
The use of electron beam lithographic graft-polymerization on thermoresponsive
polymers for regulating the directionality of cell attachment and detachment
Naokazu Idota
,
, Takahiko Tsukahara
, Kae Sato
,
, Teruo Okano
, Takehiko Kitamori
a
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo, Tokyo 113-8656, Japan
b
Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency, 4-8-1 Honcho, Kawaguchi, Saitama 332-0012, Japan
c
Center for NanoBio Integration, The University of Tokyo, 7-3-1 Hongo, Bunkyo, Tokyo 113-8656, Japan
d
Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University, 8-1 Kawadacho, Shinjuku, Tokyo 162-8666, Japan
a r t i c l e
i n f o
Article history:
Received 13 November 2008
Accepted 26 December 2008
Available online 21 January 2009
Keywords:
Electron beam
Polymerization
Thermally responsive material
Cell patterning
Cell morphology
a b s t r a c t
A simple process for nano-patterned cell culture substrates by direct graft-polymerization has been
developed using an electron beam (EB) lithography system requiring no photo-masks or EB-sensitive
resists. The compound N-isopropylacrylamide (IPAAm) was locally polymerized and grafted directly by
EB lithographic exposure onto hydrophilic polyacrylamide (PAAm)-grafted glass surfaces. The size of the
surface grafted polymers was controlled by varying the area of EB dose, and a minimal stripe pattern
with a 200 nm line-width could be fabricated onto the surface. On the stripe-patterned surfaces, above
the lower critical solution temperature (LCST), the cells initially adhered and spread with an orientation
along the pattern direction. The magnitude of the spreading angle and elongation of adhered cells
depended on the pattern intervals of the grafted PIPAAm. When culture temperature was lower than the
LCST, cultured cells detached from the surfaces with strong shrinkage along the pattern direction, and
sometimes folded and became parallel with the stripe pattern. This patterned cell recovery technique
may be useful for the construction of muscle cell sheets with efficient shrinkage/relaxation in a specific
direction and spheroidal 3D cell structures, with application to tissue engineering and microfluidic
cellular devices.
Ó 2009 Elsevier Ltd. All rights reserved.
1. Introduction
Microfabrication based on surface chemistry offers the possi-
bility of improving the chemical and structural properties of
material interfaces
. These patterning technologies have been
used routinely for cellular assays that spatially and temporally
control cell adhesion
, due to the ability to promote dense and
well-defined cell alignments. Chemical and topographic features on
patterned surfaces can provide external cues for various aspects of
cellular development, such as adhesive morphology
, prolif-
eration
, differentiation
, and gene expression
. Cells in
vivo are immobilized within tissue via extracellular matrix (ECM)
proteins, and receive biophysical cues from micrometer- and
nanometer-scale ECM structural components. Advances in cell
patterning methods hold promise for mimicking the in vivo cellular
microenvironment, and may be applied to biological assays dealing
with cell functions and to the field of regenerative medicine
Functional cell recovery systems for in vitro tissue reconstruc-
tions have been developed using thermoresponsive polymers
. Thermoresponsive poly(N-isopropylacrylamide) (PIPAAm)
responds to external temperature changes by discontinuous
changes in water solubility
. Surfaces grafted with this
compound, which exhibit significant hydrophilic/hydrophobic
property changes with temperature changes, are used in biomed-
ical applications
. Various proteins and cells can be bound
onto PIPAAm-grafted surfaces above the lower critical solution
temperature (LCST), which spontaneously release when the incu-
bation temperature is lowered below the LCST, via dynamic
hydration of graft PIPAAm layers. In these cell recovery systems, all
of the cells can be harvested as a single continuous cell sheet with
intact cell–cell junctions and ECM upon a decrease in culture
temperature after the cells reach confluency on PIPAAm-grafted
surfaces
. The harvested cell sheets maintain their function and
membrane proteins, so that they can be transferred for stratifica-
tion with other cell sheets in vitro
and for direct transplant in
vivo
. Recently, two-dimensional functional cell sheets, such as
patterned co-cultures of heterotypic cells
and vascularized
tissues consisting of endothelial cells
, have been success-
fully recovered from PIPAAm-grafted surfaces improved by
*
Corresponding author. Department of Applied Chemistry, School of Engi-
neering, The University of Tokyo, 7-3-1 Hongo, Bunkyo, Tokyo 113-8656, Japan. Tel.:
þ81 3 5841 7231; fax: þ81 3 5841 6039.
E-mail address:
(T. Kitamori).
Contents lists available at
Biomaterials
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / b i o m a t e r i a l s
0142-9612/$ – see front matter Ó 2009 Elsevier Ltd. All rights reserved.
doi:10.1016/j.biomaterials.2008.12.058
Biomaterials 30 (2009) 2095–2101

micro-patterning methods. These sheets were on a micrometer
scale for construction of a macro-ordered tissue structure. For the
next generation, nanometer-scale patterning is needed to obtain
cell sheets to allow more precise control of cellular alignment and
functionality of surface chemical and topographic features. This
patterning approach shows promise for the construction of func-
tional tissue mimicking cell sheet materials for clinical applications.
Here, we present a simple graft-polymerization method using
an electron beam (EB) lithography system to fabricate micrometer-
and nanometer-patterned PIPAAm layers. Conventional prepara-
tion of nano-patterned grafted polymer layers requires multiple
processes, such as polymerization after nanofabrication
and pre-polymerization for EB-lithographed patterned cross-link-
ing
. However, for surface modification of patterned poly-
mers,
it
is
desirable
to
perform
polymerization,
surface
modification, and pattern formation in a single process. The
simplest fabrication of nano-patterned polymer-grafted surfaces
simultaneously controls both patterning and graft-polymerization
from the monomer solution without any photo-sensitive resists. A
previous study has reported the covalent grafting and polymeri-
zation of an IPAAm monomer onto silanized glass surfaces during
EB irradiation
. To take this study a step further, here the
simultaneous local polymerization and direct surface grafting of
IPAAm monomers are examined by irradiation with a focused EB
lithography system. This EB lithographic polymerization of IPAAm
on glass substrates permits easy fabrication of patterning surfaces,
as well as the ability to harvest cell sheets with cellular morpho-
logies and functions controlled by micrometer- and nanometer-
scale surface features. A discussion then is presented on whether
designed surface patterning can regulate cellular adhesive orien-
tation and detachment behavior from these PIPAAm-grafted
surfaces.
