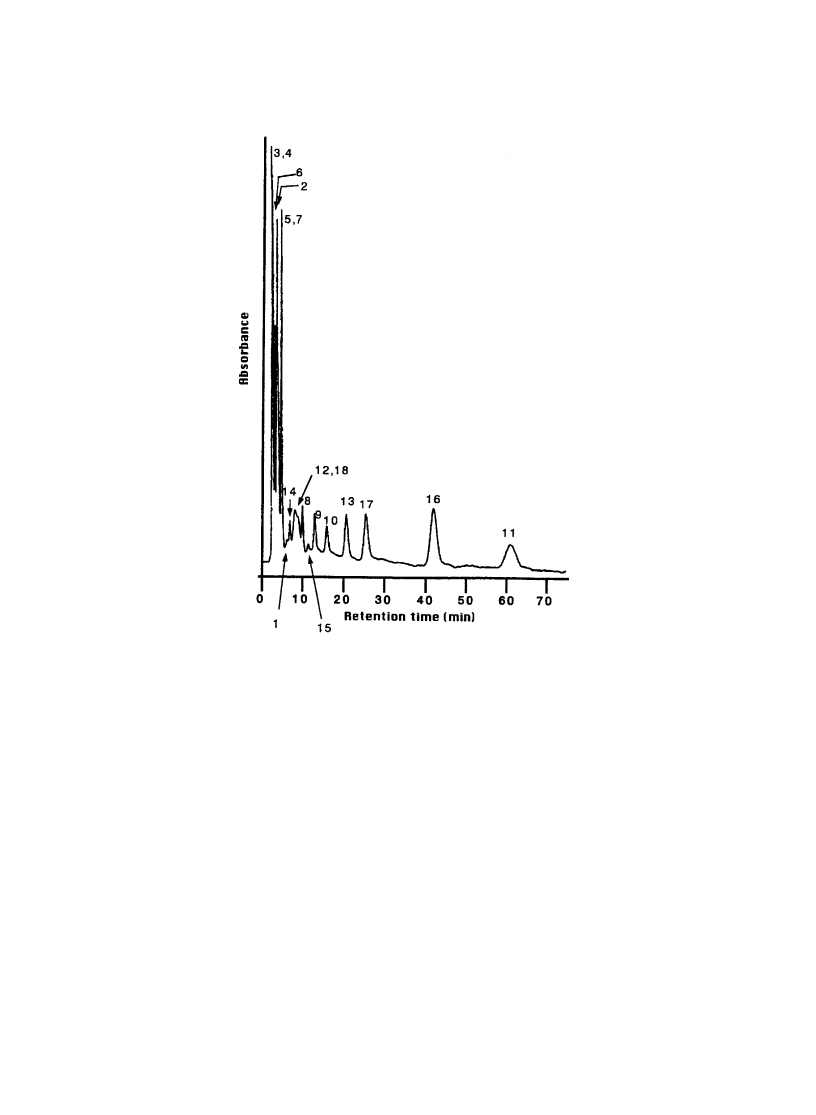
based phenylisothiocyanate degradation [50]. Fig. 11 shows the elution pro®les of 18 PTH-amino acids
from P(IPAAm-co-BMA) (BMA 3.2 mol%) column. The elution times of most of hydrophobic PTH
amino acids were extended at 30 8C than those at 5 8C from P(IPAAm-co-BMA) (BMA 3.2 mol%)
column. Since lysine has two amino groups to be reacted with phenylisothiocyanate, PTH-lysine showed
slowest elution time among the PTH amino acids examined. Step temperature gradient is also revealed to
be effective to separate PTH-amino acids within a shorter time period.
Temperature-responsive polymer-modi®ed column was further utilized to analyze endocrine disrup-
ter, bisphenol A in aqueous mobile phase at 40 8C recently [51]. Yamamoto et al. detected bisphenol A
in the extracts from a coated drink can made from steel.
Above described results strongly indicated the advantage of thermoresponsive polymer-modi®ed
column for analysis of a wide range of samples in aqueous mobile phase by changing only temperature,
no further change in elution condition is needed such as mobile phase composition. Since organic
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1180
Fig. 10. Chromatograms for the mixture of three peptides on P(IPAAm-co-BMA) (BMA 3.2 mol%)-modi®ed column with
0.5 M NaCl solution (pH 2.1) at (a) 5 8C and (b) 30 8C. Peaks: 1, insulin chain A; 2, b-endorphin fragment (1-27aa); 3, insulin
chain B. (Reprinted with permission from Ref. [48]. Copyright (1997) American Chemical Society.)
solvent is not necessary in this chromatographic system, reducing cost for preparation and waste of the
mobile phase is advantageous. Furthermore, it would be desirable for environmental reasons.
6.2. Effect of surface morphology of PIPAAm modi®ed matrix on the separation
As described in Section 4, graft conformation of PIPAAm on the surface greatly in¯uences the
temperature-responsive wettability changes. Thus, it is possible to consider that the graft conformation
of the PIPAAm on the column matrix surfaces show different elution behavior of steroids. Thus,
temperature-dependent elution behavior of steroids was examined on PIPAAm looped chain grafted
surfaces, freely PIPAAm grafted onto PIPAAm loops, and PIPAAm thin hydrogel grafted surfaces ([25]
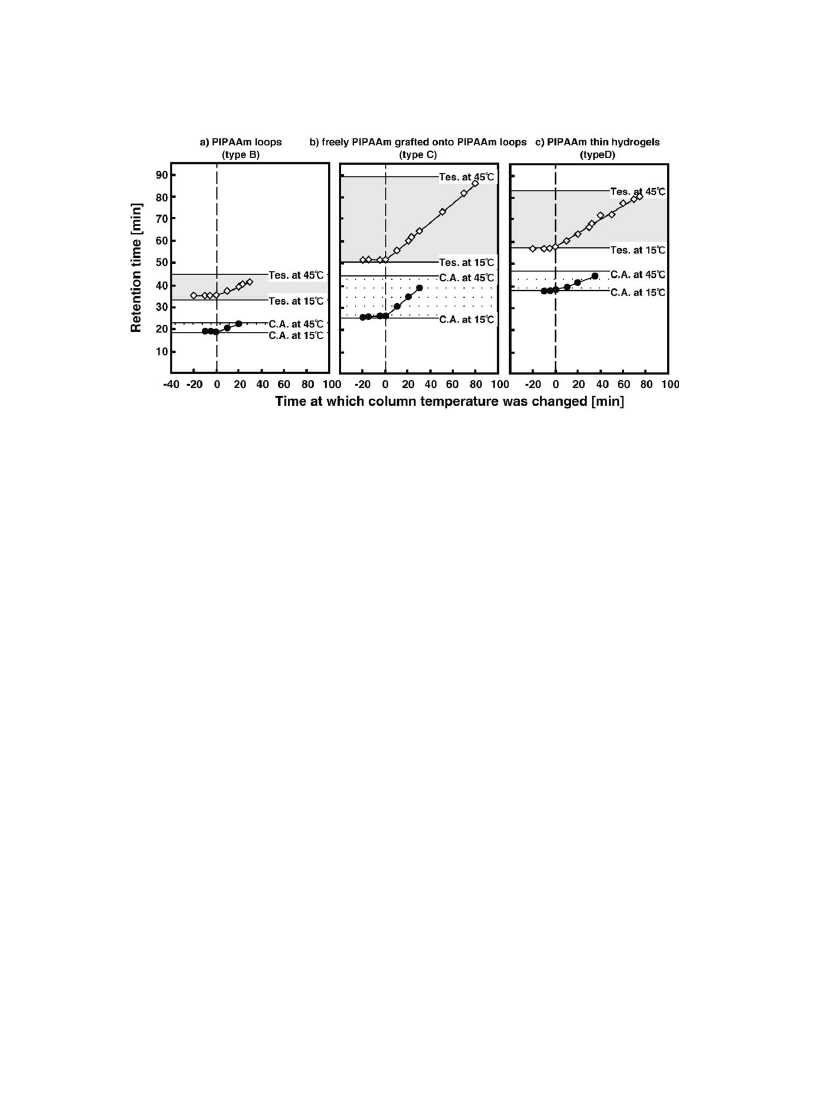
for hydrogel surface). Fig. 12 shows the chromatograms of steroids from three types of PIPAAm-
modi®ed surfaces at 5 and 45 8C, respectively. On each PIPAAm-modi®ed surface, low separation
ef®ciency is apparent from the chromatograms at 5 8C. With increasing temperature to 45 8C, elution
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1181
Fig. 11. Chromatogram for the mixture of 18 PTH-amino acids using water as a sole mobile phase at 30 8C. Peaks: 1, Ala; 2,
Asn; 3, Asp; 4, Cys; 5, Gln; 6, Glu; 7, Gly; 8, His; 9, Ile; 10, Leu; 11, Lys; 12, Met; 13, Phe; 14, Pro; 15, Thr; 16, Trp; 17, Tyr;
18, Val. (Reprinted with permission from Ref. [50]. Copyright (2000) American Chemical Society.)
