RANEY NICKEL
1
Raney Nickel
1
Ni
[106-51-4]
Ni
(MW 58.69)
InChI = 1/Ni
InChIKey = PXHVJJICTQNCMI-UHFFFAOYAH
(useful as a reducing agent for hydrogenation of organic
compounds
1
)
Solubility:
insol all organic solvents and water.
Form Supplied in:
black solid.
Preparative Methods:
there are many types of Raney nickel; they
differ based on their methods of preparation. These methods
essentially determine the hydrogen content as well as the re-
activities of various types of Raney nickel. The most popular
W-type Raney nickels can be prepared as the seven different
types listed in Table 1.
Table 1
Types of Raney nickel
NaOH
NaOH/Al
soln. conc.
Temp. (
◦
C)
Type
(molar)
(wt%)
(time, h)
Water wash method
W-1
1.35
17
115–120 (4)
Decant to neutral
W-2
1.71
20
75–80 (8–12)
Decant to neutral
W-3
1.73
20
50 (0.83)
Continuous to
neutral
W-4
1.73
20
50 (0.83)
Continuous to
neutral
W-5
1.80
21
50 (0.83)
Continuous to
neutral
W-6
1.80
21
50 (0.83)
Cont. to neut.
under H
2
W-7
1.80
21
50 (0.83)
Directly washed
with EtOH
1c
Handling, Storage, and Precautions:
Raney nickel is gener-
ally stored in an alcoholic solvent, or occasionally in water,
ether, methylcyclohexane, or dioxane. The activity of Raney
nickel decreases due to loss of hydrogen over a period of about
6 months. Raney nickel ignites on contact with air and should
never be allowed to dry.
Original Commentary
Teng-Kuei Yang & Dong-Sheng Lee
National Chung-Hsing University, Taichung, Taiwan
Desulfurization. The most widespread application of Raney
nickel is the desulfurization of a wide range of compounds
including thioacetals, thiols, sulfides, disulfides, sulfoxides, sul-
fones, thiones, thiol esters, and sulfur-containing heterocycles.
The well-known desulfurization of dithianes remains one of the
most efficient methods for reductive deoxygenation of ketones.
Recently, Rubiralta demonstrated an example in his synthesis of
the aspidosperma alkaloid framework. Dithiane (1) was reduced to
indole derivative (2) in 85% yield without reduction of the alkene
(eq 1).
2
S
S
N
Me
N
H
N
Me
N
H
(2)
(1)
W-2 Raney Ni
(1)
EtOH, reflux
85%
Raney nickel is frequently used to remove the sulfur atom of
thiols,
3
sulfides, and disulfides from a carbon skeleton, regard-
less of whether the sulfur is attached to an alkyl carbon,
4
an aryl
carbon,
5
or a carbon atom in a heterocycle.
6
Several examples are
shown in eqs 2–5. A solvent effect was observed in the Raney
nickel reduction of vinyl sulfide (11), which gave glycoside (12)
in methanol, whereas the double bond remained intact to produce
alkene (13) in THF (eq 6).
7
OH
OH
(2)
SH
Raney Ni
(3)
(4)
EtOH
90%
Raney Ni
(3)
S
OH
O
OH
O
(5)
(6)
MeOH, reflux
51%
Raney Ni
(7)
(8)
(4)
SR
SR
acetone
60%
(10)
Raney Ni
(9)
N
N
SR
R
1
OH
R
2
N
N
R
1
OH
R
2
Me
Me
(5)
EtOH
(11) Pht = phthaloyl
(13)
O
NPht
OMe
PhS
O
NPht
OMe
O
NPht
OMe
Raney Ni
(6)
Raney Ni
MeOH
87%
(12)
THF
85%
The sulfur atom can be part of a heterocycle. Högberg
8
and
Tashiro
9
used Raney nickel to remove sulfur from thiophene
derivatives (14) and (16) to give compounds (15) and (17),
respectively (eqs 7 and 8).