2. Experimental
2.1. Materials
IPAAm was obtained form Wako Pure Chemicals, Industries, Co. Ltd. (Osaka,
Japan), purified by recrystallization from n-hexane, and thoroughly dried under
vacuum at 25
C. The following reagents were used as received: 2-propanol from
Kanto Chemical Co., Inc. (Tokyo, Japan), Acrylamide (AAm) and Ammonium peroxo-
disulfate (APS) from Wako Pure Chemicals, and 3-methacryloxypropyltrimethoxy-
silane (MPTMS) from ShinEtsu Chemical Industry (Tokyo, Japan). Espacer 300,
a conductive water-soluble polymer, was obtained from Showa Denko (Tokyo,
Japan). Trypsin–EDTA solution and penicillin/streptomycin solution were obtained
from Gibco BRL (Grand Island, NY). Dulbecco’s modified Eagle’s medium (DMEM)
was purchased from Sigma Chemical Co. (St Louis, MO).
2.2. Silane immobilization onto glass surfaces
Glass substrates were treated by O
2
plasma ashing (irradiation intensity: 500 W,
oxygen pressure: 0.1 Pa) for 300 s using a plasma-etching system (NE-550; ULVAC,
Chigasaki, Japan) to activate silanol groups on the surface. These plasma-treated
glass substrates were placed in a Teflon vessel together with a glass bottle containing
1 mL MPTMS. The vessel was sealed with a cap followed by heating in an oven.
Silanization of the substrate surfaces by vaporized MPTMS was conducted for 2 h at
70
C. After the coupling reaction, the MPTMS-immobilized glass was immediately
dried at 70
C for 1 h.
2.3. Surface modification with PAAm by free radical polymerization
Hydrophilic PAAm species were covalently grafted onto MPTMS-modified glass
surfaces for preparation of protein-repelling adsorption surfaces. AAm (5 wt%) and
APS (50 mg), acting as a polymerization initiator, were dissolved in 50 mL of distilled
water, and this solution was degassed gently by bubbling with argon gas for 1 h in an
ice bath. After degassing, 0.1 mL of TEMED was added into the AAm monomer
solution. Then, the reaction solution was immediately poured into a plastic tube,
into which an MPTMS-immobilized glass was inserted, and the polymerization
reaction conducted for 2 h at 4
C. The PAAm-grafted glass substrates then were
rinsed repeatedly with distilled water, and dried at 25
C under vacuum.
2.4. EB lithographic graft-polymerization of IPAAm onto glass surfaces
A patterned thermoresponsive PIPAAm was prepared by exposure to a focused
EB using an EB lithography system (ELS-7500; Elionix, Hachioji, Japan) on PAAm-
grafted glass substrates. IPAAm (55 wt%) was dissolved in 2-propanol, followed by
filtration through a 0.25
m
m pore filter. The PAAm-grafted glass substrates were
sequentially covered with the IPAAm monomer solution and Espacer 300 using
a spin coater. This coated substrate then was placed in the vacuum chamber of an EB
lithography system, and a computer-controlled EB scanner was used to draw
a nanometer- or micrometer-sized pattern. Only the IPAAm monomers in EB-
exposed areas were directly polymerized and covalently grafted onto the PAAm-
grafted surfaces. After the exposure, unreacted IPAAm monomer and the conductive
polymer layer were removed with pure water, and the substrates were dried at 25
C
under reduced pressure. For surface characterization, MPTMS-immobilized surfaces
with patterned PIPAAm also were prepared using the same protocol.
2.5. Surface characterization
The sessile drop method was used for measuring temperature-dependent
contact angles with a contact angle meter (DropMaster500; Kyowa Interface
Science, Saitama, Japan). A drop of de-ionized water from a syringe was placed on
the surface of the plate in air. Sample temperature was regulated with a Thermo
Plate
Ò
(TOKAI HIT Co. Ltd., Shizuoka, Japan). To estimate the grafted polymer
morphologies, the patterned PNIPAAm-grafted surfaces were observed using atomic
force microscopy (AFM: SPA-400; SII NanoTechnology, Tokyo, Japan) in non-contact
mode in air. The pattern size and thickness of PNIPAAm layers were determined
from the three-dimensional surface profiles.
2.6. Cell culture
Bovine carotid aortic endothelial cells (BAECs) and NIH-3T3 mouse fibroblast
cells were purchased from the Japan Health Science Foundation (Osaka, Japan). The
cells were cultured on commercial tissue culture polystyrene (TCPS) dishes with
DMEM supplemented with 10% fetal bovine serum, 100 units mL
1
penicillin, and
100
m
g mL
1
streptomycin at 37
C in a humidified atmosphere with 5% CO
2
. The cells
were harvested from the TCPS dishes with 0.25% trypsin–EDTA in phosphate-buffered
saline. For the cell adhesion and spreading assay, cells were seeded to the patterned
PIPAAm-grafted glass substrates at a density of 1.0 10
4
cells cm
2
followed by
culturing at 37
C. Cellular morphology was monitored at intervals and photographed
using a phase-contrast microscope (Eclipse TS100; Nikon, Tokyo, Japan).