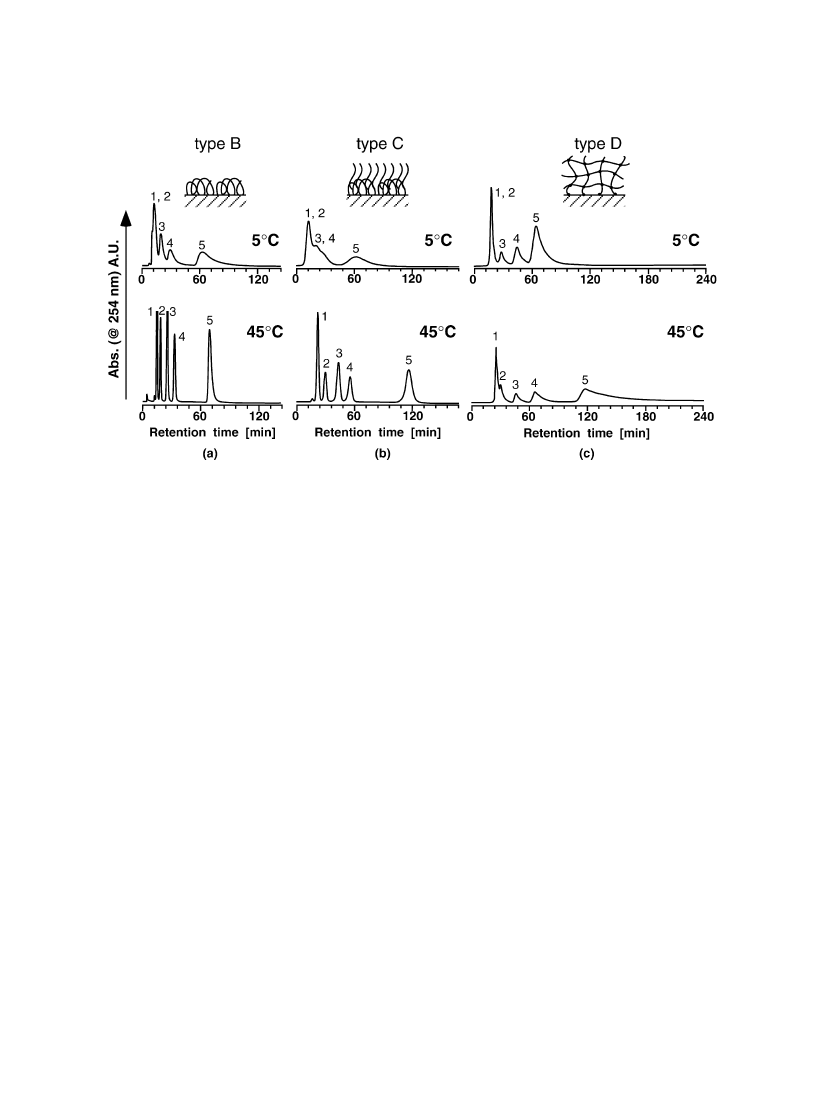
of hydrophobic steroid retarded and longer retention times were observed. As surface grafted polymer
layers have higher graft chain density than freely mobile linear PIPAAm-grafted surfaces as discussed
earlier, analyte partitioning within PIPAAm layers should affect the extension of steroids retention times
and peak broadening. As PIPAAm with free end chains were grafted onto PIPAAm loop chains, thick-
ness of PIPAAm layer increased simultaneously with the grafting reaction. Thus, longer retention was
seen on the surface with free end PIPAAm grafted onto PIPAAm loops than the surface with PIPAAm
loops alone. Signi®cant peak broadening was seen in the case of PIPAAm thin hydrogel grafted surface.
As PIPAAm hydrogel on the matrix surfaces have three-dimensional cross-linked structure, partitioning
of analyte molecules should be signi®cant. Furthermore, the mobility of the PIPAAm chains are
restricted due to the cross-linked structure, in¯uencing peak broadening as well as the retardation of
elution times of steroids. These considerations were supported by the elution pro®les of steroids after
applying step temperature gradient. Step temperature gradient from 45 to 15 8C was applied during the
elution of two steroids, cortisone acetate and testosterone. Fig. 13 shows the change in retention time
with step temperature gradient. Horizontal axis in this ®gure represents time at which column tempera-
ture was changed to 15 8C and time 0 (zero) indicates the sample injection time. Upper and lower lines of
the shaded area in the ®gure represents the isocratic elution time at 45 and 15 8C, respectively, for each
tested samples. Earlier elution of the steroids was observed than that obtained by isocratic elution at
45 8C. For each column, backpressure of the column became equilibrated values within 5 min regardless
of the surface con®guration of PIPAAm graft chains. In the case of PIPAAm loop-grafted surfaces,
retention did not return to the equilibrium retention time at 15 8C with isocratic elution even though the
column temperature was changed before sample injection. This may be due to the restricted graft chain
mobility. After that retention times changed linearly, indicating relatively rapid surface property
alterations because the slow surface property change might produce the retarded retention and retention
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1182
Fig. 12. Chromatograms of steroids from PIPAAm-modi®ed surfaces at 5 and 45 8C using water as mobile phase. (a) PIPAAm
loop grafted, (b) free PIPAAm grafted onto PIPAAm loops, (c) PIPAAm thin hydrogel grafted surfaces. Peaks: 1, cortisone; 2,
prednisolone; 3, dexamethasone; 4, cortisone acetate; 5, testosterone.
curve changes convexly. Thus, mobile phase temperature would change rapidly in this chromatographic
condition, which results in the alteration of surface properties of PIPAAm-grafted matrix. Two column
types; freely PIPAAm grafted onto PIPAAm loops, and PIPAAm thin gel layer grafted matrix showed
relatively large changes with temperature. This may be due to the thickness of the grafted polymer layer
to partitioning analyte samples. Although the present results need longer analysis time, system could be
improved to shorten analysis time with good separation factors. Molecular design of the matrix interface
with thermoresponsive polymer modi®er is promising approach to modulate interactions of and separate
analyte samples.
After Kanazawa's reports, Teal et al. [52] reported the temperature mediated hydrophobic modulation
chromatography for puri®cation of biomolecules. They utilized PIPAAm microgels instead of solid
phase materials modi®ed with PIPAAm as in the reports by Kanazawa et al. They used the prepared
PIPAAm microgel packed column to separate several sets of amino acids, proteins, and nucleotides.
Although they showed changes in elution of these biomolecules, they mixed PIPAAm microgels with
commercially available Sephadex bead matrix. This is probably because the avoidance of thermoresponsive
void volume changes inside the column since the PIPAAm gels are known to show signi®cant volume
transition with temperature changes [7,53,54]. Kanazawa et al. [45,48±50] and Yakushiji et al. [25] used
solid silica beads as base material for PIPAAm modi®cation. Furthermore, introduced PIPAAm molecules
produced signi®cantly thinner surface layers on the silica beads, minimum void volume changes is apparent
as was judged by the no change in marker molecule elution time with temperature. It is concluded that
the molecular architecture of PIPAAm as the column matrix should be one of important points for designing
aqueous temperature responsive chromatography columns for separation of diverse biomolecules.
