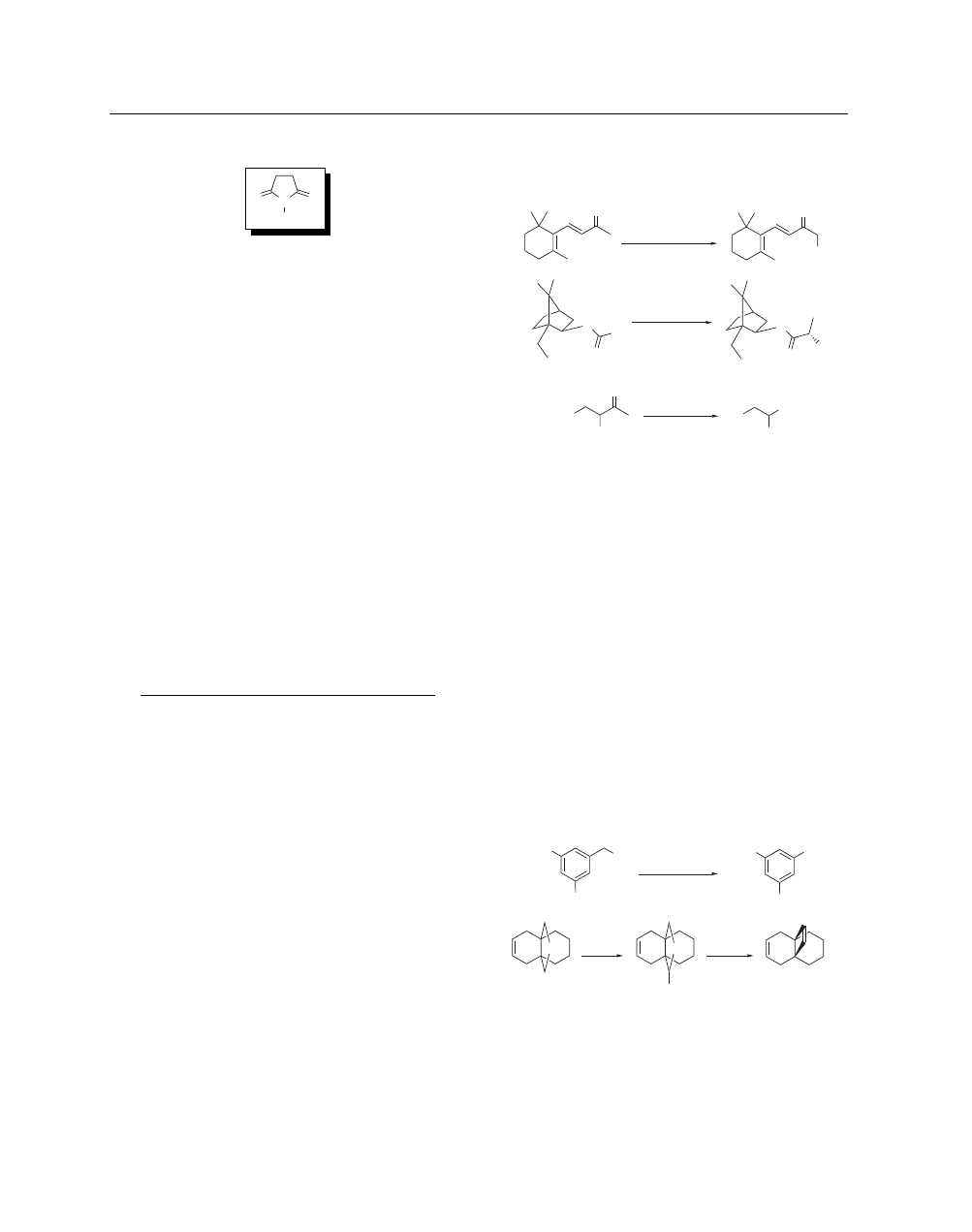
N-CHLOROSUCCINIMIDE
1
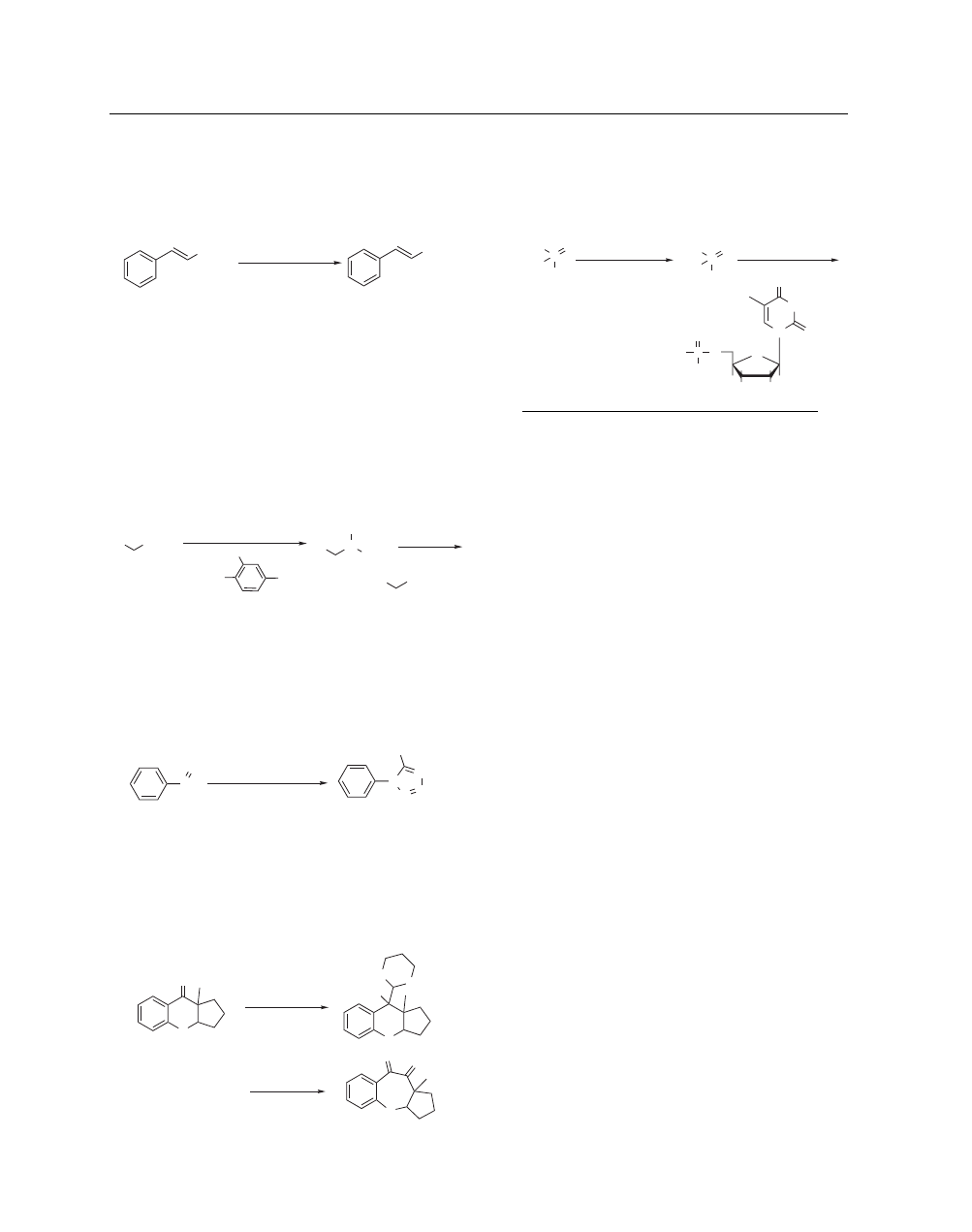
N-Chlorosuccinimide
N
O
O
Cl
[128-09-6]
C
4
H
4
ClNO
2
(MW 133.53)
InChI = 1/C4H4ClNO2/c5-6-3(7)1-2-4(6)8/h1-2H2
InChIKey = JRNVZBWKYDBUCA-UHFFFAOYAN
(electrophilic α-chlorination of sulfides, sulfoxides, and ketones;
preparation of N-chloroamines)
Alternate Name:
1-chloro-2,5-pyrrolidinedione; NCS.