3. Results and discussion
3.1. Surface characterization of patterned surfaces by EB
lithographic graft-polymerization
One of the key features of patterned cell cultures is the ability to
spatially control cell–substrate interactions. Fundamentally, cellular
responses, such as adhesion, proliferation, and differentiation, are
triggered by an interaction between the cell transmembrane and
the extracellular proteins, which are adsorbed onto the surface of
the substrates. Thus, surface modifications are needed that can
facilitate site-selection of protein adsorption. To achieve these
surface properties, protein-repulsive surfaces were prepared by
redox polymerization to modify MPTMS-immobilized substrates
with a layer of PAAm
. The wettability of these surfaces then was
investigated by measurement of static contact angles. The contact
angles were 74.7 0.7
for MPTMS-immobilized surfaces and
18.1 1.0
for PAAm-grafted surfaces. We hypothesized that the
methacryloyl groups in the surface-introduced MPTMS would react
with the growing PAAm chains during polymerization, resulting in
a hydrophilic surface. Fibroblast cells were cultured on the surfaces
to confirm that cellular adhesion was consistent with the surface
properties. While the fibroblast cells adhered, spread, proliferated,
and reached confluency on the MPTMS-immobilized surfaces,
exactly as observed on the commercial TCPS dishes, cell adhesion
onto the PAAm-grafted surfaces was completely inhibited (data not
shown). This was probably because hydration of the grafted PAAm
prevents adsorption of the serum protein in the culture medium,
inhibiting cellular adhesion.
As patterned cell-adhesive surface domains, thermoresponsive
PIPAAm was locally bound onto the cell-repulsive PAAm-grafted
N. Idota et al. / Biomaterials 30 (2009) 2095–2101
2096
surfaces in this study. The PIPAAm-grafted surfaces exhibited
temperature-induced hydrophilic/hydrophobic properties, conse-
quently providing surface adsorption of proteins at temperatures
higher than the PIPAAm transition temperature
. An attempt
was
made
to
fabricate
micrometer-
and
nanometer-scale
patterning of grafted PIPAAm by EB lithographic graft-polymeri-
zation and to confirm the surface modification of patterned PNI-
PAAm by wettability and pattern profiles of the surfaces. When
PAAm-grafted glass is used as the basal substrate for patterned
PNIPAAm grafting, the strong hydrophilicity and roughness of pre-
grafted PAAm interfere with evaluation of wettability and geometry
changes by the patterning of PIPAAm. Hence, the hydrophobic and
smooth MPTMS-immobilized glass is used as basal substrates for
the simple evaluation of a patterning process. Changes in the
contact angle of water on the patterned PIPAAm-grafted glass
substrates, caused by variations in electron beam exposure, were
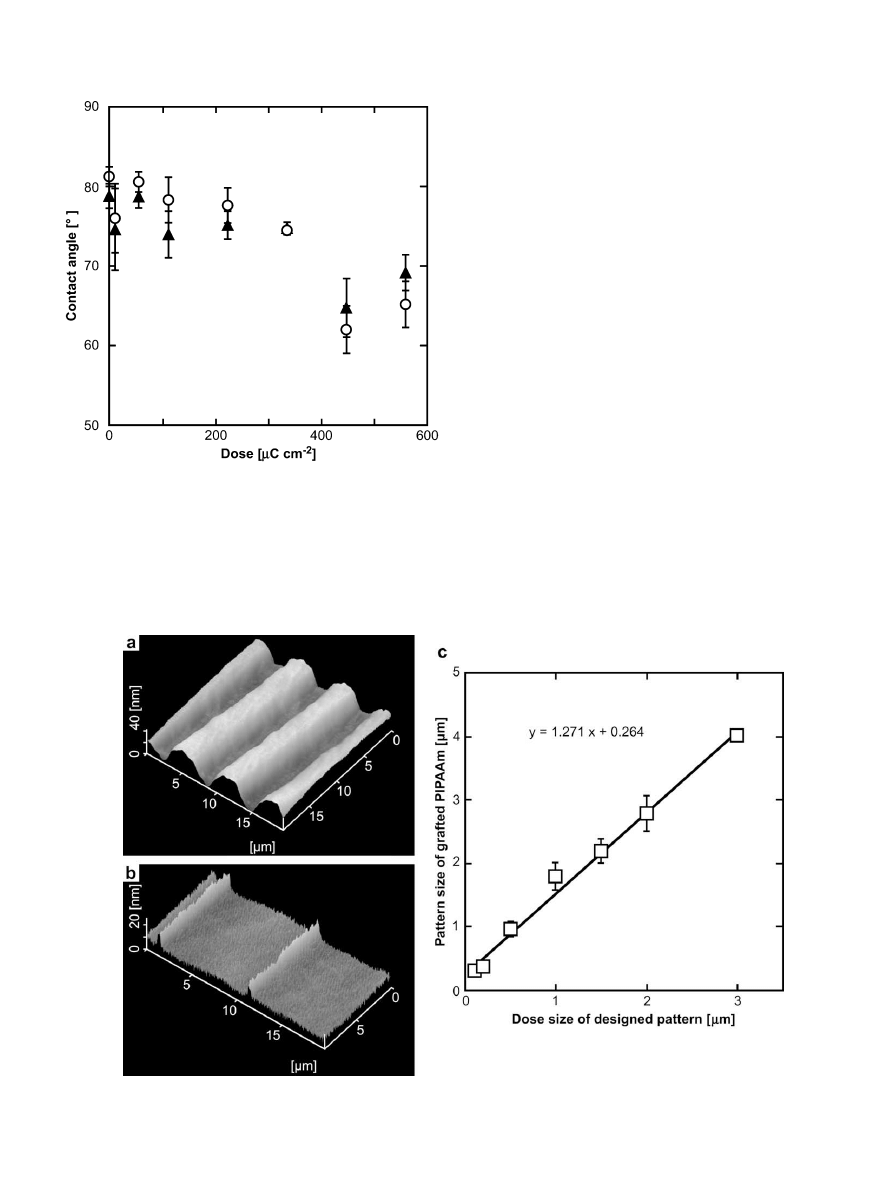
measured and results are shown in
. Results showed that the
contact angle on the surfaces decreased with increasing electron
dose at either 25
C or 37
C. The contact angles showed hydro-
phobicity below a dose of 440
m
C cm
2
, while above that dose the
aqueous wettability of the surfaces increased significantly. The
gelation dose of poly(vinylmethyl ether) was estimated based on
a comparison between EB lithography and bulky EB irradiation
(1.0
m
C cm
2
z 1.3 kGy)
. This unit conversion, used to estimate
the dose inducing the aqueous wettability transition, was ca.