6.3. Effect of surface charged groups on analyte separation
In Sections 6.1 and 6.2, mainly separation of hydrophobic substances was discussed using thermally
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1183
Fig. 13. Effects of step-temperature gradient on steroid elution from thermoresponsive columns with three different molecular
architectures using water as mobile phase. Sample was injected at time 0 (zero) in the ®gure and step-temperature gradient was
applied at given temperature indicated in horizontal axis. Tes., testosterone; C.A., cortisone acetate.

responsive and reversible solid phase surfaces with a sole aqueous mobile phase. In this section, recent
approach is described to separate charged biomolecules using charged, thermoresponsive matrix.
A wide variety of biomolecules possess both charge, and hydrophobic nature in the molecules. In
general, biomolecules are separated with reversed-phase chromatography, ion exchange chromatogra-
phy systems and these combinations. Although these procedures are currently utilized in wide ®elds, use
of organic solvents may limit further application of separated molecules due to the denaturation,
especially proteins. Thus, if electrostatic and hydrophobic interactions were modulated simultaneously
with temperature in aqueous mobile phase, the separation system would have potential application in
separation science. Described approach is to introduce weakly charged functional groups, carboxyl or
amine groups, into thermoresponsive PIPAAm derivatives onto base silica beads matrix.
Cross-linked poly(IPAAm-co-N,N-dimethylaminopropylacrylamide (DMAPAA)) with 20 mol%
DMAPAA was grafted onto silica beads to obtain cationic thermoresponsive column matrix [55].
This matrix could potentially be applied to ion exchange column in which eluent pH is changed in a
wide range. Silica base materials, however, are labile at higher pH like pH 10. Thus, the polymer
modi®ed beads as well as the base aminopropylsilica beads were immersed into alkaline solution at
pH 10 for 21 days and checked diameter change by means of scanning electron microscope (SEM) and
surface chemical composition by ESCA. Bare silica beads showed decrease in bead diameter from 5.2 to
4.2 mm, while negligible change was observed on polymer-modi®ed beads without change in chemical
composition at the surface as determined by ESCA measurement even at pH 10. Therefore, polymer
coating on the beads improved the surface stability for at least 21 days at pH 10. In the practical use, pH
of the medium is mostly neutral in the following experiments; there would be negligible size as well as
chemical composition changes during chromatographic analysis. The cationic matrix was used as
column matrix and separation of anionic substances was carried out at pH 7. With increasing tempera-
ture, elution time of anionic analytes was shortened. IPAAm sequence in the copolymer matrix dehy-
drated and surfaces became hydrophobic. Under this condition, micro environmental polarity around
amino functions decreased, which induce deprotonation of amino groups, leading to weak electrostatic
interaction with samples. Thus, retention time was shortened with temperature. Feil et al. [56] reported
the effects of pH on the swelling behavior of pH-/temperature-responsive hydrogels with temperature
changes. Swelling transition pH for pH-/temperature-responsive hydrogels was found to change with
temperature. The results strongly supported the above-mentioned discussion.
Anionic temperature-responsive polymer modi®ed column was also prepared to examine the modula-
tion of electrostatic interaction between catecholamines [57]. For this purpose, we prepared copolymer
of IPAAm and acrylic acid (AAc) with various chemical compositions. As AAc content in the copoly-
mer increased, LCST also was increased. Suf®cient soluble/insoluble phase transition was observed
below the 3 mol% AAc containing polymers. Catecholamine derivatives were used to examine
P(IPAAm-co-AAc)-modi®ed column as thermoresponsive chromatography. At lower pH where AAc
exists in protonated carboxyl, low retention with poor separation of samples was observed. At pH 7,
however, stronger interaction occurred and longer retention was observed for positively charged
samples. Electrostatic interaction is the primary force for retention of catecholamines. Since hydro-
phobic steroids have stronger interaction and increased retention time was observed with temperature at
pH 5 on P(IPAAm-co-AAc) column, sample hydrophobicity also affected on the catecholamine retention
because catecholamine also has hydrophobic benzene ring. Hence both electrostatic and hydrophobic
interactions simultaneously in¯uence partitioning of basic catecholamine derivatives. These two interaction
forces compete with each other, those interaction forces modulate retention of cationic substances.
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1184

Charged groups should be hydrophilic in aqueous solution, introduction of these groups increases the
transition temperature of matrix PIPAAm condition as described earlier. Co-incorporation of hydro-
phobic monomer, butyl methacrylate (BMA), suppresses increase in polymer transition temperature,
thus, ternary copolymer, cross-linked P(IPAAm-co-DMAPAA-co-BMA) was prepared on the silica
bead surfaces, and the modi®ed bead matrix was used for separation of adenosine nucleotides [58].
Temperature-dependent amine pK
a
shift in the copolymer was examined. Amine pK
a
decreased with
increasing temperature, especially around polymer transition temperature a drastic decrease in pK
a
was
observed. Furthermore, surface potential of the beads indicated that the amine-containing thermo-
responsive polymer-modi®ed beads showed lightly positive surface potentials below the polymer transi-
tion temperature than PIPAAm-modi®ed beads, due to the incorporation of cationic amine sites. Above
the transition temperature, surface potential decreased to negative value. These results indicated that
deprotonation of the protonated amino groups below the transition temperature occurred by only
increasing temperature. Urry et al. [59,60] reported the pK
a
shift of amino acid carboxyl groups in
hydrophobic polypeptides with temperature. They claimed that the hydrophobicity and micro environ-
mental polarity changes strongly in¯uenced to the protonation/deprotonation changes of carboxyl
groups in polypeptides. Their results strongly supported the results of the temperature-responsive charge
density control of P(IPAAm-co-DMAPAA-co-BMA)-modi®ed surfaces. Ternary polymer-modi®ed
silica beads were then used in the separation of adenosine nucleotides. Adenine nucleotides; AMP,
ADP, and ATP, play a role in energy metabolism in cellular organism, and are important with respect to
the bioanalytical and biochemical research. A mixture of adenosine nucleotides was injected into the
thermostated P(IPAAm-co-DMAPAA-co-BMA) column and monitored elution pro®les at different
temperatures. With increasing amount of phosphate group in the molecular structure, increased retention
time was observed regardless of temperature. Poor resolution of three nucleotides is observed on the
PIPAAm-modi®ed column. Thus, the electrostatic interaction dominates between positively charged
matrix surfaces and negatively charged samples. Increase in temperature resulted in the shorter retention
times and narrower peaks. The van't Hoff plots indicated the discontinuous changes in capacity factors at
temperature corresponding to the polymer transition temperature. In this case, the step temperature
gradient is effective to modulate elution of sample analytes similar to the gradient elution in
reversed-phase chromatography by altering solvent composition. Although, the system still needs longer
time for complete analysis, column condition could be optimized through chemical composition of
copolymer modi®er, size of column and silica bead diameter, and so on. Adenosine nucleotides are
currently analyzed with reversed-phase column in the absence of [61,62] or in the presence of additive
alkylamine (ion-pair RPC) [63±65] to modulate sample property and interaction with stationary phase
surfaces and thus elution times. The use of intelligent materials should prove valuable in the design of
novel aqueous `green' chromatography systems.
7. Selective adsorption/desorption of bioactive proteins
Selective protein separation and puri®cation are usually carried out using af®nity columns [66]. On the
af®nity columns, biomolecules with speci®c binding ability to target molecules are immobilized. Most
commonly used biomolecules conjugated onto the af®nity matrix are antibody. In the af®nity separation,
recovery of target proteins is commonly achieved through changing eluent composition, pH and/or ionic
strengths, or rather, urea to actively denature adsorbed proteins altering binding strength to antibody.
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1185

However, the recovery procedure frequently causes irreversible denaturation of recovered proteins,
which limits further application in biomedical purposes. Thus, af®nity separation with simple adsorp-
tion/desorption procedure is needed.