Physical Data:
mp 144–146
◦
C.
Solubility:
sol H
2
O; sl sol CCl
4
, benzene, toluene, AcOH; insol
ether.
Form Supplied in:
white powder or crystals having a weak odor
of chlorine when pure; widely available.
Purification:
the commercial reagent acquires a light yellow color
and a rather strong odor of chlorine after long storage but is
easily recrystallized from acetic acid: rapidly dissolve 200 g of
impure sample in 1 L preheated glacial AcOH at 65–70
◦
C (3–5
min); cool to 15–20
◦
C to effect crystallization; filter through a
Buchner funnel and wash the white crystals once with glacial
AcOH and twice with hexane; dry in vacuo (>85% recovery).
Analysis of Reagent Purity:
the standard iodide–thiosulfate titra-
tion method is suitable.
Handling, Storage, and Precautions:
store under refrigeration
and protect from moisture; acutely irritating solid, with toxic
effects similar to those of the free halogens; avoid inhalation;
use an efficient fume hood; perform all operations as rapidly as
possible to avoid extensive decomposition of the reagent.
Original Commentary
Scott C. Virgil
Massachusetts Institute of Technology, Cambridge, MA, USA
N
-Chlorosuccinimide is a convenient reagent for the electro-
philic substitution and addition of chlorine to organic compounds.
Other chlorinating agents of use include Chlorine, Sulfuryl Chlo-
ride, Chloramine-T, tert-Butyl Hypochlorite, and Trichloroiso-
cyanuric Acid. The primary advantages of using NCS include the
ease in handling, the mild conditions under which chlorination
proceeds, and the ease of removal of the inoffensive byproduct
succinimide.
α
α
α
-Chlorination of Carbonyl Derivatives.
Carbonyl com-
pounds can be chlorinated in the α-position by addition of NCS
directly to the lithium enolates, enoxyborinates, or more
commonly to the silyl enol ether derivatives.
1
In combination
with methods for the regiospecific generation of enolates and silyl
enol ethers, α-chloroketones of desired structure can be produced.
For example, β-ionone can be chlorinated selectively in the α
′
-
position by addition of NCS to the kinetic enolate (eq 1).
2
With the
appropriate chiral auxiliary, NCS chlorinates silyl ketene acetals
with high levels of diastereoselectivity (eq 2).
3
α
-Chloro ketones,
α
-chloro esters, and α-chloro sulfones may also be prepared by
reaction of NCS with the β-keto derivatives and in situ deacylation
in the presence of base (eq 3).
4
NCS is also an effective reagent
for the α-chlorination of acid chlorides.
5
O
(1)
O
Cl
1. LDA, THF, 0 °C
2. NCS, –70 °C
65%
O
Et
O
SO
2
NCy
2
O
O
SO
2
NCy
2
1. LDA, TMSCl
THF, –78 °C
2. NCS, –78 °C
(2)
Cl
92%
Ph
O
CO
2
Et
Ph
Cl
CO
2
Et
NCS, NaOEt
EtOH, rt
(3)
86%
Chlorination of Sulfides and Sulfoxides.
6
The reaction of
alkyl sulfides with NCS has been used extensively for the prepa-
ration of α-chloro sulfides, and NCS is generally regarded as the
reagent of choice for the preparation of these useful synthetic
intermediates (see also Trichloroisocyanuric Acid). Since
the mechanism of chlorination involves initial formation of an
S
-chlorosulfonium salt followed by a Pummerer-like rearrange-
ment, monochlorination proceeds smoothly in CCl
4
or benzene
in the absence of added acid or base.