580 kGy (acceleration voltage: 50 kV). Previous reports stated that
EB irradiation of 250–300 kGy (acceleration voltage: 150 kV)
enabled successful modification of PIPAAm on TCPS surfaces for
cellular adhesion/detachment control
. Considering
the difference in acceleration voltage, the exposure conditions used
in this study are similar to those used in the successful bulk EB-
induced polymerization of IPAAm. Therefore, it is likely that IPAAm
was successfully polymerized and grafted onto the surfaces by EB
lithographic graft-polymerization.
The patterning areas of PIPAAm-grafted onto MPTMS-immobi-
lized surfaces were controlled by changing the EB-exposed areas.
(a) and (b) shows AFM images of the PIPAAm-grafted stripe
Fig. 1. Dose-dependent static contact angles on patterned PIPAAm-grafted surfaces at
below and above LCST. Symbols: open circle, 25
C; closed triangle, 37
C.
Fig. 2. AFM images of PIPAAm-grafted surfaces with (a) 3
m
m (interval: 6
m
m) and (b) 200 nm (interval: 10
m
m) stripe pattern. (c) Effect of dose size of designed pattern on
fabricated pattern size of grafted PIPAAm.
N. Idota et al. / Biomaterials 30 (2009) 2095–2101
2097
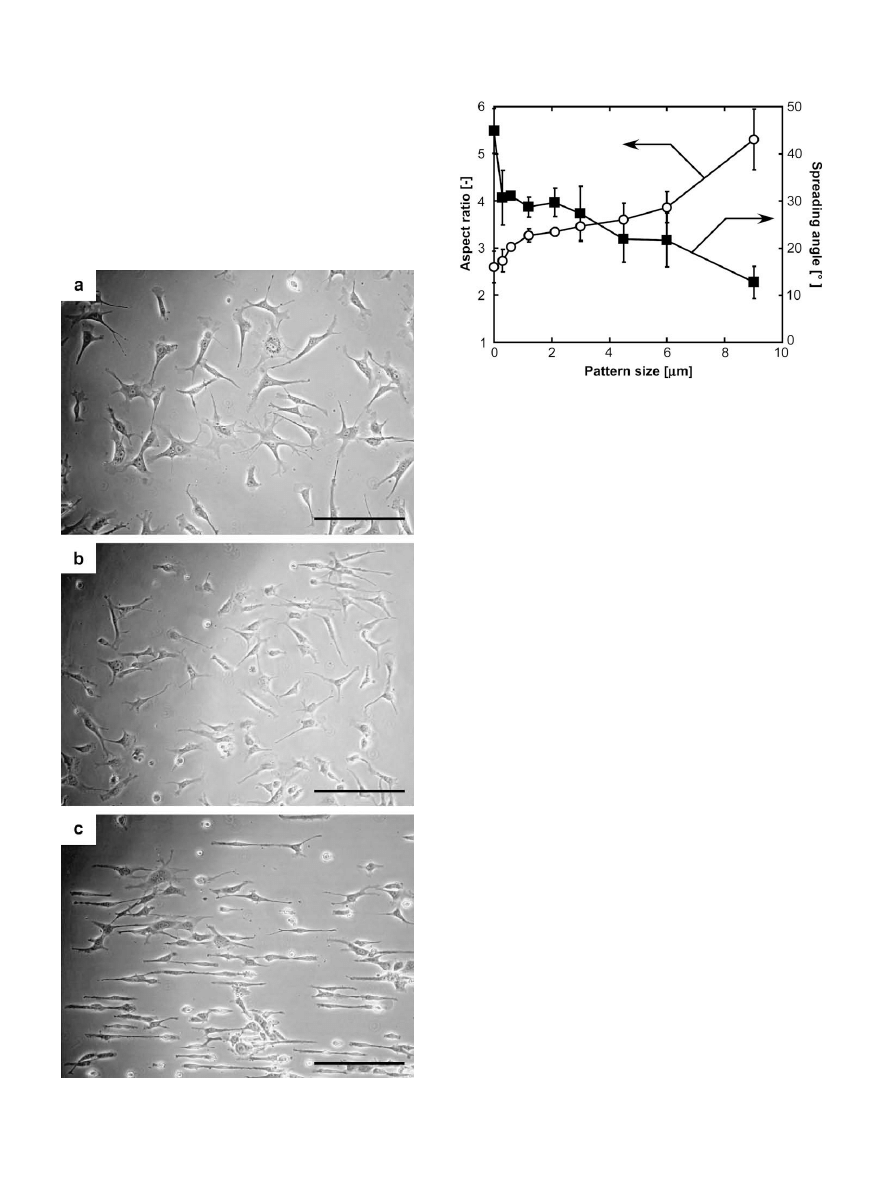
patterns with a 3
m
m and 200 nm width on the surface, respec-
tively. The patterned structures of grafted PIPAAm can be observed
by AFM, and the nanometer-scale patterning is easily fabricated
using the present technique. The designed dose vs. patterned sizes
of grafted PIPAAm stripe patterns had a linear relation, as shown in
(c), and the fabricated sizes expanded approximately 13%
compared with designated sizes. These results indicated that the
patterning area of grafted PIPAAm depended on the EB-exposed
areas, and only monomers exposed to EB were polymerized and
covalently grafted onto the surfaces. Although previous fabrication
methods of nanometer-scale patterns of polymers required
multiple difficult processes
, the method presented here of
the nanometer-scale patterned PIPAAm can be created directly on
a surface by growth of polymer chains based on a single step of EB
irradiation. The active radicals of a growing chain end propagate
toward a non-irradiated area to react with monomers, so that the
PIPAAm-grafted nanopatterns are slightly larger than the EB-
exposed areas would suggest. Even with this technique, pattern
fidelity and resolution are on the order of 200 nm, a scale much
smaller than cultured cells allowing control of chemical and topo-
logical features.