Galaev et al. [67,68] utilized temperature-responsive polymer, poly(N-vinylcaprolactam) (PVCL) as
eluent modi®er for elution of lactate dehydrogenase (LDH) bound to dye-af®nity chromatographic
column, Blue Sepharose. As above noted, to recover proteins adsorbed on the dye-conjugated column
matrixes elution with high salt concentration in buffer is frequently utilized to weaken protein±dye
interactions. However, in their system, PVCL was added to the eluent to recover proteins on the column
matrix without changing salt concentration in the eluent solution [67]. They synthesized Blue Sepharose
from Sepharose conjugated with Cibacron Blue. Then, LDH was applied onto the Blue Sepharose
packed columns. Polymer elution was carried out with 1% PVCL solution. They found that 1%
PVCL solution is a more ef®cient non-speci®c eluting agent than 1.5 M KCl solution. PVCL contains
amide polar group which interacts strongly with matrix-conjugated Cibacron Blue, therefore, PVCL
could be displaced column bound LDH. Since PVCL is a thermoresponsive polymer with a LCST
around 35±40 8C, PVCL in a polymer-enriched phase could be separated with raising solution tempera-
ture after the LDH elution with PVCL. Increasing temperature resulted in precipitation of PVCL, thus
with a low speed centrifugation precipitated polymer was separated. PVCL was completely recovered
from aqueous phase during PVCL precipitation with temperature treatment. Since the temperature was
not exceeded above 40 8C, minimum denaturation of separated enzymes was con®rmed. Recovered,
precipitated PVCL can be re-dissolved by lower temperature treatment and re-used for further chroma-
tographic runs. Galaev et al. [68] further examined by modifying Cibacron Blue-conjugated column
matrix with PVCL to shield dye molecules. Column shielding was performed with 1% PVCL solution
(ca. 50 column volumes) followed by column washing with 1.5 M KCl and re-equilibration with
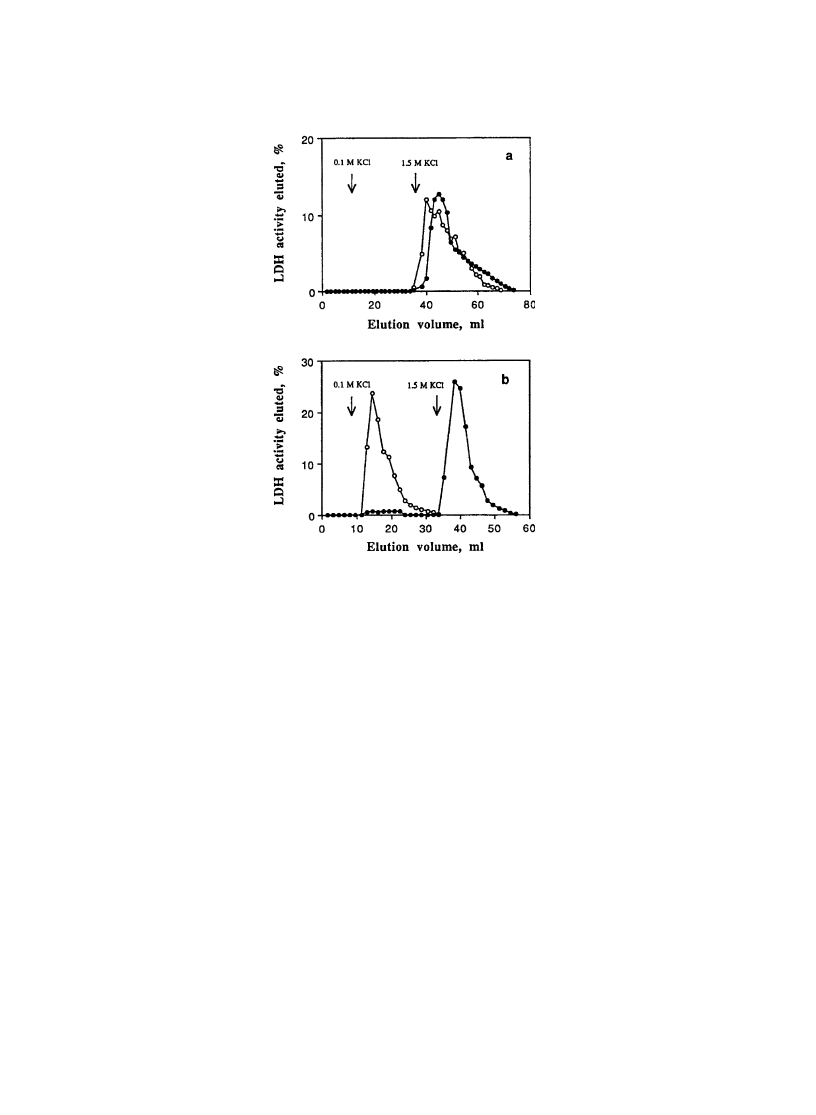
appropriate buffer solution before chromatographic runs. Fig. 14 shows the comparison of LDH elution
from unmodi®ed column and PVCL-shielded column at 23 and 40 8C. As shown in Fig. 14a, LDH
elution was observed only after the addition of 1.5 M KCl, which is required for elution of dye-bound
enzymes regardless of temperature. By strong contrast, PVCL shielding greatly affected LDH elution
from the column (Fig. 14b). At 40 8C, only a slight elution was observed, and majority of the enzymes
bound to Cibacron Blue dye on column matrixes is eluted out by the addition of 1.5 M KCl. At 23 8C,
however, elution is successful with 0.1 M KCl. This is a sharp contrast from the unmodi®ed column.
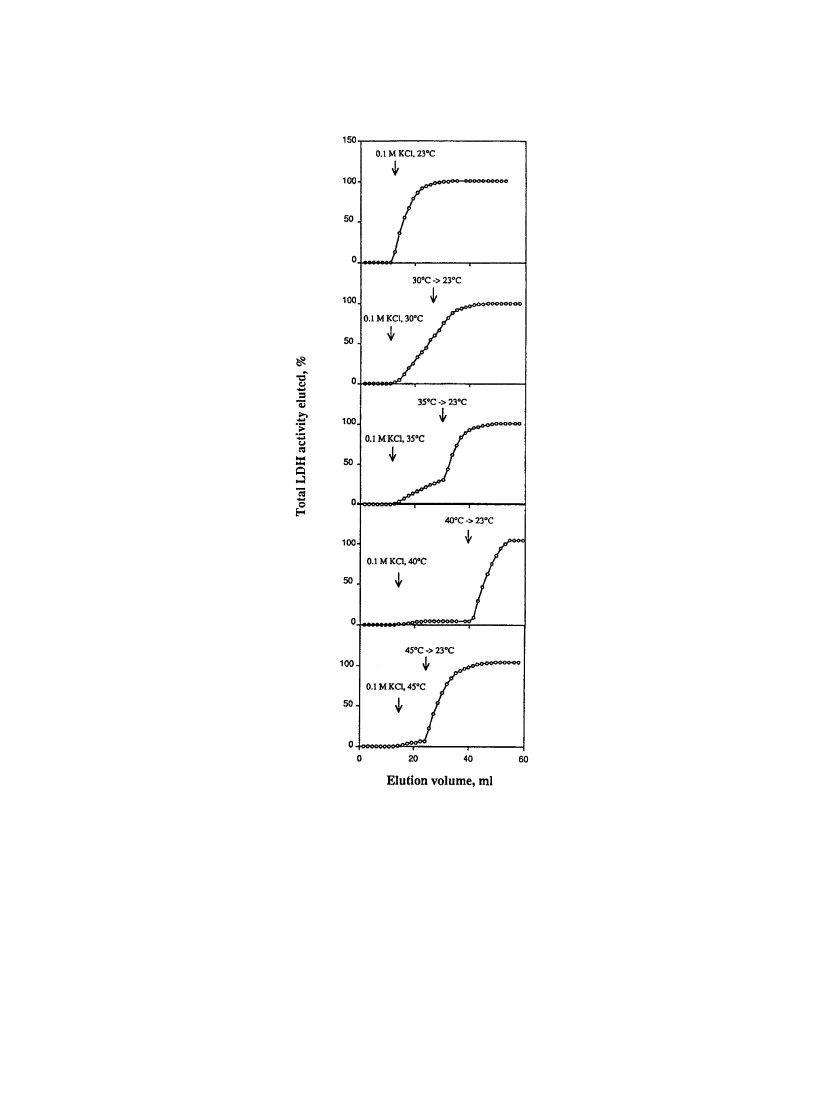
Thus, they further investigated the effect of temperature change during LDH elution with 0.1 M KCl as
eluent (Fig. 15). By raising temperature virtually no elution was obtained at 40 and 45 8C (above the
LCST of the PVCL). Only cooling column temperature is the necessary and simple method for elution of
column-bound enzymes. PVCL bound to the Cibacron Blue molecules on the column matrix surfaces
with multivalent manner. At lower temperature where PVCL is hydrated, loose coils, af®nity of the
PVCL to Cibacron Blue compete with LDH and slight decrease of LDH binding ef®ciency with 0.1 M
KCl elution is suf®cient to elute LDH from the column. By contrast, PVCL dehydrates, shrunk at higher
temperature above the LCST, which cause exposure of more amount of LDH toward Cibacron Blue on
the matrix surfaces. Therefore, stronger interaction occurs between LDH and Cibacron Blue, which lead
to the necessity of higher salt concentration of KCl for LDH elution. Galaev et al. then utilized PVCL-
shielded Blue Sepharose column for puri®cation of LDH from crude porcine muscle extracts. Crude
extract was applied to the PVCL-shielded column at 40 8C until breakthrough. Then the foreign proteins
in the crude extract were eluted out with 0.1 M KCl at 40 8C. After that the column temperature was
lowered to 23 8C, resulting in successful elution of LDH. During this procedure, the recovery of LDH
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1186
was 90%. In conclusion, shielding of Cibacron Blue dye molecules in the Blue Sepharose column with
PVCL is the simple, ef®cient method to modulate LDH elution with temperature changes.