7
The most straightfor-
ward procedure involves the addition of NCS to a solution of the
sulfide in CCl
4
at rt or reflux, followed by removal of insoluble
succinimide by filtration. The resulting α-chloro sulfides are
easily hydrolyzed and, as this is usually undesirable, α-chloro
sulfides must be prepared under strictly anhydrous conditions
and are often used without further purification. A method has
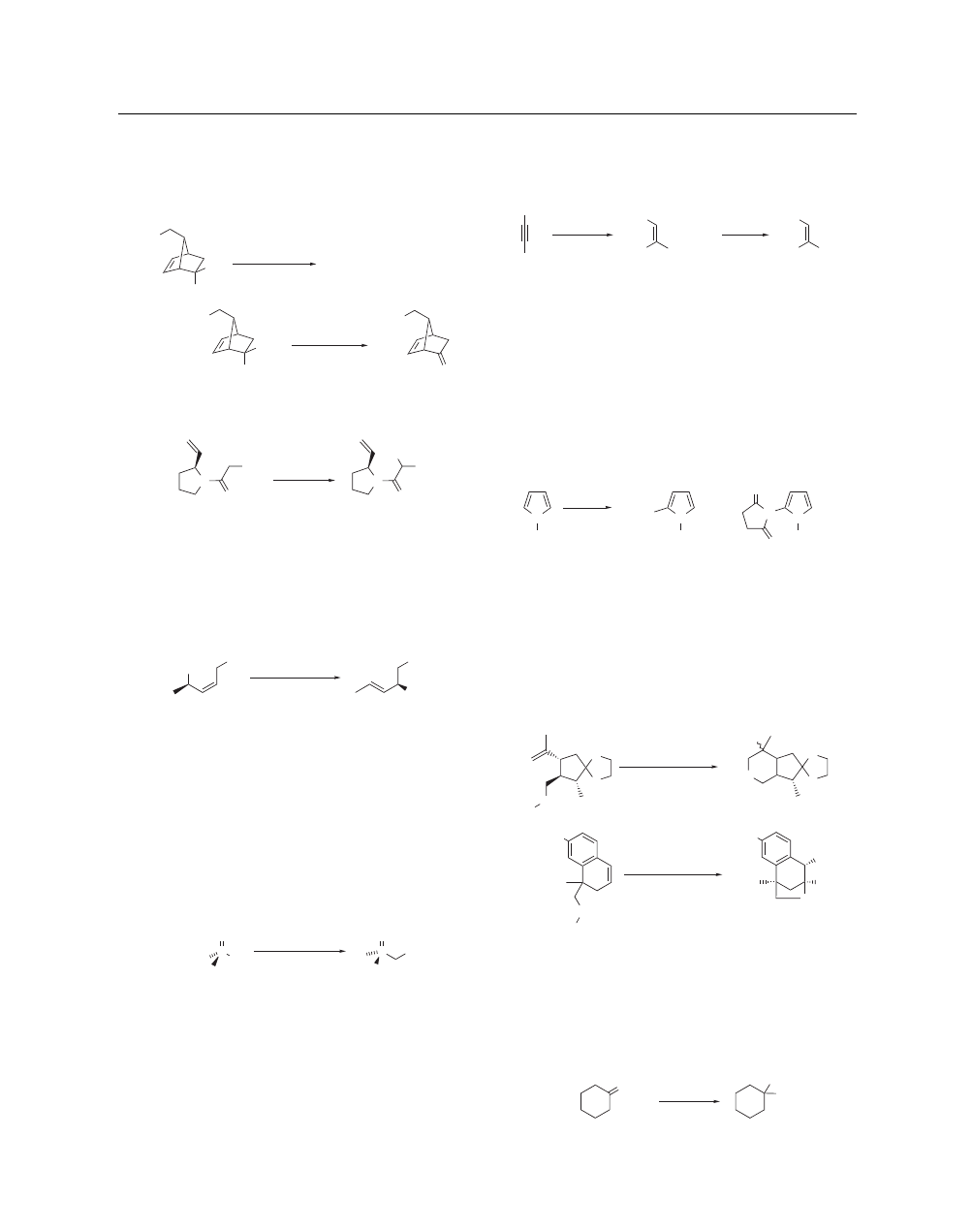
been developed for the conversion of benzylic halides to aro-
matic aldehydes (eq 4);
8
however, this transformation is more
conveniently effected in one operation with other reagents (see
Hexamethylenetetramine). Many advantages have led to the
preferred use of NCS in the Ramberg–Bäcklund rearrangement
sequence (eq 5), which has been recently reviewed.
9
t
-Bu
t
-Bu
Br
t
-Bu
CHO
t
-Bu
(4)
1. NaSPh
2. NCS, CCl
4
3. Na
2
CO
3
(aq)
85%
S
S
Cl
(5)
NCS
CCl
4
reflux
2 steps
79%
The chlorination of trimethylsilylmethyl sulfides with NCS and
trifluoroacetic acid affords the product of chlorodesilation in high
yield.
10
The degradation of carboxylic acids to ketones can be
achieved by α-sulfenation followed by reaction with NCS in the
presence of NaHCO
3
(eq 6).
11
The S-chlorosulfonium ion inter-
mediate undergoes a decarboxylative Pummerer-like rearrange-
ment to afford the ketone upon hydrolysis. α-Phenylthio esters
Avoid Skin Contact with All Reagents
2
N-CHLOROSUCCINIMIDE
and amides can be successfully α-chlorinated using NCS in CCl
4
at 0
◦
C (eq 7).
12
1,3-Dithianes are deprotected to afford ketones
by reaction with NCS alone or in combination with Silver(I)
Nitrate in aqueous acetonitrile (see also N-Bromosuccinimide,
Mercury(II) Chloride, 1,3-Diiodo-5,5-dimethylhydantoin).
13
MeO
CO
2
H
H
MeO
CO
2
H
SMe
(6)
MeO
LDA (2 equiv)
O
NCS
64%
EtOH, NaHCO
3
HMPA, THF
then MeSSMe
N
(7)
O
NCS
CCl
4
, 0 °C
SPh
N
O
SPh
Cl
100%
Sulfides can be oxidized to sulfoxides by reaction with NCS in
methanol (0
◦
C, 1 h).
14
Similarly, selenides couple with amines
when activated by NCS to form selenimide species. These have
been generated from allylic selenides in order to prepare allylic
amines and chiral secondary allylic carbamates by [2,3]-sigma-
tropic rearrangement (eq 8).
15
(8)
NCS, Cbz-NH
2
NEt
3
, MeOH, 0 °C
PhSe
Ph
Ph
NHCbz
69%
The α-chlorination of sulfoxides is generally performed in
dichloromethane in the presence of a base (either K
2
CO
3
or pyri-
dine) and proceeds more slowly than the reactions with sulfides.
16
α
-Chloro sulfoxides bearing high optical purity at sulfur are espe-
cially useful in asymmetric synthesis, but unfortunately the chlo-
rination of optically active sulfoxides is generally accompanied
by significant racemization at sulfur. Alternate procedures are
available for achieving chlorination with predominant retention
or inversion.
17
Using NCS and Potassium Carbonate the degree
of racemization is minimized and chloromethyl p-tolyl sulfoxide
can be prepared in 87% ee and 91% chemical yield (eq 9).
18
Me
S
O
Tol
S
O
Tol
(9)
:
NCS, K
2
CO
3
CH
2
Cl
2
, rt, 40 h
Cl
:
91%, 87% ee
Reaction with Vinylic and Acetylenic Derivatives. NCS is
a suitable source of chlorine for the conversion of vinylcopper
and other organometallic derivatives to the corresponding vinyl
chlorides.
19
(E)-(1-Chloro-1-alkenyl)silanes are available from
the appropriate 1-trimethylsilylalkynes by hydroalumination with
Diisobutylaluminum Hydride followed by direct treatment of the
vinylaluminum intermediate with NCS in ether at −20
◦
C (eq 10)
(the corresponding (Z)-isomer is obtained by NBS-catalyzed iso-
merization of the (E)-isomer).
20
1-Chloroalkynes can be prepared
by reaction of the corresponding lithium acetylides with NCS in
THF.
