http://jbc.sagepub.com
Compatible Polymers
Journal of Bioactive and
DOI: 10.1177/0883911507084294
2007; 22; 575
Journal of Bioactive and Compatible Polymers
Akihiko Kikuchi, Jun Kobayashi, Teruo Okano, Takeshi Iwasa and Kiyotaka Sakai
Nucleotides on Intelligent Cationic, Thermoresponsive Surfaces1
Temperature-Modulated Interaction Changes with Adenosine
http://jbc.sagepub.com/cgi/content/abstract/22/6/575
The online version of this article can be found at:
Published by:
http://www.sagepublications.com
at:
can be found
Journal of Bioactive and Compatible Polymers
Additional services and information for
http://jbc.sagepub.com/cgi/alerts
http://jbc.sagepub.com/subscriptions
http://www.sagepub.com/journalsReprints.nav
http://www.sagepub.co.uk/journalsPermissions.nav
http://jbc.sagepub.com/cgi/content/refs/22/6/575
Citations

Temperature-Modulated
Interaction Changes with
Adenosine Nucleotides on
Intelligent Cationic,
Thermoresponsive Surfaces
1
A
KIHIKO
K
IKUCHI
,
2
J
UN
K
OBAYASHI AND
T
ERUO
O
KANO
3,
*
Institute of Advanced Biomedical Engineering and Science, and Center of
Excellence Program for the 21st Century, Tokyo Women’s Medical University
8-1 Kawadacho, Shinjuku, Tokyo 162-8666, Japan
T
AKESHI
I
WASA AND
K
IYOTAKA
S
AKAI
Faculty of Science and Engineering, Department of Applied Chemistry
Waseda University, 3-4-1 Ohkubo, Shinjuku, Tokyo 169-8555, Japan
ABSTRACT: Thin layer poly(
N-isopropylacrylamide-co-n-butyl-methacrylate-
co-N,N-dimethylaminopropylacrylamide) (IBD) copolymer gels are covalently
introduced to initiator immobilized silica bead surfaces to create thermally
sensitive intelligent cationic surfaces. The surface shows thermoresponsive
changes in charge density as well as hydrophilic/hydrophobic character. The
polymer chains dehydrate and inter-/intra-molecular aggregation occurs due to
weakly deprotonated cationic amino groups in the hydrophobized circumstances,
*Author to whom correspondence should be addressed.
E-mail: tokano@abmes.twmu.ac.jp
1
In memory of the late Professor Junzo Sunamoto, Kyoto University.
2
Present address: Department of Materials Science and Technology, Tokyo University of
Science, 2641 Yamazaki, Noda, Chiba 278-8510, Japan.
3
In memoriam: I greatly appreciated the new biorelated polymer science developed and
achieved by Professor Junzo Sunamoto. We all know the outstanding achievements that
Professor Junzo Sunamoto has made based on his strong interdisciplinary expertise,
excellent professorship, and splendid worldwide relationships. It was very important to
me and many of my contemporary colleagues that he always encouraged us as young
researchers to challenge and seek innovation. I would like to express my sincere
appreciation to Professor Junzo Sunamoto who has contributed so much and achieved a
very special and outstanding academic life.
Figure 3 appears in color online: http://jbc.sagepub.com
Journal of B
IOACTIVE AND
C
OMPATIBLE
P
OLYMERS
, Vol. 22—
November 2007
575
0883-9115/07/06 0575–14 $10.00/0
DOI: 10.1177/0883911507084294
ß SAGE Publications 2007
Los Angeles, London, New Delhi and Singapore

resulting in the surface charge density decrease. This was corroborated by the
thermoresponsive p
Ka shift of dimethylamino side groups in the copolymers as
well as the surface potential changes at elevated temperature. The unique
characteristics of the IBD copolymer-immobilized surfaces were applied to
regulate adenosine nucleotides retention in high-performance liquid chromato-
graphy using aqueous mobile phase by thermal stimulus. At lower temperature,
adenosine nucleotides showed higher retention which was primarily driven by
ionic interaction with positively charged surfaces. With increasing temperatures,
their retention was shortened and a drastic change was observed above the
polymer transition temperatures. This is strong evidence that the solute
interaction is being regulated by the thermoresponsive surface charge density
changes and hydrophobic alterations. Furthermore, we confirmed the modula-
tion of nucleotide retention by step-temperature gradient without changing
mobile phase composition. These findings should be beneficial in utilizing this
stimuli responsive surface for the separation of bioactive compounds in aqueous
system and environmental impact.
KEY WORDS: thermoresponsive polymer, poly(n-isopropylacrylamide), adeno-
sine nucleotides, separation, electrostatic interaction, hydrophobic interaction.
INTRODUCTION
M
aterials that respond specifically and dynamically to external
physical and/or chemical stimuli by altering their structure
and/or properties are often referred to as intelligent materials.
Poly(
N-isopropylacrylamide) (PIPAAm) is a thermoresponsive polymer
exhibiting a lower critical solution temperature at 328C in aqueous
milieu that has received considerable attention in the context of
intelligent materials [1]. PIPAAm’s well-known reversible soluble/
insoluble changes have been exploited to produce reversible surface
hydrophilic/hydrophobic properties in thermally modulated cultured cell
recovery systems [2–6] and in stationary column chromatography
matrices that effectively separate hydrophobic analytes in aqueous
milieu without use of organic mobile phases or gradient elution [7–10],
and even in microcapillary columns [11]. When weakly charged
co-monomer groups are introduced into PIPAAm, charge group
dissociation states are drastically altered via temperature-responsive
hydrophilic/hydrophobic changes in these new copolymers.