In their method, there is a possibility of co-elution of surface bound PVCL molecules during the
protein elution. Galaev et al. concluded that, as PVCL is temperature-responsive polymer, raising
temperature of the ef¯uent followed by low speed centrifuge is suf®cient to separate co-eluted PVCL.
This might be tedious procedure if the method extends to commercial use.
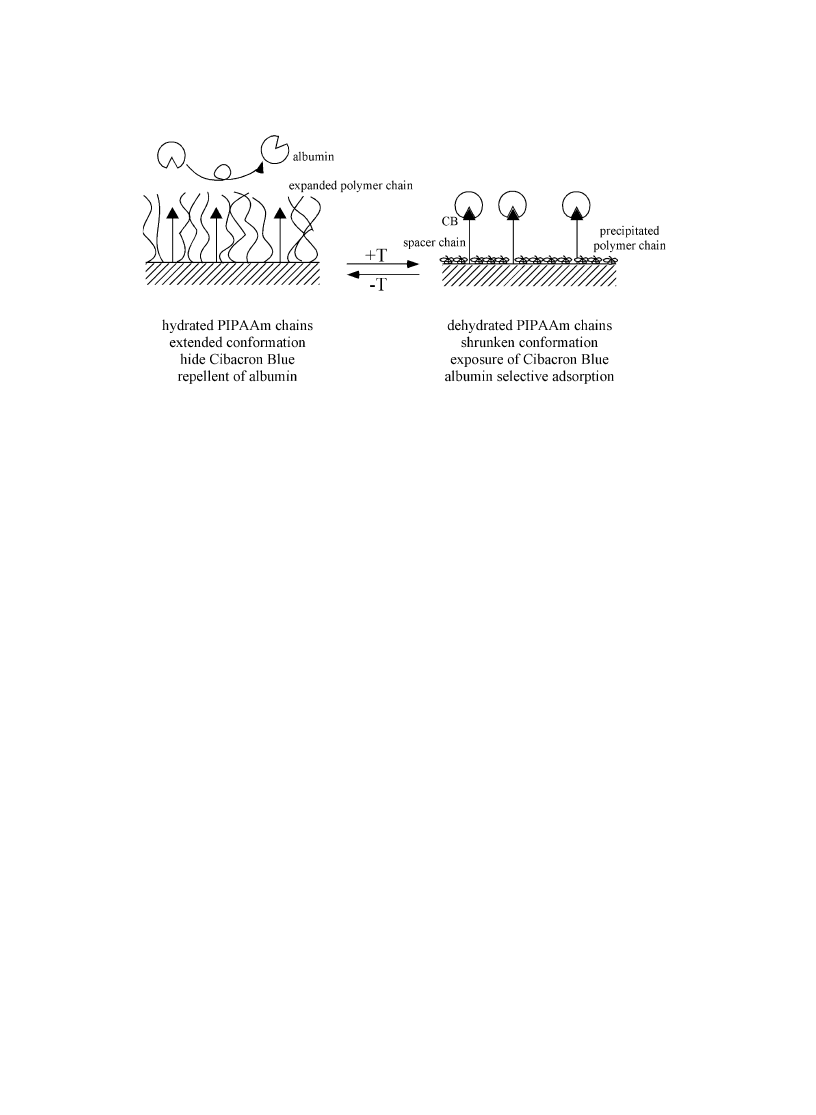
PIPAAm's soluble/insoluble changes have been recently utilized to control af®nity of target molecule
by modulating the surrounding temperature [69]. In this system, adsorbed biomolecules could be
completely recovered by lowering temperature through `kicking-out' effect of samples with expanding
PIPAAm chains (Fig. 16). To do this, Cibacron Blue is immobilized onto amino functionalized poly-
methacrylate bead matrixes with spacers having different spacer sizes. Cibacron Blue is known to have
higher af®nity to serum albumin molecules and used in dye-af®nity chromatography [70,71]. The matrix
surfaces are co-immobilized with end-carboxyl PIPAAm. End-carboxyl PIPAAm was synthesized
through radical telomerization using MPA as chain transfer agents in N,N-dimethylformamide (DMF)
[46]. The PIPAAm chain lengths are controlled by the molar ratio of thiol compounds to IPAAm
monomer. In our study, the molecular weight of PIPAAm was 1900 with molecular weight distribution
index of 3.8 as determined by terminal carboxyl quanti®cation by titration and GPC measurement.
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1187
Fig. 14. Elution pro®le of LDH at 23 8C (open circle) and 40 8C (closed plot) from (a) unmodi®ed and (b) PVCL-shielded Blue
Sepharose column with 0.1 M KCl, followed by elution with 1.5 M KCl. Arrows in the ®gure indicate when elution with 0.1 or
1.5 M KCl was begun. (Reprinted from Yu, et al. Temperature-induced displacement of proteins from dye-af®nity columns
using an immobilized polymeric displacer. J Chromatogr A 1994;684:37±43 with permission from Elsevier Science.)
Polymethacrylate beads with epoxy side chains are aminated with 1,6-hexamethylenediamine for further
conjugation of spacer molecules and active esteri®ed PIPAAm molecules. Spacers used were 1,3-
butadiene diepoxide and ethylene glycol diglycidylether. Those spacer molecules were used to change
the distance between bead surfaces and af®nity molecule, Cibacron Blue. Assuming that the surface-
grafted PIPAAm is fully expanded below the LCST, its length is comparable or slightly longer than the
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1188
Fig. 15. Elution pro®les of LDH from PVCL-shielded Blue Sepharose with 0.1 M KCl at different temperatures. Arrows in the
®gures indicated when elution was interrupted, the column was cooled to 23 8C and elution was continued at this temperature.
(Reprinted from Yu, et al. Temperature-induced displacement of proteins from dye-af®nity columns using an immobilized
polymeric displacer. J Chromatogr A 1994;684:37±43 with permission from Elsevier Science.)
Cibacron Blue bound with 1,3-butadiene diepoxide. However, in case of ethyleneglycol diglycidylether
as the spacer, the length of Cibacron Blue is much longer than the fully expanded PIPAAm molecules.