21
TMS
Bu
TMS
Al(i-Bu)
2
Bu
TMS
Cl
Bu
(10)
DIBAL
84%
– 20 °C
Et
2
O, 40 °C
NCS
Chlorination of Aromatic Compounds. NCS has also been
used for the chlorination of pyrroles and indoles; however, the
reaction is less straightforward than when NBS and N-Iodosuccin-
imide are used.
22
In the chlorination of 1-methylpyrrole, it has
been demonstrated that basic conditions (NaHCO
3
, CHCl
3
) lead
to the formation of 1-methyl-2-succinimidylpyrrole (eq 11).
23
In
the presence of catalytic amounts of perchloric acid, thiophenes
and other electron-rich aromatic compounds have been chlori-
nated with NCS.
24
(N-Chlorosuccinimide–Dimethyl Sulfide is
used for the selective o-substitution of phenols.)
N
Me
N
Me
Cl
N
Me
N
O
O
(11)
NCS, rt
+
THF
CHCl
3
, NaHCO
3
89%
–
3%
76%
Synthesis of N-Chloroamines. The conversion of secondary
amines to N-chloroamines by reaction with NCS in ether or
dichloromethane has many advantages over the use of aqueous
hypochlorite, including ease of isolation. This method has been
used repeatedly in the preparation of N-chloroamines for alkene
amination (eqs 12 and 13)
25
and other reactions.
26
1. NCS
CH
2
Cl
2
, 0 °C
2. Ag
2
O, dioxane (aq)
O
O
O
O
MeN
(12)
NH
Me
HO
59%
1. NCS
CH
2
Cl
2
, 0 °C
2. Ag
2
O, THF (aq)
(13)
BnO
BnO
NMe
H
OH
NH
Me
83%
Other Oxidation and Chlorination Reactions.
27
gem
-
Chloronitro compounds are prepared by treating nitronate
anions with NCS in aqueous dioxane, or alternatively by reac-
tion of ketoximes with NCS (eq 14).
28
Oxidative decarboxylation
of carboxylic acids with Lead(IV) Acetate and NCS has been used
effectively for the synthesis of tertiary alkyl chlorides (eq 15).
29
NCS
H
2
O, C
6
H
6
NOH
NO
2
Cl
(14)
100%
A list of General Abbreviations appears on the front Endpapers
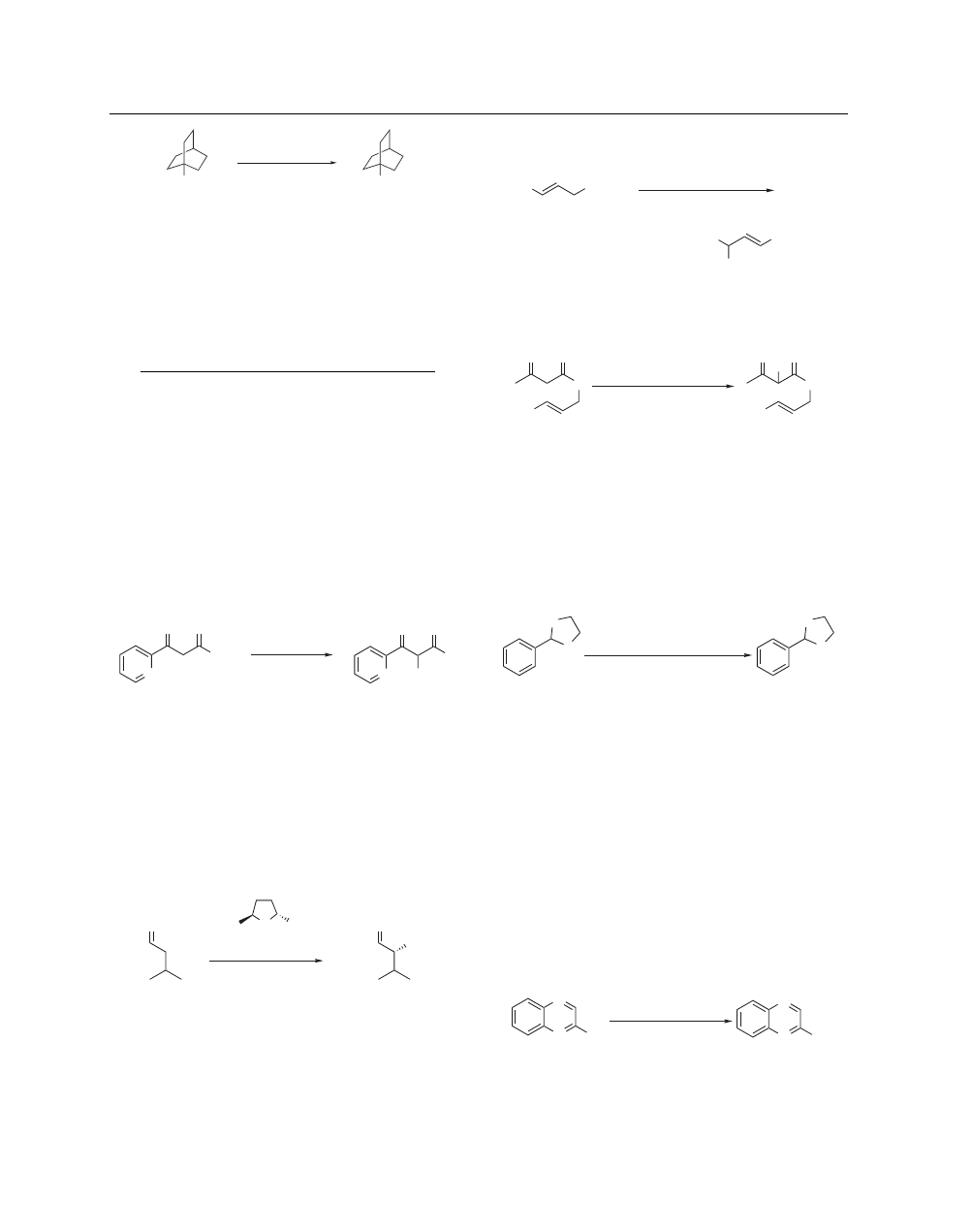
N-CHLOROSUCCINIMIDE
3
Pb(OAc)
4
, NCS
DMF, AcOH, 50 °C
CO
2
H
Cl
(15)
95%
NCS is also regularly used for the direct oxidation of alco-
hols to ketones. The presence of Triethylamine serves to acti-
vate the reagent for rapid quantitative oxidation of catechols and
hydroquinones to o- and p-quinones, respectively, and for the oxi-
dation of benzophenone hydrazone to diphenyldiazomethane.