Here, the introduction of co-monomer,
N,N-dimethylaminopropyl-
acrylamide, into PIPAAm copolymers is shown to control both
temperature-responsive surface charge density as well as hydrophilic/
hydrophobic property alterations. These unique thermal properties are
exploited to modulate interactions between surfaces of chromatography
resins grafted with these polymers and charged bioactive compounds,
576
A. K
IKUCHI ET AL
.

producing effective separations in an intelligent thermoresponsive
‘‘green’’ chromatography system operating exclusively under aqueous
conditions.
PIPAAm is soluble in water at temperatures below 328C due to
sufficient hydration of amide side groups and hydrophobic hydration
around isopropyl side groups in these homopolymers. With increasing
temperature, hydrophobic hydration is suddenly thermally disrupted,
and dehydrated polymeric isopropyl groups spontaneously produce
intra- and inter-molecular aggregation that reversibly precipitates
these polymer molecules from water [1]. We have already reported
on–off switched drug release from PIPAAm hydrogels, thermally
controlled by the rapid chain transitions [12,13]. Furthermore, we
have altered PIPAAm chain collapse–rehydration phenomena using
grafted PIPAAm hydrogels with freely mobile PIPAAm chains to
produce new rapid gel deswelling controls [14]. This same principle
can be applied to rapidly change solid surface wetting transitions by
modulating grafted PIPAAm hydrophilic/hydrophobic properties using
temperature changes [15,16]. PIPAAm-modified surfaces exhibit dra-
matic wettability changes near 328C. This hydrophilic/hydrophobic
alteration of PIPAAm-grafted surfaces has been utilized for cultured cell
attachment/detachment control [2,3,17] and for tissue engineering
applications using cultured cell sheet manipulation [4–6,18–20].
Furthermore, the thermoresponsive wettability changes of PIPAAm
surfaces are useful to control solute interaction in aqueous hydrophobic
chromatography.
The objective is to provide a suitable environmentally friendly
separation alternative to conventional reversed-phase chromatography
(RPC) in organic solutions [7–9]. Effective separations, modulated by
temperature in purely aqueous mobile phases, would reduce waste-
handling costs as well as environmental impact. Since thermo-modulation
produces change only in the vicinity of the PIPAAm-immobilized surface
zone, negligible volume changes in the stationary phase occur, an
important characteristic for a modified chromatography matrix.
EXPERIMENTAL
Materials
The
N-isopropylacrylamide (IPAAm), and N,N-dimethylaminopropyl-
acrylamide (DMAPAA) were kindly provided by KOHJIN (Tokyo,
Japan). IPAAm was purified by recrystallization from
n-hexane.
DMAPAA was purified by distillation under reduced pressure and the
Temperature-Modulated Interaction Changes
577

fractional distillate at 1138C (1 mmHg) was collected.
n-Butyl metha-
crylate (BMA) was purchased from Kanto Chemicals (Tokyo, Japan) and
the fractional distillate at 548C (11 mmHg) was used. 4,4
0
-Azobis(4-
cyanovaleric acid) was obtained from Wako Pure Chemical Industries
Co. Ltd. (Osaka, Japan). All other reagents were analytical grade from
Wako Pure Chemical Industries and used without further purification.
Aminopropylsilica beads (average diameter 5 mm, lot. 6015) were
purchased from Nishio Industry Co. (Tokyo, Japan).
Immobilization of Thin Polymer Layer on Aminopropylsilica
Beads Surfaces
Azo polymerization initiator, 4,4
0
-azobis(4-cyanovaleric acid) (2.1 g)
and 3.7 g of 1-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ;
Tokyo Kasei, Co., Tokyo, Japan) as condensing agent were dissolved
in 100 mL of DMF to attach the initiator onto aminopropylsilica
beads (5.0 g) through amide bond formation. Introduction of the
azo-initiator onto the bead surface was confirmed by quantifying
residual amino groups on the bead surface using sulfo-
N-succinimidyl-
4-
O-(4,4
0
-dimethoxytrityl)butyrate (sulfo-SDTB; Pierce) [21]. Radical
copolymerization of IPAAm, DMAPAA, and BMA was carried out in
the presence of initiator-immobilized silica beads in ethanol for 15 h
at 708C after degassing by freeze-thaw cycles three times. Total
monomer concentration is set at 88.4 mM, DMAPAA 7.5 mol%,
IPAAm/BMA ¼ 95/5 (mol/mol).
N,N
0
-Methylenebisacrylamide (1 mol%
to total monomer) was used as the cross-linker. Terpolymer-modified
silica beads are abbreviated as IBD7.5. The IPAAm homopolymer
hydrogel-modified silica beads were also prepared as a reference surface
in a similar manner [9]. These prepared surfaces were extensively
washed with ethanol to remove unreacted monomers and ungrafted
polymers, then dried under vacuum at 258C for at least 12 h.
The surface potential of the polymer-modified beads were measured
using electrophoretic light scattering spectrophotometer (ELS-8000,
Otsuka Electronics Co., Osaka, Japan) with sample suspension in
10 mmol/L KCl at pH 7.0. Cell temperature was controlled with
thermostated water bath within 0.18C.