Thus, by changing temperature, the number of af®nity molecules on the bead surfaces could be regu-
lated. In fact, no albumin adsorption onto PIPAAm-grafted surfaces was evident, however, temperature
dependent adsorption was obvious for Cibacron Blue co-immobilized beads surfaces. At higher
temperature than PIPAAm's LCST, albumin adsorption is large, and the amount decreased with lower-
ing temperature below the LCST. Adsorbed albumin at higher temperature was easily desorbed with low
temperature treatment, where PIPAAm molecules hydrate and expand to outward. Expansion of
PIPAAm molecules induced albumin conjugated to Cibacron Blue to be pushed out. Almost all albumin
molecules on the Cibacron Blue immobilized with 1,3-butadiene diepoxide spacers were desorbed with
only decreasing temperature. No modi®cation to eluent pH, or ionic strength is applied. When ethylene
glycol diglycidylether was used as spacer molecule for Cibacron Blue immobilization, the limited
number of albumin adsorbed on the bead surfaces can be desorbed with lowering temperature. This
is probably due to the insuf®cient expansion of PIPAAm molecules at lower temperature since the
expanded chain length of hydrated PIPAAm is calculated to be shorter than Cibacron Blue with ethylene
glycol diglycidylether spacers. Thus, the size of spacer molecules and PIPAAm chain length are both
dominant factors for controlled albumin adsorption/desorption behavior.
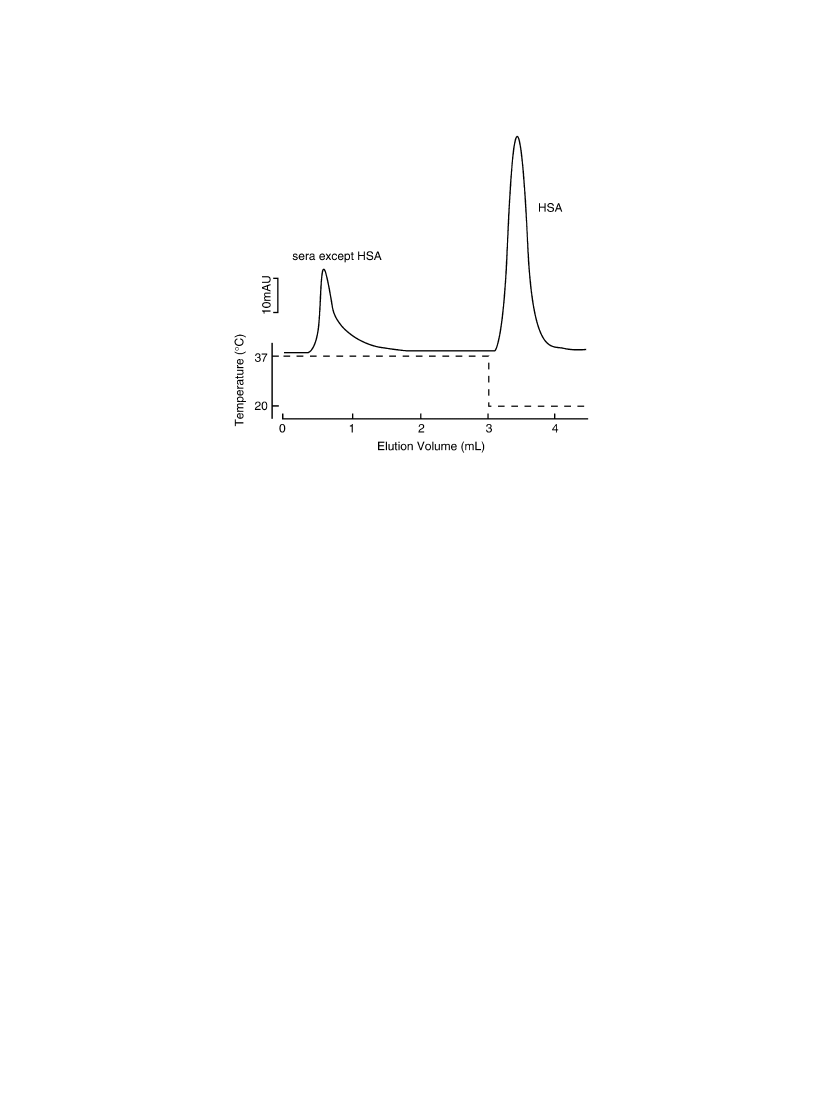
The human sera were then used to separate human serum albumin from the sera (Fig. 17). At higher
temperature (37 8C), only one peak was appeared. Using SDS-PAGE, this peak contains two different
molecular weight substances other than albumin. After 3 ml elution, column temperature was decreased
to 20 8C and a single, major peak was obtained. In this peak only one molecular weight protein was
found in SDS-PAGE, which corresponds to the human albumin. This result strongly supports the new
thermoresponsive chromatography with `selective catch and release' mechanism other than hydrophobic
interaction or pore size control with thermoresponsive PIPAAm grafts.
Under this procedure, no contamination of PIPAAm was achieved because PIPAAm was covalently
bound on the matrix surfaces and unbound molecules were extensively washed out during matrix
preparation. Thus, this method is feasible to extend a large number of proteins.
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1189
Fig. 16. Schematic representation of the concept for selective adsorption/desorption control with thermoresponsive PIPAAm
co-grafted with af®nity dye, Cibacron Blue.
8. Conclusion
In this review, surface modi®cation with stimuli-responsive `intelligent materials' is introduced. Modi®ed
surfaces and interfaces were utilized to control permeation, diffusion of substances to be separated and/or
released. Special attention was made on the thermo-responsive polymer, PIPAAm, as a surface modi®er, and
on PIPAAm-modi®ed surfaces for possible use in chromatographic matrix with sole aqueous mobile
phase. Successful modulation of interaction between PIPAAm-modi®ed surfaces and samples was
apparent with alteration of surface property through temperature control in aqueous solution. This
feature should be feasible to develop `green' chromatography systems with sole aqueous mobile phase.
Acknowledgements
Part of the present work on thermoresponsive aqueous chromatography was carried out by collabora-
tion with Professors Hideko Kanazawa and Yoshiazu Matsushima of Kyoritsu College of Pharmacy, Drs
Yukio Hasegawa, Yasuro Shinohara, Kimihiro Yoshizako, and Yoshikatsu Akiyama of Amersham
Biosciences Co., and Dr Taiji Yakushiji, and Professor Kiyotaka Sakai of Waseda University. Financial
support was provided from the Japan Chemical Innovation Institute (JCII) under the New Energy and
Industrial Technology Development Organization (NEDO) from ®scal year of 1996±2000 to which the
authors would express sincere acknowledgement.
References
[1] Okano T, Yoshida R. Intelligent polymeric materials for drug delivery. In: Tsuruta T, Hayashi T, Kataoka K, Ishihara K,
Kimura Y, editors. Biomedical applications of polymeric materials. Boca Raton, FL: CRC Press, 1993. p. 407±28.
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1190
Fig. 17. Effect of step temperature gradient on selective `catch and release' of target molecule, human serum albumin, for
PIPAAm-co-grafted Cibacron Blue af®nity column.

[2] Okano T. Molecular design of temperature-responsive polymers as intelligent materials. In: Dusek K, editor. Advances in
polymer science, responsive gels; volume transition II. Berlin: Springer, 1993. p. 179±97.
[3] Okano T, Yui N, Yokoyama M, Yoshida R. Advances in polymeric systems for drug delivery. Yverdon, Switzerland:
Gordon & Breach, 1994.
[4] Okano T, editor. Biorelated polymers and gels: controlled release and applications in biomedical engineering. Chestnut
Hill, MA: Academic Press, 1998.
[5] Hoffman AS. Intelligent polymers in medicine and biotechnology. Macromol Symp 1995;98:645±64.
[6] Heskins M, Guillet JE, James E. Solution properties of poly(N-isopropylacrylamide). J Macromol Sci, Chem A
1968;2:1441±5.
[7] Bae YH, Okano T, Kim SW. Temperature dependence of swelling of crosslinked poly(N,N-alkyl substituted acrylamides)
in water. J Polym Sci: Part B, Polym Phys 1990;28:923±36.