30
N-Chlorosuccinimide–Dimethyl Sulfide is also used in the mild
oxidation of alcohols, as well as in the conversion of allylic alco-
hols to allylic chlorides.
First Update
Terry V. Hughes
J&JPRD, Raritan, NJ, USA
α
α
α
-Chlorination of Carbonyl Derivatives. The direct chlori-
nation of β-keto esters and cyclic ketones by NCS proceeds readily
at room temperature under acid catalysis by Amberlyst-15
©
. The
reaction is general and works for acyclic, cyclic, and heterocyclic
β
-keto esters. For example, 3-oxo-3-pyridin-2-yl-propionic acid
ethyl ester was α-chlorinated in excellent yield (eq 16).
31
N
O
OEt
O
N
O
OEt
O
Cl
Amberlyst-15,
EtOAc, NCS, rt
85%
(16)
The enantioselective α-chlorination of β-keto esters was
achieved with up to 88% ee using NCS with a commercially
available TADDOL ligand.
32
The chiral bisoxazoline copper(II)
complexes have also been reported to induce the asymmetric α-
chlorination of β-keto esters when reacted with NCS.
33
The asym-
metric α-chlorination of aldehydes has been achieved using NCS
and (2R,5R)-diphenylpyrrolidine as a chiral catalyst. For example,
the enantioselective chlorination of 3-methylbutanal with NCS
proceeds in 95% yield and 94% ee (eq 17).
34
O
N
H
Ph
Ph
O
Cl
NCS, DCE, rt, 30 min
95% yield, 94% ee
(17)
The enantioselective α-chlorination reaction was also reported
to proceed for β-keto phosphonates using NCS and bisoxazoline
zinc(II) complexes in 70–91% ee.
35
Phenylselenyl chloride has
been shown to enhance the electrophilicity of NCS in chlorination
reactions. Allylic chlorination of olefins with NCS catalyzed by
PhSeCl was reported to occur with ene regiochemistry in high
yields at room temperature. For example, methyl oct-3-enoate was
smoothly converted to methyl 4-chloro-oct-2-enoate in excellent
yield with no α-chlorination to the carbonyl detected (eq 18).
36
Bu
CO
2
Me
Bu
CO
2
Me
Cl
PhSeCl, NCS, DCM, rt, 4 h
89%
(18)
Interestingly, NCS catalyzed by phenylselenyl chloride selec-
tively α-chlorinates β-keto esters in the presence of olefins with
no allylic chlorination observed (eq 19).
37
O
Ph
O
O
O
Ph
O
O
Cl
PhSeCl, NCS, MeOH, rt, 16 h
87%
(19)
Chlorination of Sulfides. The treatment of 1,3-oxathioacetals
or dithioacetals with NCS in the presence of MeOH, EtOH, 1,2-
ethanediol, or 1,3-propanediol results in the clean conversion to
the corresponding acetal or cyclic acetal. The protecting group
conversion occurs quickly and in excellent yield. For example,
the reaction of 2-phenyl-1,3-dithiolane with 1 equiv of NCS and 3
equiv of 1,2-ethanediol in dichloromethane proceeds readily to
afford 2-phenyl-1,3-dioxolane in almost quantitative yield
(eq 20).
38
S
S
O
O
NCS, 1,2-ethanediol, DCM, rt, 5 min
95%
(20)
In a similar reaction, 1,3-oxathioacetals or dithioacetals can
be deprotected with 10 mol % NCS in chloroform with 5 equiv
of DMSO to yield the corresponding carbonyl compound in
excellent yield. The reaction is chemoselective and works in the
presence of O,O-acetals.
39
Conversion of Alcohols and Thiols to Chlorides. Primary
and secondary alcohols are converted to the corresponding alkyl
chlorides with the inversion of configuration when reacted with
NCS and triphenylphosphine under Mitsunobu-type conditions.
40
The NCS and triphenylphosphine combination also transforms
certain hydroxyheterocycles to the corresponding chloroheterocy-
cle. The structural requirement for this transformation is that the
hydroxyl needs to be ortho to a nitrogen atom in the heterocycle.
For example, quinoxalin-2-ol is converted to 2-chloroquinoxaline
in good yield when treated with NCS and triphenylphosphine in
refluxing dioxane (eq 21).
41
N
N
OH
N
N
Cl
NCS, PPh
3
, dioxane, reflux
63%
(21)
Benzylic, primary, and secondary thiols are readily converted
to the corresponding alkyl chlorides when treated with NCS and
triphenylphosphine in dichloromethane. The reaction for benzylic
thiols is immediate and occurs within 24 h for secondary thiols.
Avoid Skin Contact with All Reagents
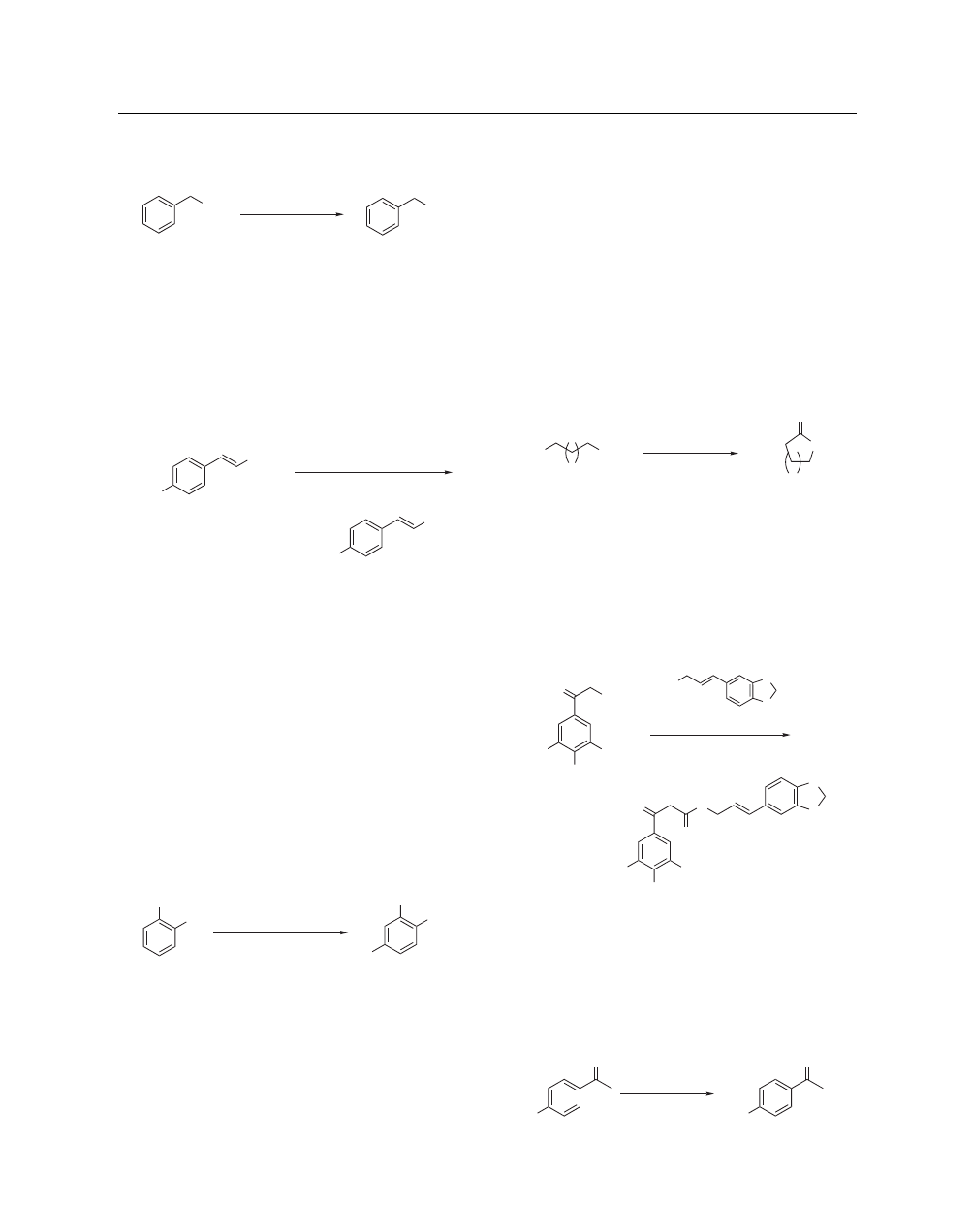
4
N-CHLOROSUCCINIMIDE
For example, α-toluenethiol is immediately converted to benzyl
chloride in 90% yield when treated with NCS and triphenylphos-
phine at room temperature (eq 22).