3.2. Cellular orientation and elongation dependent on pattern size
In the patterning technique based on EB lithographic graft-
polymerization, the fabrication of patterned polymer layers can be
controlled on a nanometer-scale area without any EB-sensitive
resist. The utility of this technique for biomedical applications was
demonstrated by regulating the adhesion and detachment of cell
sheets in response to patterning features induced by temperature
changes. Topographic and chemical nanometer-scale patterned
surfaces have been fabricated previously to control the adhesion
and spreading behavior of NIH-3T3 cells
. Such patterning
enables NIH-3T3 cells to attach and elongate along the stripe, with
their morphologies dependent upon stripe width and spacing size.
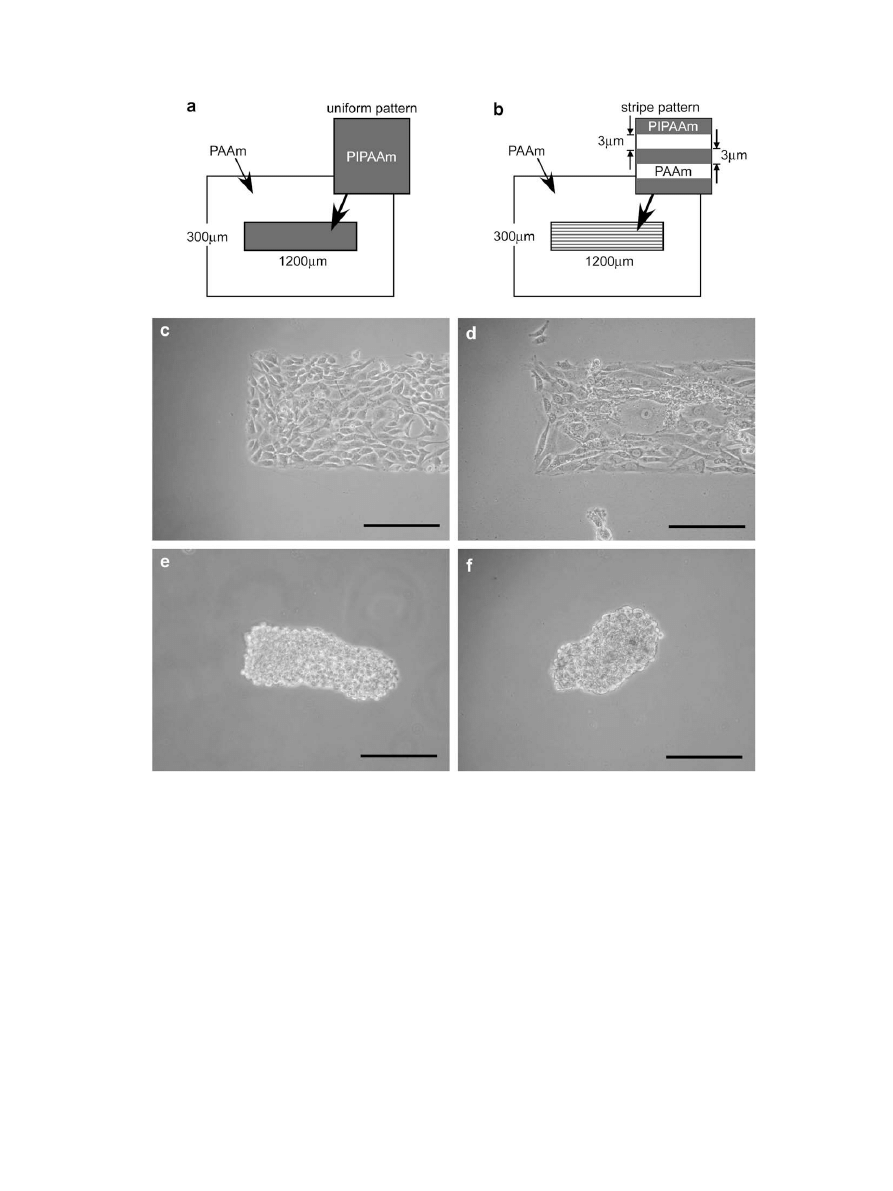
For this study, striped nanometer-scale patterns of PIPAAm on
hydrophilic PAAm-grafted glass substrates were fabricated by the
EB lithographic graft-polymerization method. The BAECs were
cultured on the surface at 37
C, and their adhesive morphology
was observed one day after seeding. Most of the BAECs on the non-
patterned surfaces adhered randomly and spread uniformly by
occupying large adhesion areas [
(a)]. The nanometer-scale
stripe patterns showed that the adhered cells possessed a relatively
slender shape in random directions [
(b)]. In contrast, a large
number of adhered BAECs visibly elongated and oriented them-
selves along the stripe lines of the micrometer-patterned surfaces
[
(c)]. The PAAm-grafted surfaces prevented protein adsorption
and cellular adhesion, allowing cells to adhere on the PIPAAm-
grafted surfaces in a manner similar to that seen in ungrafted
culture dishes at 37
C
. Hence, the cultured cells can be
expected to selectively change their shapes and adhesive directions
Fig. 3. Photographs of BAECs’ adhesion onto (a) 0
m
m, (b) 0.6
m
m and (c) 9
m
m stripe-
patterned PIPAAm-grafted surfaces. Scale bar: 200
m
m.
Fig. 4. Effect of stripe pattern size of grafted PIPAAm on aspect ratio and spreading
angle of adhered BAECs. Symbols: open circle, aspect ratio; closed square, spreading
angle.
N. Idota et al. / Biomaterials 30 (2009) 2095–2101
2098
depending on the spacing of PIPAAm-grafted surfaces above their
LCST, to areas that exhibit greater cell adhesion properties, and thus
maintain more stable adhesive morphology than in the PAAm-
grafted domains.