(7)
H
2
, Raney Ni
OH
S
OH
(14)
(15)
MeOH
85%
Avoid Skin Contact with All Reagents
2
RANEY NICKEL
S
OMe
t
-Bu
OMe
t
-Bu
Br
Br
(8)
H
2
, W-7 Raney Ni
(16)
(17)
EtOH
84%
Raney nickel can remove the sulfinyl and sulfonyl groups
from sulfoxides and sulfones under neutral conditions (eqs 9 and
10).
10,11
Cox demonstrated the cleavage of both sulfur–carbon
bonds in sulfoxide (22) and noted that the stereogenic center
remained untouched (eq 11).
12
O
CO
2
Me
RO
RO
OH
S
Ph
(9)
O
RO
RO
OH
CO
2
Me
O
Raney Ni
(18)
(19)
THF
83%
(10)
N
SO
2
Ph
R
SPh
SO
2
Ph
N
H
R
1. Raney Ni
EtOH, reflux
71–91%
(20) R = alkyl, aryl
(21) 3 examples
2. NaOH, H
2
O
reflux
S
OTBDPS
OTBDPS
(23)
Ph
Ph
O
(11)
Raney Ni
(22)
EtOH, reflux
83%
Some thioamides are reduced by Raney nickel to the corre-
sponding imines. Two typical examples are shown in eqs 12 and
13.
13,14
Thiones have been reported to give alkanes, but only low
or unstated yields are reported (eqs 14 and 15).
15,16
(25)
(12)
N
H
N
Ph
Ph
S
Me
O
N
Ph
Ph
O
Raney Ni
(24)
EtOH, reflux
71%
(13)
N
NH
S
NH
2
N
N
Sugar
N
N
NH
2
N
N
Sugar
H
2
O, 50 °C
73%
(26)
(27)
Raney Ni
Raney Ni
(28)
(29)
(14)
N
H
S
N
H
25%
Raney Ni
(15)
S
(31)
(30)
Liu and Luo
17
used Raney nickel to reduce glycidic thioester
(32) to the corresponding 1,3-diol (33) in good yield (eq 16).
With Sodium Borohydride at ambient temperature or Lithium
Aluminum Hydride at −78
◦
C, glycidic thioester (32) was re-
duced chemoselectively to furnish 2,3-epoxy alcohol (34) in 82%
yield (eq 16).
17
O
S
O
t
-Bu
OH
HO
O
OH
(16)
(33)
(34)
W-2 Raney Ni
95% EtOH, rt
87%
NaBH
4
, rt
82%
(32)
Deoxygenation and Deamination. Besides the widely used
desulfurization process, Raney nickel can be used to reduce ben-
zylic nitrogen and oxygen atoms. Behren’s report shows the partial
deoxygenation of diol (35) to mono alcohol (36) in 75–96% yields
(eq 17).
18
Ikeda used W-2 Raney nickel to remove the benzyl pro-
tecting group from compound (37) However, partial epimerization
occurred in this reaction to produce a 3.7:1 mixture of the 6α- and
6β-alcohols (38) (eq 18).
19
Azetidine (39) was opened by Raney
nickel in refluxing ethanol, to give acyclic amine (40) in 88% yield
(eq 19).
20
H
2
, Raney Ni
2.5N NaOH, EtOH
R
OH
HO
R
HO
(17)
(35)
(36)
R = H, CN, NH
2
, CH
2
NH
2
20 °C
75–96%
N
O
Me
H
BnO
OMe
MeO
N
O
Me
H
HO
OMe
MeO
(18)
W-2 Raney Ni
(37)
(38)
α:β = 3.7:1
EtOH, reflux
A list of General Abbreviations appears on the front Endpapers
RANEY NICKEL
3
Raney Ni
(39)
(40)
N
HN
Ac
Ph
R
HN
HN
Ac
Ph
R
(19)
EtOH, reflux
88%
Krafft reported that tertiary alcohols were also deoxygenated
to alkanes by Raney nickel (eq 20). On the other hand, primary
alcohols were oxidized to aldehydes and then subsequently
decarbonylated (eq 21), and secondary alcohols were oxidized
to the corresponding ketones (eq 22).