Temperature-responsive HPLC for Adenosine Nucleotides
The polymer-modified beads were packed into stainless steel column
(4.6 mmf 150 mm) by introducing at 350 kg/cm
2
from water/methanol
slurry of polymer-modified beads. After extensive washing with distilled
578
A. K
IKUCHI ET AL
.
water, the polymer-modified bead-packed column was connected to
an aqueous HPLC system (JASCO Co., Ltd., Tokyo, Japan). Three
adenosine nucleotides, adenosine-5
0
-monophosphate (AMP, 26.3 ng/mL),
adenosine-5
0
-diphosphate (ADP, 183.7 ng/mL), and adenosine-5
0
-tripho-
sphate (ATP, 790.0 ng/mL), all obtained from Sigma, were dissolved in
degassed Na
2
HPO
4
/citric acid buffer (
I ¼ 0.1) at pH 7.0. Samples (20 mL)
were injected with an auto-sampler (AS-950) and pumped into the
column at a flow rate of 0.5 mL/min with an intelligent pump (PU-980).
Nucleotide elution was monitored by the UV absorption at 254 nm with
an UV/Vis spectrophotometer (UV-970) and analyzed with BORWIN
analysis software (Ver. 1.21, JASCO). Column temperature was
controlled by connecting the column circulating water jacket to a
thermostated water bath (Coolnics Circulator, CTE42A, Komatsu-
Yamato, Japan) to within 0.18C. Capacity factor,
k, for each analyte
was calculated by the following equation:
k ¼
R
t
ð
R
t
R
0
Þ
ð
1Þ
where
R
t
is the retention time of each analyte at determined
temperature, and
R
0
is the retention time of deuterium oxide (D
2
O)
that was used as the internal standard, since retention time of D
2
O did
not change with temperature.
Effect of Step Temperature Gradient on Nucleotide Retention
After AMP and ADP were eluted with relatively good separation at
208C, column temperature was changed to 508C at 35 min postsample
injection. Stationary surface properties (polymer collapse) changed
immediately with this temperature change, resulting in an earlier
ATP elution than the isocratic elution at 208C. Column temperature was
changed using two thermostated water baths connected to the column
jacket via a three-way stopcock.
RESULTS AND DISCUSSION
Thermo-responsive Characteristics of IBD Copolymer-modified
Surfaces
External temperature not only modulated PIPAAm surface hydro-
philic/hydrophobic changes but also the surface charge density distribu-
tions in IPAAm copolymers bearing charged functional groups.
To achieve this for ‘‘green’’ chromatography and separations, the
Temperature-Modulated Interaction Changes
579
cationic monomer,
N,N-dimethylaminopropylacrylamide (DMAPAA),
was copolymerized by radical copolymerization with IPAAm to produce
cationic thermosensitive copolymers useful for stationary phase surface
modification (Figure 1). Immobilization of azo-initiator was determined
from the consumption of surface amino groups indicating that the
initiator was successfully attached to the silica bead surface. After
polymerization, silica bead size was estimated from the scanning
electron micrographs; negligible diameter changes were observed. This
result suggests that the surface-grafted polymer layer was very thin.
In addition, ESCA analysis revealed that N/C atomic ratio was higher
for the cationic thermoresponsive surfaces of IBD surfaces than for the
IPAAm homopolymer surfaces. This data indicated the successful
introduction of cationic thermoresponsive polymers on the silica bead
surfaces. Similarly, we introduced anionic thermoresponsive polymers
onto silica bead surfaces and found that the surface-grafted polymer
amount was approximately 600 mg/m
2
[10]. In this case, we did not
measure the grafted amount on the silica, though it should be similar.
Using these surface polymer-grafted silica beads, thermoresponsive
changes in polymer charge density and wetting were investigated.
Previous reports documented the weakening of tertiary amine basicity
in IPAAm copolymers at elevated temperature (above LCST) with no
observable change in amine basicity in water-soluble polyacrylamide
derivatives over a wide temperature range [22]. The decrease in amino
group basicity in the case of the PIPAAm derivatives is due to the
decrease in the dielectric constant around the amino groups at higher
temperatures where the polymer loses hydration and becomes hydro-
phobic. Urry [23] reported a carboxyl p
Ka shift in synthetic polypeptides
by
modulating
peptide
hydrophobicity.