To evaluate the dependence of cellular morphology on pattern
area, the magnitude of orientation and elongation of adhered cells
was determined using two measurements: the average value of the
angle of cellular spreading with respect to the stripe pattern
direction, and the aspect ratio (ratio of the long to the short axis of
the adhered cells)
. The results are summarized in
. The
BAECs adhered and spread in many directions on non-patterned
PIPAAm-grafted surfaces, a process resulting in a spreading angle of
ca. 45
. In contrast, enlarging the pattern area decreased the
spreading angles, which indicates that the cells aligned parallel to
the pattern direction. The aspect ratios of the BAECs cultured on the
patterned PIPAAm-grafted surfaces increased with enlargements in
pattern size. The PAAm and PIPAAm stripe patterns occur at equal
intervals, so that the BAECs spread to overlap the PAAm-grafted
regions, since the nanometer-scale patterning is much smaller than
the cell size. As pattern size increased, the BAECs adhered selec-
tively onto a line-shape patterned surface grafted with PIPAAm,
resulting in their orientation and elongation along the pattern.
However, the cultured cells could not reach confluency on the large
spacing of patterned surfaces, due to the difficulty of contact with
other cells by crossing over the stripe patterns. Thus, few
micrometer-scale stripe patterns should be required to construct
a cell sheet with intact cell–cell junctions with controllable adhe-
sive morphologies.
3.3. Direction control of adhesion/detachment of cell sheets on
patterned surfaces
A decrease in culture temperature below the LCST can release
cultured cells from PIPAAm-grafted surfaces as a monolayer
Fig. 5. Photographs of detached fibroblast cell sheets from patterned PIPAAm-grafted surfaces by reducing temperature from (c, d) 37
C to (e, f) 25
C. Pattern design: (a, c, e)
uniform pattern, (b, d, f) stripe pattern. Scale bar: 200
m
m.
N. Idota et al. / Biomaterials 30 (2009) 2095–2101
2099
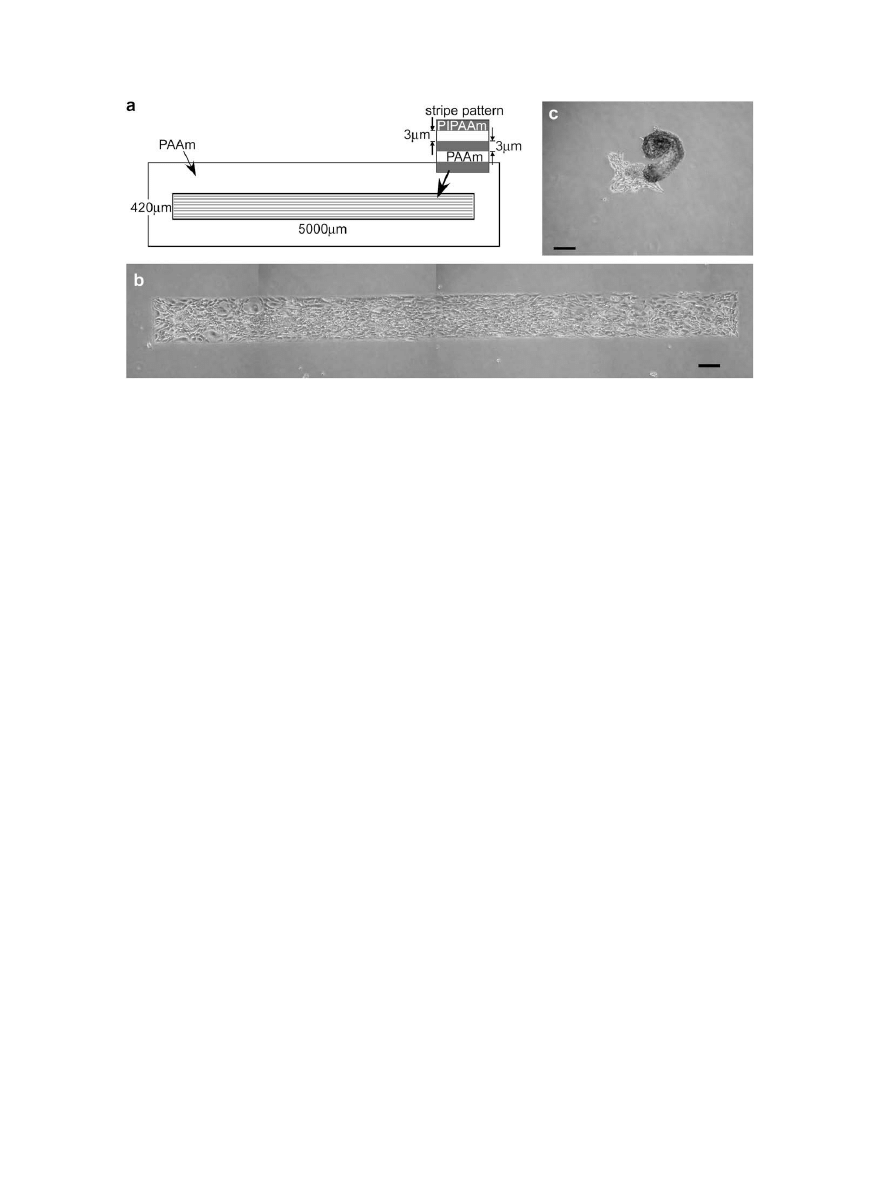
without the need for enzymatic digestion. Thus, detachment of
fibroblast cells from patterned thermoresponsive surfaces was
examined upon reducing the temperature from 37 to 25
C. These
surfaces consisted of a 3
m
m pitch stripe-patterning of grafted
PIPAAm and PAAm [
(b)]. For comparison, patterned PIPAAm-
grafted surfaces without stripes were prepared on PAAm-grafted
substrates [
(a)] by commercial photolithographic techniques
similar to those previously reported
. The fibroblast cells were
placed on both pattern-grafted surfaces at 37
C; the cells adhered,
spread, proliferated, and reached confluency only within the
PIPAAm-grafted domains [
(c) and (d)]. The cells growing on
the striped surfaces loosely maintained their orientation along the
pattern direction. When the culture temperature decreased to
25
C after the cells reached confluency, the patterned cells
detached from both PIPAAm-grafted surfaces as a continuous cell
sheet with intact cell–cell junctions [
(e) and (f)]. The cell
sheets obtained could be re-cultured on other TCPS surfaces, indi-
cating that the patterned surfaces can be harvested as intact cell
sheets non-invasively. To evaluate cellular detachment behavior,
the shrinkage ratios of the patterned cell sheets were measured,
which indicates the ratios of the length of the detached cell sheet to
the cell adhesion area along its long and short axes. The shrinkage
ratios of the patterned cell sheets from the uniform PIPAAm-
patterned surfaces were 60.7 3.6% and 54.2 6.1% for the long
and short axes, respectively (n ¼ 10), a result indicating isotropic
shrinkage. In contrast, shrinkage ratios from the striped PIPAAm-
patterned surfaces were 73.6 2.3% and 47.8 3.7% for the long
and short axes, respectively (n ¼ 7). The cell sheets were strongly
contracted in the stripe pattern direction. After treatment at
temperatures below the LCST, the remaining intact cell–cell junc-
tions and peripheral actin filament bands in the cells induced
contractile forces that caused shrinkage of entire cell sheets
On stripe-patterned PIPAAm surfaces, such contractile forces
worked mainly along the stripe pattern direction, because the cells
oriented and elongated at a culture temperature of 37
C. Thus,
strong contractile forces in a specific direction can be controlled by
appropriate patterning design of stripe width and spacing, and is
useful for actuation controls using muscle cell sheets (i.e., tissue
engineering of cardiac muscle
and microfluidic pumping using
cardiomyocyte sheets
). In addition, 3
m
m pitch stripe-
patterning surfaces with a large aspect ratio of cell culture area
were prepared [
(a)], and fibroblast cells cultured on those
surfaces for 5 days at 37
C [
(b)]. In some cases, after the cell
sheets gradually detached from the edges of the pattern domain at
culture temperatures below 25
C, they then folded and rolled in
parallel with the stripe-patterning [
(c)]. When a cell sheet
detached from its edges, the folding of the cells in their elongated
direction was more stable stereoscopically at the confluent state.