42
SH
Cl
NCS, PPh
3
, DCM, rt
90%
(22)
Hunsdiecker Reactions.
The Hunsdiecker reaction is the
decarboxylative halogenation of metal carboxylate salts. The
reaction of α,β-unsaturated carboxylic acids with NCS and
catalytic lithium acetate in acetonitrile–water provides the corres-
ponding β-halostyrenes in moderate yields under mild conditions.
The reaction proceeds with a good degree of stereospecificity. For
example, the reaction of 3-(4-methoxy-phenyl)-acrylic acid with
NCS with a catalytic amount of lithium acetate at room tempera-
ture provides 1-(2-chloro-vinyl)-4-methoxybenzene in good yield
(eq 23).
43
CO
2
H
MeO
Cl
MeO
NCS, LiOAc, CH
3
CN, H
2
O, rt, 6 h
65%
(23)
A modification of the Hunsdiecker reaction uses NCS
catalyzed with tetrabutylammonium trifluoroacetate (TBATFA)
and gives β-chlorostyrenes in excellent yields.
44
The use of the
NCS/TBATFA-catalyzed Hunsdiecker reaction has been extended
to various heterocyclic α,β-unsaturated carboxylic acids.
45
Aromatic Chlorination. Many aromatic and heteroaromatic
chlorinations using NCS are catalyzed by acetic acid.
46,47
Ferric
chloride and ammonium nitrite have also been used to catalyze
the chlorination of various heterocycles with NCS.
48
Although
NCS has been used for halogenation of electron-rich aromatics,
the halogenation of electron-poor aromatic systems with NCS has
been difficult to achieve. However, the chlorination of various de-
activated aromatic systems can be achieved when NCS is acid
catalyzed with boron trifluoride monohydrate. The reaction is
impressive in that even the deactivated 1-fluoro-2-nitrobenzene
is chlorinated to afford 4-chloro-1-fluoro-2-nitrobenzene in
81% yield after 18 h at 100
◦
C (eq 24).
49
NO
2
F
NO
2
F
Cl
NCS, BF
3
_
H
2
O, 100
°C, 18 h
81%
(24)
Oxidation of Alcohols. The oxidation of primary, benzylic,
and allylic alcohols to aldehydes can be selectively achieved when
the alcohol is treated with NCS catalyzed by TEMPO. Reaction
conditions are mild and do not chlorinate olefins or allylic po-
sitions. The reaction is run under typical phase-transfer condi-
tions using a dichloromethane–water mixture and TBACl as the
phase-transfer agent. The aqueous layer for the biphasic reaction is
buffered at pH 8.6 with NaHCO
3
–K
2
CO
3
. Primary alcohols were
selectively oxidized to aldehydes in the presence of secondary
alcohols and only 0–5% of the ketone resulting from oxidation
of the secondary alcohol was observed.
50
Alternatively the oxi-
dation of alcohols with NCS to the corresponding carbonyl com-
pounds can be catalyzed with N-tert-butylbenzenesulfenamide.
This reaction presumably proceeds via an initial oxidation of the
sulfur atom of the catalyst. The N-tert-butylbenzenesulfenamide-
catalyzed oxidation is selective for primary alcohols over sec-
ondary alcohols, works on a variety of substrates, and has the
advantage that it can be performed without using phase-transfer
conditions.
51
An interesting variant to the oxidation of alcohols
to carbonyl compounds with NCS is the oxidation of diols to
lactones. The reaction of 1,4-butanediol and 1,5-pentanediol with
NCS in dichloromethane at room temperature provided the corres-
ponding five- and six-membered-ring lactones in excellent yield
(eq 25).
52
HO
OH
n
n
= 2 or 3
O
O
n
n
= 1 or 2
NCS, DCM, rt, 5 h
86–88%
(25)
Miscellaneous Uses. NCS catalyzes the transesterification of
β
-keto ethyl esters with substrate alcohols under neutral condi-
tions in refluxing toluene in excellent yields. The ethanol formed
during the reaction is removed by distillation. Surprisingly, the re-
action conditions are selective and the chlorination of allylic posi-
tions or olefins is not observed. Additionally, the reaction proceeds
with only 1 equiv of the substrate alcohol allowing for complex
esters to be readily formed (eq 26).
53
MeO
OMe
OMe
O
CO
2
Et
O
O
HO
MeO
OMe
OMe
O
O
O
O
O
(26)
NCS, toluene, reflux, 7 h
81%
Oximes are converted to the corresponding carbonyl compound
when treated with NCS in CCl
4
at room temperature in excellent
yields. The workup of these deoximation reactions is especially
simple with the removal of insoluble succinimide and concentra-
tion of the solvent to afford the product carbonyl compound in high
purity. For example, 4-methoxyacetophenone oxime was readily
converted to the corresponding ketone in 4 h at room temperature
(eq 27).