Therefore,
temperature
(CH
2
(CH
2
)
3
(CH
2
)
3
(CH
2
)
2
CH
CH
N
C=O
C=O
C=O
C=O
C=O
O
NH
NH
NH
NH
CH
CH)
n
C
CH
2
H
3
C
H
3
C
CH
3
CH
3
CH
3
CH
3
CH CH
2
CH
2
CH
2
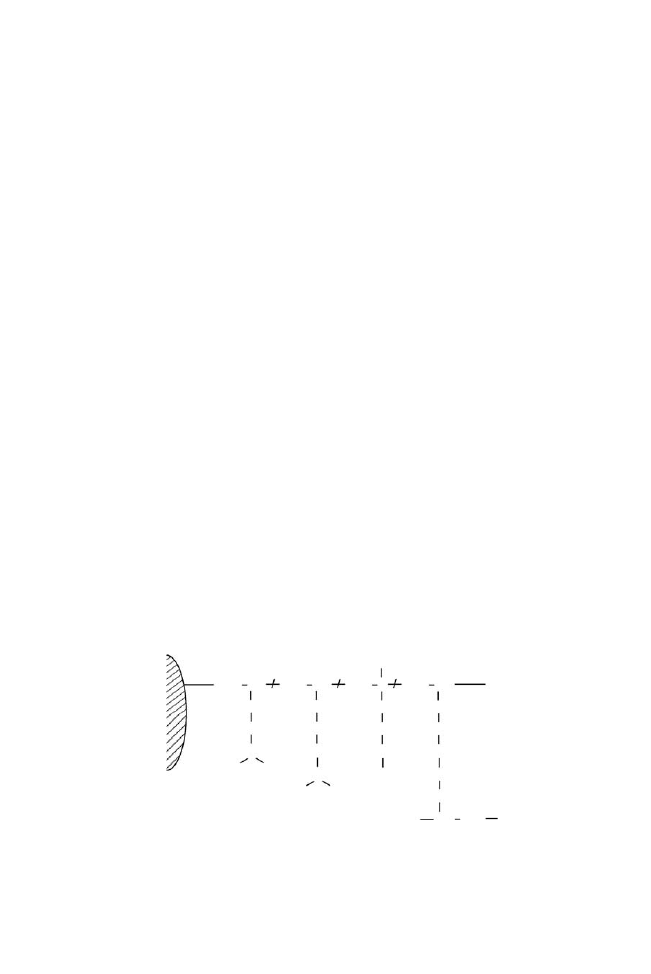
Figure 1. Schematic drawing of crosslinked poly(IPAAm-
co-DMAPAA-co-BMA)-modified
silica beads.
580
A. K
IKUCHI ET AL
.

modulated changes based on the surface charge density of grafted
PIPAAm derivatives containing ionizable groups is possible.
Poly(IPAAm-
co-DMAPAA-co-butyl methacrylate (BMA)) containing
7.5 mol% DMAPAA in the copolymer (termed IBD7.5) was synthesized
in the presence of azo-initiator-immobilized silica beads with a
modification to our previous report [9]. The hydrophobic BMA
co-monomer was introduced to control total matrix hydrophobicity
and its transition temperature. Soluble IBD7.5 linear copolymers were
prepared by free radical polymerization that had a number averaged
molecular weight of 7.08 10
4
and a polydispersity index (PDI) of 1.52
after dialysis. Copolymer IBD7.5 exhibited a transition temperature at
29.58C in distilled water as determined by solution transmittance-
turbidity measurement at 500 nm. IBD hydrogel-surface modified beads
were assessed using surface potential measurements with electro-
phoretic laser light scattering spectrophotometry at various tempera-
tures (Figure 2(b)). The introduction of cationic amino groups into
the copolymer produced a slightly higher surface potential in the
IBD-modified beads than that for the pure PIPAAm-modified beads.
Both bead samples showed reductions in the surface potential above the
respective polymer transition temperature, 308C, for PIPAAm-
modified beads and, 358C, for IBD-modified beads using this method.
The
reduction
in
surface
potential
indicated
a
compression
in the surface electrostatic double layer. This was due to hydrophobic
aggregation of the IPAAm sequences in the polymer chains above the
collapse transition, accompanied by local reduction in the surface zone
dielectric constant around the copolymer amino groups. Deprotonation
of the IBD amino groups on the derivatized bead surface was apparent
above the polymer transition temperature. This correlated with
significant changes in the apparent p
Ka values for the tertiary amines
above the polymer transition temperature determined by direct acid-
base titration, shown in Figure 2(a). At higher temperatures, dehydra-
tion of IPAAm isopropyl groups occurred which locally increased
the
hydrophobic
microenvironment
around
the
polymer
amino
groups and enhanced the deprotonation of the amino groups at the
higher temperature. Consequently, the surface charge density and the
hydrophobicity were altered by changing temperature over small
increments.
Temperature Responsive Elution of Adenosine Nucleotides
We
applied
this
unique
temperature
controlled
surface
property alteration to regulate solute–surface interactions in a
Temperature-Modulated Interaction Changes
581
separation mode. Three adenosine nucleotide model analytes with
different charge densities – adenosine 5
0
-monophosphate (AMP),
adenosine
5
0
-diphosphate
(ADP),
and
adenosine
5
0
-triphosphate
(ATP) – share identical base adenosine chemistry but different
numbers of anionic phosphate units. These models were selected
due to their importance in cellular metabolism and current attention
in bioanalytical and biochemical research. Shown in Figures 3(a) and
(b) are the chromatograms generated for these adenosine nucleotides
in aqueous buffer (pH 7.0) using only changing the temperatures of
the IBD7.5 column and PIPAAm column, respectively. Analyte
retention times increased with increasing numbers of adenosine
phosphate
units
involved,
the
increasing
order
was;
AMP5ADP5ATP for the IBD column regardless of column tem-
perature, while poor separation was observed on PIPAAm column at
all temperatures. Since adenosine phosphate groups are negatively
7.0
7.5
8.0
8.5
9.0
−20
−15
−10
−5
0
5
10
(a)
(b)
b)
70
60
50
40
30
20
10
0
Temperature (
°C)
ζ potential (mV)
pKa
Figure 2. (a) Temperature-induced p
Ka shifts for amino side groups in terpolymer IBD7.5
in 100 mM KCl solution. (b) Effect of temperature on the potential changes (electrostatic
double layer thickness) for thermoresponsive polymer-modified silica beads. Open circle:
PIPAAm-modified silica beads, closed square: IBD7.5-modified silica beads.
582
A. K
IKUCHI ET AL
.