[8] Kanazawa H, Matsushima Y, Okano T. Temperature-responsive chromatography. In: Brown PR, Grushka E, editors.
Advances in chromatography. New York: Marcel Dekker, 2001. p. 311±36.
[9] Iwata H, Matsuda T. Preparation and properties of novel environment-sensitive membranes prepared by graft polymer-
ization onto a porous membrane. J Membr Sci 1988;38:185±99.
[10] Iwata H, Hirata I, Matsuda Y. Atomic force microscopic images of solvated polymer brushes. Langmuir
1997;13:3063±6.
[11] Ito Y, Casolaro M, Chung DJ, Imanishi Y. Design and synthesis of glucose-sensitive insulin-releasing systems. Drug
Deliv Syst 1988;3(3):391±7.
[12] Ito Y, Casolaro M, Kono K, Imanishi Y. An insulin-releasing system that is responsive to glucose. J Control Release
1989;10:195±203.
[13] Okahata Y, Noguchi H, Seki T. Thermoselective permeation from a polymer-grafted capsule membrane. Macromolecules
1986;19:493±4.
[14] Iwata H, Oodate M, Uyama Y, Amemiya H, Ikada Y. Preparation of temperature-sensitive membranes by graft poly-
merization onto a porous membrane. J Membr Sci 1991;55:119±30.
[15] Fong RB, Ding Z, Long CJ, Hoffman AS, Stayton PS. Thermoprecipitation of streptavidin via oligonucleotide-mediated
self-assembly with poly(N-isopropylacrylamide). Bioconj Chem 1999;10:720±5.
[16] Stayton PS, Shimoboji T, Long C, Chilkoti A, Chen G, Harris JM, Hoffman AS. Control of protein-ligand recognition
using a stimuli-responsive polymer. Nature 1995;378:472±4.
[17] Chilkoti A, Chen G, Stayton PS, Hoffman AS. Site-speci®c conjugation of a temperature-sensitive polymer to a geneti-
cally-engineered protein. Bioconj Chem 1994;5:504±7.
[18] Gewehr M, Nakamura K, Ise N, Kitano H. Gel permeation chromatography using porous glass beads modi®ed with
temperature-responsive polymers. Makromol Chem 1992;193:249±56.
[19] Hosoya K, Sawada E, Kimata K, Araki T, Tanaka N, Frechet JMJ. In situ surface-selective modi®cation of uniform size
macroporous polymer particles with temperature-responsive poly-N-isopropylacrylamide. Macromolecules
1994;27:3973±6.
[20] Hosoya K, Kimata K, Araki T, Tanaka N, Frechet JMJ. Temperature-controlled high-performance liquid chromatography
using a uniformly sized temperature-responsive polymer-based packing material. Anal Chem 1995;67:1907±11.
[21] Lakhiari H, Okano T, Nurdin N, Luthi C, Descouts P, Muller D, Jozefonviz J. Temperature-responsive size-exclusion
chromatography using poly(N-isopropylacrylamide) grafted silica. Biochim Biophys Acta 1998;1379:303±13.
[22] Go H, Sudo Y, Hosoya K, Ikegami T, Tanaka N. Effects of mobile-phase composition and temperature on the selectivity
of poly(N-isopropylacrylamide)-bonded silica gel in reversed-phase liquid chromatography. Anal Chem 1998;70:4086±
93.
[23] Takei YG, Aoki T, Sanui K, Ogata N, Sakurai Y, Okano T. Dynamic contact angle measurement of temperature-
responsive surface properties for poly(N-isopropylacrylamide) grafted surfaces. Macromolecules 1994;27(21):6163±6.
[24] Yakushiji T, Sakai K, Kikuchi A, Aoyagi T, Sakurai Y, Okano T. Graft architectural effects on thermo-responsive
wettability changes of poly(N-isopropylacrylamide)-modi®ed surfaces. Langmuir 1998;14(16):4657±62.
[25] Yakushiji T, Sakai K, Kikuchi A, Aoyagi T, Sakurai Y, Okano T. Effects of cross-linked structure on temperature-
responsive hydrophobic interaction of PIPAAm hydrogel modi®ed surfaces with steroids. Anal Chem 1999;71(6):1125±
30.
[26] Morra M, Cassinelli C. Thermal recovery of cells cultured on poly(N-isopropylacrylamide) surface-grafted polystyrene
dishes. Polym Prepr 1995;36(1):55±6.
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1191

[27] Volpe CD, Cassinelli C, Morra M. Wilhelmy plate measurements on poly(N-isopropylacrylamide)-grafted surfaces.
Langmuir 1998;14(16):4650±6.
[28] Liang L, Feng X, Liu J, Rieke PC, Fryxell GE. Reversible surface properties of glass plate and capillary tube grafted by
photopolymerization of N-isopropylacrylamide. Macromolecules 1998;31:7845±50.
[29] Pan YV, Wesley RA, Luginbuhl R, Denton DD, Ratner BD. Plasma polymerized N-isopropylacrylamide: synthesis and
characterization of a smart thermally responsive coating. Biomacromolecules 2001;2(1):32±6.
[30] Takezawa T, Yamazaki M, Mori Y, Yonaha T, Yoshizato K. Morphological and immuno-cytochemical characterization
of a heterospheroid composed of ®broblasts and hepatocytes. J Cell Sci 1992;101:495±503.
[31] Takezawa T, Mori Y, Yoshizato K. Cell culture on a thermo-responsive polymer surface. Biotechnology
1990;8:854±6.
[32] Yamada N, Okano T, Sakai H, Karikusa F, Sawasaki Y, Sakurai Y. Thermo-responsive polymeric surfaces; control of
attachment and detachment of cultured cells. Makromol Chem, Rapid Commun 1990;11:571±6.
[33] Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene
dishes grafted with poly(N-isopropylacrylamide). J Biomed Mater Res 1993;27:1243±51.
[34] Okano T, Yamada N, Okuhara M, Sakai H, Sakurai Y. Mechanism of cell detachment from temperature-modulated,
hydrophilic±hydrophobic polymer surfaces. Biomaterials 1995;16:297±303.
[35] von Recum H, Kikuchi A, Okuhara M, Sakurai Y, Okano T, Kim SW. Retinal pigmented epithelium cultures on thermally
responsive polymer porous substrates. J Biomater Sci, Polym Ed 1998;9(11):1241±53.
[36] Kushida A, Yamato M, Konno C, Kikuchi A, Sakurai Y, Okano T. Temperature-responsive culture dishes allow non-
enzymatic harvest of differentiated Madin±Darby canine kidney (MDCK) cell sheets. J Biomed Mater Res 2000;51:216±
23.
[37] Kikuchi A, Okuhara M, Karikusa F, Sakurai Y, Okano T. Two-dimensional manipulation of con¯uently cultured vascular
endothelial cells using temperature-responsive poly(N-isopropylacrylamide)-grafted surfaces. J Biomater Sci, Polym Ed
1998;9(12):1331±48.
[38] Kushida A, Yamato M, Konno C, Kikuchi A, Sakurai Y, Okano T. Decrease in culture temperature releases monolayer
endothelial cell sheets together with deposited ®bronectin matrix from temperature-responsive culture surfaces. J Biomed
Mater Res 1999;45:355±62.
[39] Hirose M, Kwon OH, Yamato M, Kikuchi A, Okano T. Creation of designed shape cell sheets that are noninvasively
harvested and moved onto another surface. Biomacromolecules 2000;1(3):377±81.
[40] Kwon OH, Kikuchi A, Yamato M, Okano T. Rapid cell sheet detachment from poly(N-isopropylacrylamide)-grafted
porous cell culture membranes. J Biomed Mater Res 2000;50:82±9.
[41] Yamato M, Utsumi M, Kushida A, Konno C, Kikuchi A, Okano T. Thermo-responsive culture dishes allow the intact
harvest of multilayered keratinocyte sheets without dispose by reducing temperature. Tissue Engng 2001;7(4):473±80.