54
MeO
NOH
MeO
O
NCS, CCl
4
, rt, 4 h
(27)
93%
A list of General Abbreviations appears on the front Endpapers
N-CHLOROSUCCINIMIDE
5
Alkenyl boronic acids are converted to the corresponding alkyl
chlorides when treated with NCS and TEA in good to excellent
yields. The reaction proceeds with retention of configuration at
room temperature in 30 min. For example, (E)-β-styryl boronic
acid is readily converted to (E)-β-chlorostyrene in 85% yield after
30 min at room temperature (eq 28).
55
B(OH)
2
Cl
NCS, TEA, rt, 30 min
82%
(28)
The conversion of a primary amine to the corresponding alkyl
chloride can be achieved through NCS chemistry. N-Substituted-
N
-tosylhydrazines are readily available from the reaction of
primary amines with tosyl chloride followed by subsequent
amination O-(2,4-dinitrophenyl)hydroxylamine. Treatment of N-
substituted-N-tosylhydrazines with NCS at room temperature
affords the corresponding alkyl chlorides in good yields. Solvent
choice for the reaction is critical with THF giving optimum results.
It is presumed that the chlorodeamination reaction proceeds via a
radical mechanism with the loss of nitrogen. The overall reaction
sequence for conversion of a primary amine to the corresponding
primary chloride is shown in (eq 29).
56
R
NH
2
H
2
NO
O
2
N
NO
2
R
N
Tosyl
NH
2
R
Cl
1. TosylCl
2.
NCS, THF, rt
(29)
A new synthesis of 5-chloro-1-phenyltetrazole, a useful
activating group for the hydrogenolysis of phenols, was reported
using NCS-mediated chemistry. The phase-transfer reaction of
NCS with sodium azide in chloroform generates chloroazide in
situ. The transient chloroazide reacts with phenyl isocyanide via a
1,3-dipolar cycloaddition at 0
◦
C to afford 5-chloro-1-phenyltetra-
zole in 69% yield (eq 30).
57
N
C
NCS, CHCl
3
, H
2
O, NaN
3
69%
N
N
N
N
Cl
(30)
An interesting rearrangement of cyclic dithiane alcohols to the
corresponding one-carbon ring expanded 1,2-diketones is cat-
alyzed by NCS. The reaction appears to be quite general and pro-
vides 1,2-diketones in high yields in a two-step sequence from
cyclic ketones. The two-step reaction sequence from a cyclic
ketone to a 1,2-diketone is high yielding and uses readily available
reagents (eq 31).
58
O
O
O
HO
S
S
O
O
O
n
-BuLi, 1,3-dithiane
NCS, DCM, H
2
O
74%
89%
(31)
Chlorination of dialkylphosphites with NCS affords the corres-
ponding dialkylchlorophosphate. The dialkylchlorophosphates
generated react with alcohols to give phosphonate esters. The
direct chlorination of dibenzylphosphite with NCS was used in
the synthesis of phosphate prodrugs of the anti-HIV drug 3
′
-azido-
2
′
,3
′
-dideoxythymidine (AZT) (eq 32).
59
NH
O
O
N
O
H
N
3
H
H
H
H
O
P
BnO
OBn
O
P
H
BnO
O
BnO
P
Cl
BnO
O
BnO
NCS, toluene, rt, 18h
AZT, pyridine, rt, 19 h
(32)
1.
(a) Hambly, G. F.; Chan, T. H., Tetrahedron Lett. 1986, 27, 2563. (b)
Hooz, J.; Bridson, J. N., Can. J. Chem. 1972, 50, 2387. (c) Ohkata, K.;
Mase, M.; Akiba, K., J. Chem. Soc., Chem. Commun. 1987, 1727.
2.
Vaz, A. D. N.; Schoellmann, G., J. Org. Chem. 1984, 49, 1286.
3.
Oppolzer, W.; Dudfield, P., Tetrahedron Lett. 1985, 26, 5037.
4.
Mignani, G.; Morel, D.; Grass, F., Tetrahedron Lett. 1987, 28, 5505.
5.
Harpp, D. N.; Bao, L. Q.; Black, C. J.; Gleason, J. G.; Smith, R. A., J.
Org. Chem. 1975
, 40, 3420.
6.
Dilworth, B. M.; McKervey, M. A., Tetrahedron 1986, 42, 3731.
7.
Tuleen, D. L.; Stephens, T. B., J. Org. Chem. 1969, 34, 31.
8.
Paquette, L. A.; Klobucar, W. D.; Snow, R. A., Synth. Commun. 1976,
6
, 575.
9.
Paquette, L. A., Org. React. 1977, 25, 1.
10.
Ishibashi, H.; Nakatani, H.; Maruyama, K.; Minami, K.; Ikeda, M., J.
Chem. Soc., Chem. Commun. 1987
, 1443.
11.
(a) Trost, B. M.; Tamaru, Y., J. Am. Chem. Soc. 1977, 99, 3101. (b) Trost,
B. M.; Crimmin, M. J.; Butler, D., J. Org. Chem. 1978, 43, 4549.
12.
Ishibashi, H.; Uemura, N.; Nakatani, H.; Okazaki, M.; Sato, T.;
Nakamura, N.; Ikeda, M., J. Org. Chem. 1993, 58, 2360.
13.
Corey, E. J.; Erickson, B. W., J. Org. Chem. 1971, 36, 3553.
14.
Harville, R.; Reed, S. F., Jr., J. Org. Chem. 1968, 33, 3976.
15.
(a) Fitzner, J. N.; Shea, R. G.; Fankhauser, J. E.; Hopkins, P. B., J. Org.
Chem. 1985
, 50, 418. (b) Spaltenstein, A.; Carpino, P. A.; Hopkins,
P. B., Tetrahedron Lett. 1986, 27, 147.
16.
(a) Tsuchihashi, G.; Ogura, K., Bull. Chem. Soc. Jpn. 1971, 44, 1726.
(b) Ogura, K.; Imaizumi, J.; Iida, H.; Tsuchihashi, G., Chem. Lett. 1980,
1587.
17.
Calzavara, P.; Cinquini, M.; Colonna, S.; Fornasier, R.; Montanari, F., J.
Am. Chem. Soc. 1973
, 95, 7431.
18.
(a) Satoh, T.; Oohara, T.; Ueda, Y.; Yamakawa, K., Tetrahedron Lett.
1988, 29, 313. (b) Drabowicz, J., Synthesis 1986, 831.
19.
Levy, A. B.; Talley, P.; Dunford, J. A., Tetrahedron Lett. 1977, 3545.