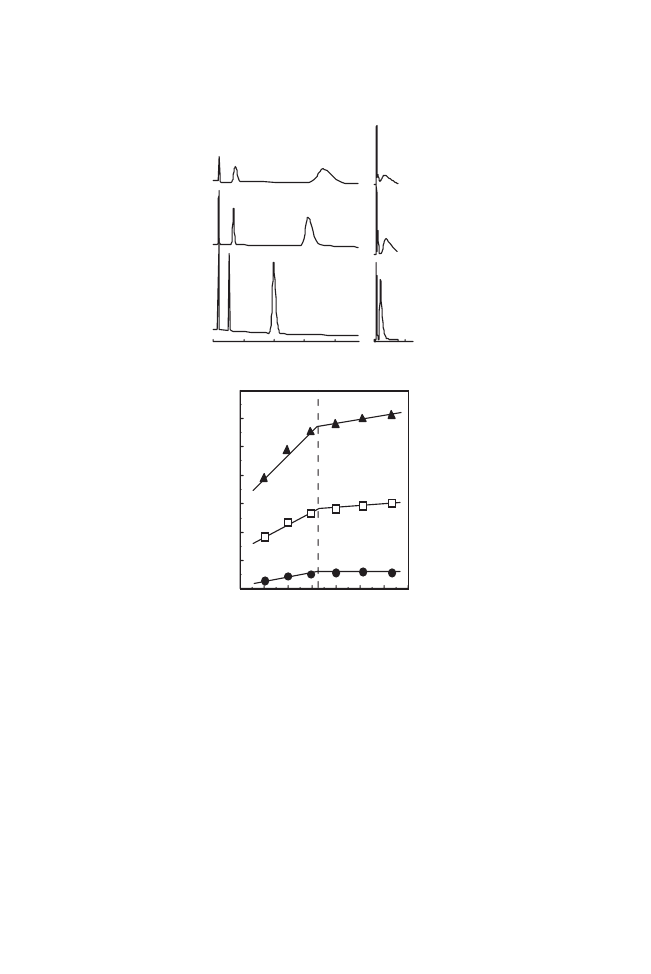
charged at pH 7.0, the electrostatic interactions occurred predomi-
nately with the positive charges on the copolymer column matrix
surfaces. As the column temperature increased, the solute retention
was shortened and peaks become narrower, concomitant with polymer
dehydration with increasing temperature. The polymer dehydration
correlated with the observed reduction in apparent amino group p
Ka
0
50
100 150 200
0
50
1
2
3
1
2
3
1
2
3
50
°C
10
°C
30
°C
1
2
3
1
2
3
1
2
3
(a) IBD7.5
(b) PIPAAm
Retention time (min)
4.5
1.0
1.5
2.0
2.5
3.0
3.5
4.0
2.9 3.0 3.1 3.2 3.3 3.4 3.5 3.6
ADP
ATP
AMP
1/T [
×10
−3
K
−1
]
(c)
ln
k
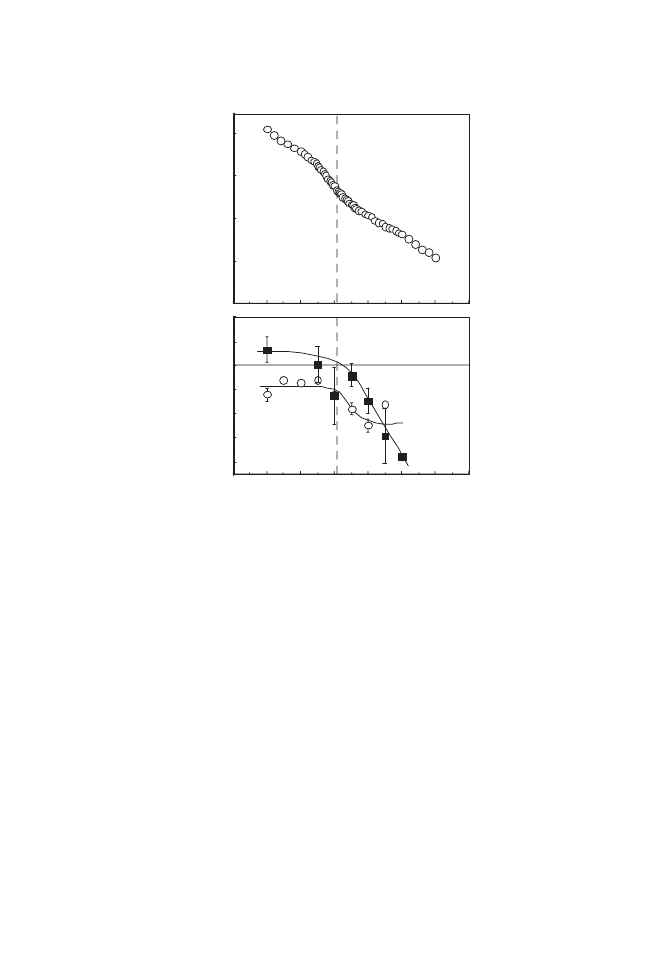
Figure 3. Temperature-responsive elution profiles of adenosine nucleotides, 1; AMP,
2; ADP, and 3; ATP from (a) IBD7.5-modified silica bead column, and (b) PIPAAm-
modified bead column in aqueous buffer at pH 7.0; (c) the van’t Hoff plots for nucleotides
on IBD7.5-modified silica bead column. In Figure 3(c), the natural logarithm of the
capacity factor,
k, for adenosine nucleotides are plotted against reciprocal of temperature.