The cell sheets with a large aspect ratio consequently formed rolled
structures through continuous folding behavior. Regulation of the
folding behavior of cell sheets is possible using local temperature,
which controls the starting point of cell detachment from the
stripe-patterned PIPAAm surfaces. Evidently, the shrinking and
folding direction of detached cell sheets can be controlled on the
striped PIPAAm-grafted surfaces simply by reducing the tempera-
ture, resulting in the formation of spheroidal cell structures.
4. Conclusions
Temperature-responsive PIPAAm was applied directly onto glass
surfaces on nanometer-scale stripe patterns using EB lithographic
graft-polymerization. This modification method is simple and can
be applied to a wide variety of polymers without photo-sensitive
resist. The patterned area of the grafted PIPAAm can be controlled
on a nanometer-scale by changes in the irradiated area. At
temperatures above the LCST, cells adhered on these surfaces were
oriented and elongated along the stripes of grafted PIPAAm. Below
the LCST, they detached with shrinkage and folding along the
pattern direction. This patterning and cell recovery system could be
useful for applications such as engineering of functional cell sheets,
cell-based bioreactors, and the construction of spheroidal 3D cell
structures.
References
[1] Zhao B, Brittain WJ. Polymer brushes: surface-immobilized macromolecules.
Prog Polym Sci 2000;25:677–710.
[2] Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in
biology and biochemistry. Annu Rev Biomed Eng 2001;3:335–73.
[3] Ginger DS, Zhang H, Mirkin CA. The evolution of dip-pen nanolithography.
Angew Chem Int Ed 2004;43:30–45.
[4] Suh KY, Seong J, Khademhosseini A, Laibinis PE, Langer R. A simple soft
lithography route to fabrication of poly(ethylene glycol) microstructures for
protein and cell patterning. Biomaterials 2004;25:557–63.
[5] Falconnet D, Csucs G, Grandin HM, Textor M. Surface engineering approaches
to micropattern surface for cell-based assays. Biomaterials 2006;27:3044–63.
Fig. 6. Photographs of folding and rolling detachment of fibroblast cell sheets from patterned PIPAAm-grafted surfaces by reducing temperature. (a) Pattern design. Culture
temperature: (b) 37
C, (c) 25
C. Scale bar: 200
m
m.
N. Idota et al. / Biomaterials 30 (2009) 2095–2101
2100

[6] Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. Epithelial contact
guidance on well-defined micro- and nanostructured substrates. J Cell Sci
2003;116:1881–92.
[7] Goto M, Tsukahara T, Sato K, Kitamori T. Micro- and nanometer-scale
patterned surface in a microchannel for cell culture in microfluidic devices.
Anal Bioanal Chem 2008;390:817–23.
[8] Gerecht S, Bettinger CJ, Zhang Z, Borenstein JT, Vuniak-Novakvoic G, Langer R.
The effect of actin disruption agents on contact guidance of human embryonic
stem cells. Biomaterials 2007;28:4068–77.
[9] Goto M, Tsukahara T, Sato K, Konno T, Ishihara K, Sato K, et al. Nanometer-scale
patterned surfaces for control of cell adhesion. Anal Sci 2007;23:245–7.
[10] Zinger O, Zhao G, Schwartz Z, Simpson J, Wieland M, Landolt D, et al. Differ-
ential regulation of osteoblasts by substrate microstructural features. Bioma-
terials 2005;26:1837–47.
[11] Dalby MJ, Riehle MO, Yarwood SJ, Wilkinson CDW, Curtis ASG. Nucleus
alignment and cell signaling in fibroblasts: response to a micro-grooved
topography. Exp Cell Res 2003;284:274–82.
[12] Andersson H, van den Berg A. Microfabrication and microfluidics for tissue
engineering: state of the art and future opportunities. Lab Chip 2004;4:98–103.
[13] Khadembosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies
for tissue engineering and biology. Proc Natl Acad Sci U S A 2006;103:2480–7.
[14] Yamada N, Okano T, Sakai H, Karikusa F, Sawasaki Y, Sakurai Y. Thermo-
responsive polymer surface: control of attachment and detachment of
cultured cells. Makromol Chem Rapid Commun 1990;11:571–6.
[15] Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured
cells using plasma-treated polystyrene dishes grafted with poly(N-iso-
propylacrylamide). J Biomed Mater Res 1993;27:1243–51.