[42] Nandkumar MA, Yamato M, Kushida A, Konno C, Hirose M, Kikuchi A, Okano T. Two-dimensional cell sheet
manipulation of heterotypically co-cultured lung cells utilizing temperature-responsive culture dishes results in long-
term maintenance of differentiated epithelial cell functions. Biomaterials 2002;23(4):1121±30.
[43] Rollason G, Davis JE, Sefton MV. Preliminary report on cell culture on a thermally reversible copolymer. Biomaterials
1993;14(2):153±5.
[44] Ito Y, Chen G, Guan Y, Imanishi Y. Patterned immobilization of thermoresponsive polymer. Langmuir 1997;13:2756±9.
[45] Kanazawa H, Yamamoto K, Matsushima Y, Takai N, Kikuchi A, Sakurai Y, Okano T. Temperature-responsive chroma-
tography using poly(N-isopropylacrylamide)-modi®ed silica. Anal Chem 1996;68(1):100±5.
[46] Takei YG, Aoki T, Sanui K, Ogata N, Okano T, Sakurai Y. Temperature-responsive bioconjugates. 1. Synthesis of
temperature-responsive oligomers with reactive end groups and their coupling to biomolecules. Bioconj Chem
1993;4:42±46.
[47] Hansch C, Leo A, Hoekman D. Exploring QSARÐhydrophobic, electrostatic, and steric constants. Washington, DC:
American Chemical Society, 1995.
[48] Kanazawa H, Kashiwase Y, Yamamoto K, Matsushima Y, Kikuchi A, Sakurai Y, Okano T. Temperature-responsive
liquid chromatography. 2. Effect of hydrophobic groups in N-isopropylacrylamide copolymer-modi®ed silica. Anal Chem
1997;69(5):823±30.
[49] Kanazawa H, Yamamoto K, Kashiwase Y, Matsushima Y, Takai N, Kikuchi A, Sakurai Y, Okano T. Analysis of peptides
and proteins by temperature-responsive chromatographic system using N-isopropylacrylamide polymer-modi®ed
columns. J Pharm Biomed Anal 1997;15:1545±50.
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1192

[50] Kanazawa H, Sunamoto T, Matsushima Y, Kikuchi A, Okano T. Temperature-responsive chromatographic separation of
amino acid phenylthiohydantoins using aqueous media as the mobile phase. Anal Chem 2000;72(24):5961±6.
[51] Yamamoto K, Kanazawa H, Matsushima Y, Oikawa K, Kikuchi A, Okano T. Temperature-responsive chromatographic
separation of bisphenol A with water as a sole mobile phase. Environ Sci 2000;7(1):47±56.
[52] Teal HR, Hu Z, Root DD. Native puri®cation of biomolecules with temperature-mediated hydrophobic modulation liquid
chromatography. Anal Biochem 2000;283:159±65.
[53] Sato-Matsuo E, Tanaka T. Kinetics of discontinuous volume-phase transition of gels. J Chem Phys 1988;89(3):1695±703.
[54] Yoshida R, Uchida K, Kaneko Y, Sakai K, Kikuchi A, Sakurai Y, Okano T. Comb-type grafted hydrogels with rapid
temperature responses. Nature 1995;374(6519):240±2.
[55] Kobayashi J, Nakayama S, Kikuchi A, Kanazawa H, Matsushima Y, Sakai K, Okano T. Separation of acidic bioactive
compounds by aqueous chromatography using thermo-responsive cationic surfaces. Therapeu Engng 2000;12(1):670±6
(in Japanese).
[56] Feil H, Bae YH, Feijen J, Kim SW. Mutual in¯uence of pH and temperature on the swelling of ionizable and thermo-
sensitive hydrogels. Macromolecules 1992;25:5528±30.
[57] Kobayashi J, Sakai K, Kikuchi A, Okano T. Aqueous chromatography utilizing pH-/temperature-responsive polymer
stationary phases to separate ionic bioactive compounds. Anal Chem 2001;73(9):2027±33.
[58] Kikuchi A, Iwasa T, Kobayashi J, Sakai K, Okano T. Submitted for publication.
[59] Urry DW. Free energy transduction in polypeptides and proteins based on inverse temperature transitions. Prog Biophys
Mol Biol 1992;57:23±57.
[60] Urry DW, Gowda DC, Peng S, Parker TM, Jing N, Harris RD. Nanometric design of extraordinary hydrophobic-induced
pK
a
shifts for aspartic acid: relevance to protein mechanisms. Biopolymers 1994;34:889±96.
[61] Leoncini G, Buzzi E, Maresca M, Mazzei M, Balbi A. Alkaline extraction and reverse-phase high-performance liquid
chromatography of adenine and pyridine nucleotides in human platelets. Anal Biochem 1987;165:379±83.
[62] Teerlink T, Hennekes M, Bussemaker J, Groeneveld J. Simultaneous determination of creatine compounds and adenine
nucleotides in myocardial tissue by high-performance liquid chromatography. Anal Biochem 1993;214:278±83.
[63] Tekkanat KK, Fox IH. Isocratic separation of ATP and its degradation products from biological ¯uids by automated liquid
chromatography. Clin Chem 1988;34(5):925±32.
[64] Schobert B. Enzymatic synthesis of ATP analogs and their puri®cation by reversed-phase high-performance liquid
chromatography. Anal Biochem 1995;226:288±92.
[65] Blanco J, Canela EI, Sayos J, Mallol H, Lluis C, Franco R. Adenine nucleotides and adenosine metabolism in pig kidney
proximal tubule membranes. J Cell Physiol 1993;157:77±83.
[66] Matejtschuk P. Af®nity chromatogaphyÐa practical approach. Oxford: IRL Press, 1997.
[67] Galaev IY, Mattiasson B. Effect of synthetic polymers, poly(N-vinyl pyrrolidone) and poly(N-vinyl caprolactam), on
elution of lactate dehydrogenase bound to blue Sepharose. J Chromatogr 1993;648:367±71.
[68] Galaev IY, Warrol C, Mattiasson B. Temperature-induced displacement of proteins from dye-af®nity columns using an
immobilized polymeric displacer. J Chromatogr 1994;684:37±43.
[69] Yoshizako K, Akiyama Y, Yamanaka H, Shinohara Y, Hasegawa Y, Carredano E, Kikuchi A, et al. Submitted for
publication.
[70] Allary M, Saint-Blancard J, Boschetti E, Girot P. Bioseparations 1991;2:167.
[71] Matejtschuk P, Feldman PA, Rott J, More J. Af®nity separations of proteins. In: Matejtschuk P, editor. Af®nity separa-
tionsÐa practical approach. Oxford: IRL Press, 1997. p. 61±97.
A. Kikuchi, T. Okano / Prog. Polym. Sci. 27 (2002) 1165±1193
1193
Wyszukiwarka
Podobne podstrony:
Solid phase microextraction for the analysis of biological s
The use of electron beam lithographic graft polymerization on thermoresponsive polymers for regulati
EU funding 'Orwellian' artificial intelligence plan to monitor public for abnormal behaviour xxx
NFPA 20 (2003) Stationary Pumps for Fire Protection
HS SPME procedures for gas chromatographic analysis of biolo
The American Society for the Prevention of Cruelty
Party Games for Large Groups of Teenagers
[Pargament & Mahoney] Sacred matters Sanctification as a vital topic for the psychology of religion
International Convention for the Safety of Life at Sea
Chromatografia cienkowarstwowa, biologia, biochemia
Broad; Arguments for the Existence of God(1)
ESL Seminars Preparation Guide For The Test of Spoken Engl
Kinesio taping compared to physical therapy modalities for the treatment of shoulder impingement syn
GB1008594A process for the production of amines tryptophan tryptamine
Popper Two Autonomous Axiom Systems for the Calculus of Probabilities
Anatomical evidence for the antiquity of human footwear use
The Reasons for the?ll of SocialismCommunism in Russia
1543 save all your kisses for me brotherhood of man CSMF5XGQF7DB2OTIUZDPNBSSS2SOAZAQPLXL2KA
więcej podobnych podstron