Temperature-Modulated Interaction Changes
583

values in the copolymer matrix as described above. Any decrease in
the amount of protonated amino groups in the copolymer solid phase
reduced the effective positive charge density which lead to the
observed decrease in solute retention time. The p
Ka change-induced
decreases in analyte retention were apparent above the polymer
transition temperature, and seen in the van’t Hoff plots of nucleotides
separated on the copolymer-modified column (Figure 3(c)). The
dashed line in Figure 3(c) represents transition temperature of
IBD7.5. The slope of each plot changed both below and above the
transition temperature. As seen in Figure 2(a), the p
Ka of the amino
groups changed with temperature and the electrostatic interaction
changed continuously with temperature. However, above the polymer
transition temperature, such interactions were largely changed to
a hydrophobic mode. Thus such surface property changes affected the
analyte interactions.
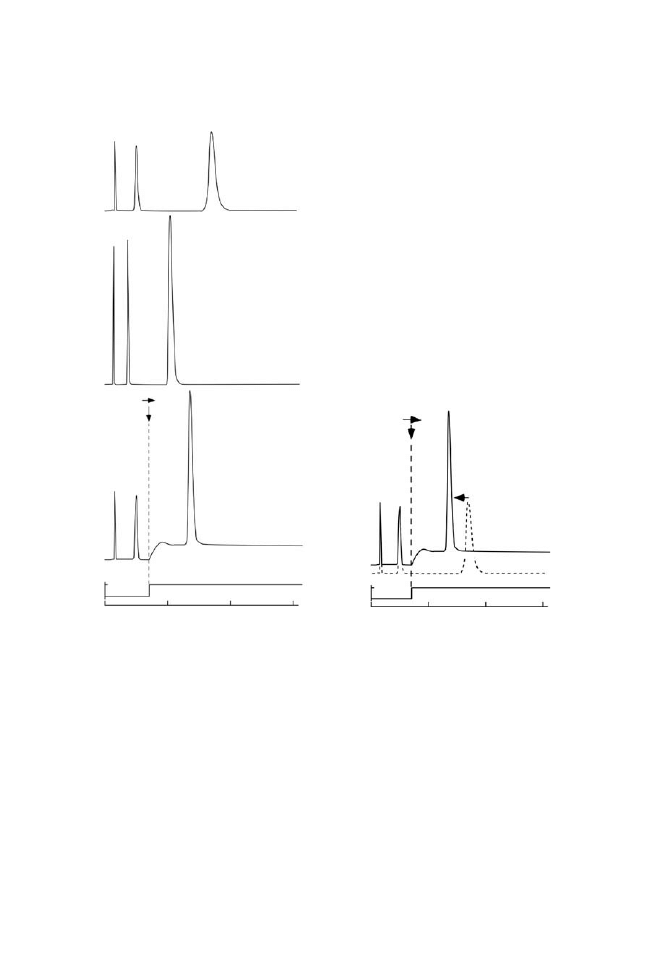
Effect of Step Temperature Gradient on Nucleotide Elution
Shown in Figure 4 are the chromatograms of the adenosine
nucleotides produced
using
a
step
temperature
gradient.
This
instantaneous temperature gradient induced dynamic stationary
phase surface property alterations during solute elution similar to
the gradient elution in RPC. The thin copolymer layer formed on the
solid phase responded rapidly to the applied thermal gradient on
the column. As seen in this figure, ATP eluted much faster after the
temperature was stepwise changed to 508C (35 min after injection)
which is significantly less than that for 208C. Although a baseline
drift was observed after this temperature change, sufficient separa-
tion was obtained. Stationary phase surface wettability as well as
charge density immediately changed by increasing the column
temperature which altered the solute interactions with the altered,
hydrophobic solid phase. This was seen by the changing retention
times as analyte interactions were modulated by changing tempera-
ture during separation procedure.
These nucleotides are frequently analyzed by RPC [24,25], ion-pair
RPC [26–28], or ion exchange chromatography [29]. However, RPC
requires organic solvents for mobile phase preparation and/or hydro-
phobic ion-pairing agents to control solute elution by modulating solute
hydrophobicity and interaction with solid phases. Ion exchange columns
frequently require very long analysis times. These are disadvantageous
for separations in terms of experimental analytical period, waste
handling costs, and eluting solvent disposal. In the present system,
584
A. K
IKUCHI ET AL
.
only aqueous mobile phase is required for baseline separations without
use of organic solvents: the IBD copolymer-modified surface hydro-
phobicity and charge density are readily controlled by changing column
temperature to effect separation. Although optimization of several
characteristics of the surface-grafted polymers and separation condi-
tions are still needed for IBD copolymer columns, use of intelligent
1
2
3
3
3
1
2
2
1
3
3
50
20
0
50
Retention time (min)
100
150
0
50
Retention time (min)
100
150
2
1
(a) 20
°C
(b) 50
°C
(c) 20
50
°C
20
°C
50
°C
20
Temperature (
°C)
50
20
Temperatrue (
°C)
Figure 4. Modulation of nucleotide elution by applying a step temperature gradient on
the IBD7.5 column. Numbers in the figure indicate nucleotide analytes analogous to those
in Figure 3(a): (a) elution profiles of nucleotide at a different temperature,
(b) superimposed curves of chromatogram observed for isocratic elution at 208C, and
(c) step temperature gradient from 20 to 508C. Dotted line indicates the chromatogram of
adenosine nucleotides at 208C.