[16] Heskins M, Guillet
JE, James E.
Solution properties of
poly(N-iso-
propylacrylamide). J Macromol Sci Chem 1968;A2:1441–5.
[17] Kikuchi A, Okano T. Intelligent thermoresponsive polymeric stationary phases
for aqueous chromatography of biological compounds. Prog Polym Sci
2002;27:1165–98.
[18] Ebara M, Hoffman JM, Hoffman AS, Stayton PS. Switchable surface traps for
injectable bead-based chromatography in PDMS microfluidic channels. Lab
Chip 2006;6:843–8.
[19] Kushida A, Yamato M, Konno C, Kikuchi A, Sakurai Y, Okano T. Decrease in
culture temperature releases monolayer endothelial cell sheets together with
deposited fibronectin matrix from temperature-responsive culture surfaces.
J Biomed Mater Res 1999;45:355–62.
[20] Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, et al. Fabrication of
pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manip-
ulation technique and temperature-responsive cell culture surfaces. Circ Res
2002;90:e40–8.
[21] Yang J, Yamato M, Shimizu T, Sekine H, Ohashi K, Kanzaki M, et al. Recon-
struction of functional tissues with cell sheet engineering. Biomaterials
2007;28:5033–43.
[22] Tsuda Y, Kikuchi A, Yamato M, Nakao A, Sakurai Y, Umezu M, et al. The use of
patterned dual thermoresponsive surfaces for the collective recovery as
co-cultured cell sheets. Biomaterials 2005;26:1885–93.
[23] Tsuda Y, Yamato M, Kikuchi A, Watanabe M, Chen G, Okano T. Thermores-
ponsive microtextured culture surfaces facilitate fabrication of capillary
networks. Adv Mater 2007;19:3633–6.
[24] Tsuda Y, Shimizu T, Yamato M, Kikuchi A, Sasagawa T, Sekiya S, et al. Cellular
control of tissue architectures using a three-dimensional tissue fabrication
technique. Biomaterials 2007;28:4939–46.
[25] Kaholek M, Lee WK, Ahn SJ, Ma H, Caster KC, LaMattina B, et al. Stimuli-
responsive poly(N-isopropylacrylamide) brushes and nanopatterns prepared
by surface-initiated polymerization. Chem Mater 2004;16:3688–96.
[26] He Q, Ku¨ller A, Grunze M, Li J. Fabrication of thermoresponsive polymer
nanopatterns through chemical lithography and atom transfer radical poly-
merization. Langmuir 2007;23:3981–7.
[27] Ballav N, Schilp S, Zharnilkov M. Electron-beam chemical lithography with
aliphatic self-assembled monolayers. Angew Chem Int Ed 2008;47:1421–4.
[28] Schmidt T, Mo¨nch JI, Arndt KF. Temperature-sensitive hydrogel pattern by
electron-beam lithography. Macromol Mater Eng 2006;291:755–61.
[29] Tirumala VR, Divan R, Ocola LE, Mancini DC. Direct-write e-beam patterning of
stimuli-responsive hydrogel nanostructure. J Vac Sci Technol B 2005;23:
3124–8.
[30] Idota N, Kikuchi A, Kobayashi J, Sakai K, Okano T. Microfluidic valves
comprising nanolayered thermoresponsive polymer-grafted capillaries. Adv
Mater 2005;17:2723–7.
[31] Xiao D, Zhang H, Wirth M. Chemical modification of the surface of poly-
(dimethylsiloxane) by atom-transfer radical polymerization of acrylamide.
Langmuir 2002;18:9971–6.
[32] Kawaguchi H, Fujimoto K, Mizuhara Y. Hydrogel microspheres III. Tempera-
ture-dependent
adsorption of proteins on poly-N-isopropylacrylamide
hydrogel microspheres. Colloid Polym Sci 1992;270:53–7.
[33] Au HTH, Cheng I, Chowdhury MF, Radisic M. Interactive effects of surface
topography and pulsatile electrical filed stimulation on orientation and
elongation of fibroblasts and cardiomyocytes. Biomaterials 2007;28:4277–
302.
[34] Itoga K, Kobayashi J, Yamato M, Kikuchi A, Okano T. Maskless liquid-crystal-
display projection photolithography for improved design flexibility of cellular
micropatterns. Biomaterials 2006;27:3005–9.
[35] Tanaka Y, Sato K, Shimizu T, Yamato M, Okano T, Kitamori T. A micro-spherical
heart pump powered by cultured cardiomyocytes. Lab Chip 2007;7:207–12.
N. Idota et al. / Biomaterials 30 (2009) 2095–2101
2101
Document Outline
- The use of electron beam lithographic graft-polymerization on thermoresponsive polymers for regulating the directionality of cell attachment and detachment
Wyszukiwarka
Podobne podstrony:
Tomaszewski P (2008) Child visual discourse The use of language, gestures, and vocalizations
Use of exponential, Page’s and diffusional models to simulate the drying kinetics of kiwi fruit
VERDERAME Means of substitution The use of figurnes, animals, and human beings as substitutes in as
71 1021 1029 Effect of Electron Beam Treatment on the Structure and the Properties of Hard
Dispute settlement understanding on the use of BOTO
Illiad, The Analysis of Homer's use of Similes
Resuscitation- The use of intraosseous devices during cardiopulmonary resuscitation, MEDYCYNA, RATOW
or The Use of Ultrasound to?celerate Fracture Healing
The use of Merit Pay Scales as Incentives in Health?re
Kinesio® Taping in Stroke Improving Functional Use of the Upper Extremity in Hemiplegia
A Common Use of the 'den', ❑Języki, ►Język turecki, turkish grammar
The use of 了 to imply completed?tionx
Capo Ferro Great Representation Of The Art & Of The Use Of Fencing
A Common Use of the 'den' (2)
Oren The use of board games in child psychotherapy
or The Use of Extracorporeal Shock Wave Therapy to Improve Fracture Healing
William Varner The Christian Use Of Jewish Numerology
więcej podobnych podstron