20.
Zweifel, G.; Lewis, W., J. Org. Chem. 1978, 43, 2739.
21.
(a) Murray, R. E., Synth. Commun. 1980, 10, 345. (b) Verboom, W.;
Westmijze, H.; De Noten, L. J.; Vermeer, P.; Bos, H. J. T., Synthesis
1979, 296.
22.
(a) Gilow, H. M.; Burton, D. E., J. Org. Chem. 1981, 46, 2221. (b) Powers,
J. C., J. Org. Chem. 1966, 31, 2627.
23.
De Rosa, M.; Nieto, G. C., Tetrahedron Lett. 1988, 29, 2405.
24.
Goldberg, Y.; Alper, H., J. Org. Chem. 1993, 58, 3072.
25.
(a) Kametani, T.; Suzuki, Y.; Ban, C.; Honda, T., Heterocycles 1987, 26,
1491. (b) Honda, T.; Yamamoto, A.; Cui, Y.; Tsubuki, M., J. Chem. Soc.,
Perkin Trans. 1 1992
, 531.
Avoid Skin Contact with All Reagents

6
N-CHLOROSUCCINIMIDE
26.
(a) Wolff, M. E., Chem. Rev. 1963, 63, 55. (b) Stella, L., Angew. Chem.,
Int. Ed. Engl. 1983
, 22, 337.
27.
Filler, R., Chem. Rev. 1963, 63, 21.
28.
(a) Amrollah-Madjdabadi, A.; Beugelmans, R.; Lechevallier, A.,
Synthesis 1986
, 828. (b) Amrollah-Madjdabadi, A.; Beugelmans, R.;
Lechevallier, A., Synthesis 1986, 826. (c) Corey, E. J.; Estreicher, H.,
Tetrahedron Lett. 1980
, 21, 1117.
29.
Becker, K. B.; Geisel, M.; Grob, C. A.; Kuhnen, F., Synthesis 1973, 493.
30.
Durst, H. D.; Mack, M. P.; Wudl, F., J. Org. Chem. 1975, 40, 268.
31.
Meshram, H. M.; Reddy, P. N.; Sadashiv, K.; Yadav, J. S., Tetrahedron
Lett. 2005
, 46, 623.
32.
Hintermann, L.; Togni, A., Helv. Chim. Acta 2000, 83, 2425.
33.
Marigo, M.; Kumaragurubaran, N.; Jørgensen, K. A., Chem. Eur. J. 2004,
10
, 2133.
34.
Halland, N.; Braunton, A.; Bachmann, S.; Marigo, M.; Jørgensen, K. A.,
J. Am. Chem. Soc. 2004
, 126, 4790.
35.
Bernardi, L.; Jørgensen, K. A., Chem. Commun. 2005, 1324.
36.
Tunge, J. A.; Mellegaard, S. R., Org. Lett. 2004, 6, 1205.
37.
Wang, C.; Tunge, J., Chem. Commun. 2004, 2694.
38.
Karimi, B.; Seradj, H.; Maleki, J., Tetrahedron 2002, 58, 4513.
39.
Iranpoor, N.; Firouzabadi, H.; Shaterian, H. R., Tetrahedron Lett. 2003,
44
, 4769.
40.
Mihovilovic, M. D.; Rudroff, F.; Grötzl, B.; Stanetty, P., Eur. J. Org.
Chem. 2005
, 5, 809.
41.
Sugimoto, O.; Mori, M.; Tanji, K., Tetrahedron Lett. 1999, 40, 7477.
42.
Iranpoor, N.; Firouzabadi, H.; Aghapour, G., Synlett 2001, 1176.
43.
Chowdhury, S.; Roy, S., J. Org. Chem. 1997, 62, 199.
44.
Naskar, D.; Chowdhury, S.; Roy, S., Tetrahedron Lett. 1998, 39, 699.
45.
Naskar, D.; Roy, S., Tetrahedron 2000, 56, 1369.
46.
Day, R. A.; Blake, J. A.; Stephens, C. E., Synthesis 2003, 1586.
47.
Menichincheri, M.; Ballinari, D.; Bargiotti, A.; Bonomini, L.; Ceccarelli,
W.; D’Alessio, R.; Fretta, A.; Moll, J.; Polucci, P.; Soncini, C.; Tibolla,
M.; Trosset, J.-Y.; Vanotti, E., J. Med. Chem. 2004, 47, 6466.
48.
Tanemura, K.; Suzuki, T.; Nishida, Y.; Satsumabayashi, K.; Horaguchi,
T., Chem. Lett. 2003, 32, 932.
49.
Prakash, G. K. S.; Mathew, T.; Hoole, D.; Esteves, P. M.; Wang, Q.;
Rasul, G.; Olah, G. A., J. Am. Chem. Soc. 2004, 126, 15770.
50.
Einhorn, J.; Einhorn, C.; Ratajczak, F.; Pierre, J.-L., J. Org. Chem. 1996,
61
, 7452.
51.
Matsuo, J.; Iida, D.; Yamanaka, H.; Mukaiyama, T., Tetrahedron 2003,
59
, 6739.
52.
Kondo, S.; Kawasoe, S.; Kunisada, H.; Yuli, Y., Synth. Commun. 1995,
25
, 719.
53.
Bandgar, B. P.; Uppalla, L. S.; Sadavarte, V. S., Synlett 2001, 1715.
54.
Bandgar, B. P.; Kunde, L. B.; Thote, J. L., Synth. Commun. 1997, 27,
1149.
55.
Petasis, N. A.; Zavialov, I. A., Tetrahedron Lett. 1996, 37, 567.
56.
Collazo, L. R.; Guziec, F. S.; Hu, W.; Pankayatselvan, R., Tetrahedron
Lett. 1994
, 35, 7911.
57.
Collibee, W. L.; Nakajima, M.; Anselme, J.-P., J. Org. Chem. 1995, 60,
468.
58.
Ranu, B. C.; Jana, U., J. Org. Chem. 1999, 64, 6380.
59.
Cardona, V. M. F.; Ayi, A. I.; Aubertin, A.-M.; Guedj, R., Antiviral Res.
1999, 42, 189.
A list of General Abbreviations appears on the front Endpapers
Wyszukiwarka
Podobne podstrony:
chloroform eros rc105
chloromethane eros rc113
chloroform eros rc105
Fluorescencja chlorofilu
Chloroplast
W4 Mitochondria i chloroplasty
benzyl chloride eros rb050
hydrobromic acid eros rh031
magnesium eros rm001
oxalyl chloride eros ro015
potassium permanganate eros rp244
peracetic acid eros rp034
p toluenesulfonic acid eros rt134
więcej podobnych podstron