Temperature-Modulated Interaction Changes
585

materials should prove valuable in the design of novel ‘‘green’’
separation systems.
ACKNOWLEDGMENTS
This work was financially supported in part by the National Institute
of Environmental Science (NIES), commissioned with the Ministry of
Environment
(MENV),
Japan,
‘‘The
Development
of
New
Environmental Technology Using Nanotechnology Project’’. The
authors are grateful to Professor H. Kanazawa, Kyoritsu University of
Pharmacy and Professor D.W. Grainger, University of Utah for their
scientific discussions.
REFERENCES
1. Heskins, M., Guillet, J.E. and James, E. (1968). Solution Properties of poly
(N-isopropylacrylamide).
Journal of Macromolecular Science, Chemistry,
A2(8): 1441–1445.
2. Okano, T., Yamada, N., Sakai, H. and Sakurai, Y. (1993). A Novel
Recovery System for Cultured Cells using Plasma-treated Polystyrene
Dishes Grafted with poly(N-isopropylacrylamide),
Journal of Biomedical
Materials Research, 27(10): 1243–1251.
3. Okano, T., Yamada, N., Okuhara, M., Sakai, H. and Sakurai, Y. (1995).
Mechanism of Cell Detachment from Temperature-modulated, Hydrophilic-
hydrophobic Polymer Surfaces,
Biomaterials, 16(4): 297–303.
4. Kikuchi, A., Okuhara, M., Karikusa, F., Sakurai, Y. and Okano, T. (1998).
Two-dimensional Manipulation of Confluently Cultured Vascular Endothelial
Cells using Temperature-responsive poly(N-Isopropylacrylamide)-Grafted
Surfaces,
Journal of Biomaterials Science, Polymer Edition, 9(12): 1331–
1348.
5. von Recum, H., Kikuchi, A., Okuhara, M., Sakurai, Y., Okano, T. and Kim,
S.W. (1998). Retinal Pigmented Epithelium Cultures on Thermally
Responsive Polymer Porous Substrates,
Journal of Biomaterials Science,
Polymer Edition, 9(11): 1241–1253.
6. Yamato, M., Kushida, A., Konno, C., Kikuchi, A., Sakurai, Y. and Okano, T.
(1999). A Novel Tool of Temperature-responsive Cell Culture Surfaces and its
Application to Matrix Biology,
Connective Tissue, 31(1): 13–6.
7. Kanazawa, H., Yamamoto, K., Matsushima, Y., Takai, N., Kikuchi, A.,
Sakurai, Y. and Okano, T. (1996). Temperature-responsive Chromatography
using poly(N-isopropylacrylamide)-modified Silica,
Analytical Chemistry,
68(1): 100–105.
586
A. K
IKUCHI ET AL
.

8. Kanazawa, H., Kashiwase, Y., Yamamoto, K., Matsushima, Y., Kikuchi, A.,
Sakurai, Y. and Okano, T. (1997). Temperature-Responsive Liquid
Chromatography.
2.
Effect
of
Hydrophobic
Groups
in
N-
Isopropylacrylamide Copolymer-Modified Silica,
Analytical Chemistry,
69(5): 823–830.
9. Yakushiji, T., Sakai, K., Kikuchi, A., Aoyagi, T., Sakurai, Y., and Okano, T.
(1999). Effects of Cross-Linked Structure on Temperature-Responsive
Hydrophobic Interaction of Pipaam Hydrogel Modified Surfaces with
Steroids,
Analytical Chemistry, 71(6): 1125–1130.
10. Kobayashi, J., Kikuchi, A., Sakai, K. and Okano, T. (2003). Aqueous
Chromatography Utilizing Ph/Temperature-Responsive Polymer Stationary
Phases to Separate Ionic Bioactive Compounds,
Analytical Chemistry, 73(9):
2027–2033.
11. Idota, N., Kikuchi, A., Kobayashi, J., Akiyama, Y., Sakai, K. and Okano, T.
(2006). Thermally Modulated Interaction of Aqueous Steroids Using
Polymer-Grafted Capillaries,
Langmuir, 22(1): 425–430.
12. Bae, Y.H., Okano, T., Kim, S.W. (1991). ‘‘On-off’’ Thermocontrol
of
Solute
Transport.
I.
Temperature
Dependence
of
Swelling
of
N-Isopropylacrylamide Networks Modified with Hydrophobic Components
in Water,
Pharmaceutical Research, 8(4): 531–537.
13. Bae, Y.H.,. Okano, T. and Kim, S.W. (1991). ‘‘On–off ’’ Thermocontrol of
Solute Transport. II. Solute Release from Thermosensitive Hydrogels,
Pharmaceutical Research, 8(5): 624–628.
14. Yoshida, R., Uchida, K., Kaneko, Y., Sakai, K., Kikuchi, A., Sakurai. Y. and
Okano, T. (1995). Comb-type Grafted Hydrogels with Rapid Temperature
Responses,
Nature, 374(6519): 240–242.
15. Takei, Y.G., Aoki, T., Sanui, K., Ogata, N., Sakurai, Y. and Okano, T. (1994).
Dynamic Contact Angle Measurement of Temperature-Responsive Surface,
Properties
for
Poly(
N-Isopropylacrylamide)
Grafted
Surfaces.
Macromolecules, 27(21): 6163–6166.
16. Yakushiji, T., Sakai, K., Kikuchi, A., Aoyagi, T., Sakurai, Y. and Okano, T.
(1998). Graft Architectural Effects on Thermo-Responsive Wettability
Changes of poly(
N-isopropylacrylamide)-modified surfaces, Langmuir,
14(16): 4657–4662.
17. Yamada, N., Okano, T., Sakai, H., Karikusa, F., Sawasaki, Y. and
Sakurai, Y. (1990). Thermo-responsive Polymeric Surfaces; Control of
Attachment and Detachment of Cultured Cells,
Makromolekulare Chemie,
Rapid Communications, 11(11): 571–576.
18. Hirose, M., Kwon, O.H., Yamato, M., Kikuchi, A. and Okano, T. (2000).
Creation of Designed Shape Cell Sheets that are Noninvasively Harvested
and Moved onto Another Surface,
Biomacromolecules, 1(3): 377–381.
19. Yamato, M. and Okano, T. (2004). Cell Sheet Engineering,
Materials Today,
7(5): 42–47.
Temperature-Modulated Interaction Changes
587

20. Nishida, K., Yamato, M., Hayashida, Y., Watanabe, K., Yamamoto, K.,
Adachi, E., Nagai, S., Kikuchi, A., Maeda, N., Watanabe, H., Okano, T. and
Tano, Y. (2004). Corneal Reconstruction with Tissue-engineered Cell Sheets
Composed of Autologous Oral Mucosal Epithelium,
The New England
Journal of Medicine, 351(12): 1187–1196.
21. Gaur, R.K. and Gupta, K.C. (1989). A Spectrophotometric Method for the
Estimation of Amino Groups on Polymer Supports,
Analytical Biochemistry,
180(2): 253–258.
22. Feil, H., Bae, Y.H., Feijen, J. and Kim, S.W. (1992). Mutual Influence of Ph
and Temperature on the Swelling of Ionizable and Thermosensitive
Hydrogels,
Macromolecules, 25(20): 5528–5530.
23. Urry, D.W. (1992). Free Energy Transduction in Polypeptides and Proteins
Based on Inverse Temperature Transitions,
Progress in Biophysics and
Molecular Biolology, 57(1): 23–57.
24. Leoncini, G., Buzzi, E., Maresca, M., Mazzei, M. and Balbi, A. (1987).
Alkaline
Extraction
and
Reverse-Phase
High-Performance
Liquid
Chromatography
of
Adenine
and
Pyridine
Nucleotides
in
Human
Platelets,
Analytical Biochemistry, 165(2): 379–383.
25. Teerlink, T., Hennekes, M., Bussemaker, J. and Groeneveld, J. (1993).
Simultaneous
Determination
of
Creatine
Compounds
and
Adenine
Nucleotides
in
Myocardial
Tissue
by
High-Performance
Liquid
Chromatography,
Analytical Biochemistry, 214(1): 278–283.
26. Tekkanat, K.K. and Fox, I.H. (1988). Isocratic Separation of ATP and its
Degradation Products from Biological Fluids by Automated Liquid
Chromatography,
Clinical Chemistry, 34(5): 925–932.
27. Schobert, B. (1995). Enzymatic Synthesis of ATP Analogs and their
Purification
by
Reversed-Phase
High-Performance
Liquid
Chromatography,
Analytical Biochemistry, 226(2): 288–292.
28. Blanco, J., Canela, E.I., Sayos, J., Mallol, H., Lluis, C. and Franco, R. (1993).
Adenine Nucleotides and Adenosine Metabolism in Pig Kidney Proximal
Tubule Membranes,
Journal of Cellular Physiology, 157(1): 77–83.
29. Fujimori, H., Sasaki, T., Hibi, K., Senda, M. and Yoshioka, M. (1990). Direct
Injection
of
Blood
Samples
into
a
High-Performance
Liquid
Chromatographic Adenine Analyser to Measure Adenine, Adenosine, and
the Adenine Nucleotides with Fluorescence,
Journal of Chromatography,
515: 363–373.
588
A. K
IKUCHI ET AL
.
Wyszukiwarka
Podobne podstrony:
Sea Change with Monsters Paul J McAuley
Please visit our website for an interactive text with links
1880 2004 temperature anomaly climate change global warming
Numerical Simulation of Interacting Bodies with Delays;
Virato, Swami Interview With Sogyal Rinpoche On The Tibetan Book Of Living And Dying (New Frontier
Wójcik, Marcin; Suliborski, Andrzej The Origin And Development Of Social Geography In Poland, With
Exergetic efficiency of high temperature lift chemical heat pump (CHP) based on CaO CO2 and CaO H2O
Turing Machines and Undecidability with Special Focus on Computer Viruses
Stein Wilkeshuis M A Viking age Treaty Between Constantinople and Northern Merchants, With its Provi
07 Kolar K i inni Influence of separation agents on quality of concrete surface
Interacting With Folks on Different Levels of the Poker Food Chain
The effect of the interaction of various spawn grains with different culture medium on carpophore
Changes in passive ankle stiffness and its effects on gait function in
Improving Grape Quality Using Microwave Vacuum Drying Associated with Temperature Control (Clary)
anyway on with the show
Amon Amarth With Oden on Our Side
O&O Services Single Sign On on Linux using LDAP with Active Directory (2002)
Change Text on XP Start Button
WITH ON IN
więcej podobnych podstron