21
Raney Ni
OH
(20)
(41)
(42)
toluene, reflux
90%
Raney Ni
MeO
OH
O
MeO
O
( )
9
( )
9
(21)
(43)
(44)
toluene, reflux
73%
Raney Ni
OH
O
(22)
(45)
(46)
benzene, reflux
95%
Recently, Ohta reported Raney nickel would deoxygenate
N
-oxide (47) to pyrazine (48), while Phosphorus(III) Bromide
gave many side products (eq 23).
22
Raney Ni
N
N
OH
O
–
N
N
OH
(23)
(47)
(48)
+
EtOH
62%
Cleavage of Heteroatom–Heteroatom Bonds.
Both N–N
and N–O bonds can be cleaved by Raney nickel in the presence
of hydrogen. Alexakis reported that hydrazine (49) was easily
cleaved to the free amine by Raney nickel under hydrogen
atmosphere, then protected to give carbamate (52) (eq 24).
23
In
addition, he found even hindered hydrazines (50) and (51) were
successfully deaminated to free amines (56) and (57),
respectively, without racemization if the reactions were assisted
by ultrasound.
24
The N–O bonds in 1,2-oxazine (58) and isooxazolidine (60)
were cleaved by Raney nickel via a radical mechanism to pro-
duce 1,4-diketone (59) and β-lactam (61), respectively (eqs 25
and 26).
25,26
N
N
Ph
Ph
Me
Me
R
HN N
Me
Me
N
N
Ph
Ph
Me
Me
R
HN Boc
N
N
Ph
Ph
Me
Me
R
NH
2
73%
0%
0%
(52) R = Me
(53) R = t-Bu
(54) R = Ph
(55) R = Me
(56) R = t-Bu
(57) R = Ph
72%
66%
70%
(49) R = Me
(50) R = t-Bu
(51) R = Ph
1. H
2
, 40 atm
Raney Ni
40 °C, MeOH
2. (Boc)
2
O
1. H
2
, 1 atm
Raney Ni
ultrasound
20 °C, MeOH
2. (Boc)
2
O
(24)
H
2
, Raney Ni
(58)
(59)
(25)
CO
2
Et
O
N
O
TMSO
CO
2
Et
O
O
O
2N HCl, EtOH, rt
68%
N
O
F
F
PhS
PhS
Ph
Me
N
PhS
PhS
O
Me
Ph
Raney Ni
(26)
(60)
(61)
acetone
50%
Hydrogenation of Multiple Bonds. Applications in this area
are not very popular for Raney nickel. Raney nickel in dilute base
is, however, an effective reagent for reduction of pyridines to the
corresponding piperidines. The reaction is accelerated by sub-
stituents in the 2-position and by electron-withdrawing groups in
the 3- and 4-positions, while electron-donating groups in the 3-
and 4-positions retard the reaction (eq 27).
27
Occasionally, Raney
nickel is used to reduce acyclic multiple bonds. An example for
selective reduction of triple bonds to cis double bonds is shown in
eq 28.
28
N
(27)
(62)
N
H
1. Raney Ni
KOH, H
2
O
(63)
•HCl
77%
18 other examples
2. HCl, H
2
O
PPh
3
Cl
–
Raney Ni
PPh
3
Cl
–
+
+
(28)
(64)
(65)
MeOH
80%
Avoid Skin Contact with All Reagents
4
RANEY NICKEL
Deselenation. Similar to the desulfurization process, Raney
nickel can be used to remove selenium from selenoketones, dise-
lenides, selenides, and selenooxides. Typical examples are shown
in eqs 29–33.
29−31
Moreover, Raney nickel has been used for
a hydrodetelluration of chiral compound (76) without any race-
mization (eq 34).
32
(29)
Raney Ni
Se
O
(66)
(67)
PBr
3
15%
(30)
Se
Se
Raney Ni
benzene, EtOH
2
(68)
(69)
reflux
76%
(31)
Se
Raney Ni
benzene, EtOH
(70)
(71)
reflux
87%
(73)
Se
Raney Ni
H
2
(50 psi)
NO
2
(72)
NH
2
(32)
benzene, reflux
63%
(33)
Se
Raney Ni
benzene, EtOH
(74)
O
(75)
reflux
72%
PhCO
2
Cl
Cl
3
Te
PhCO
2
Cl
(34)
Raney Ni
(76)
(77)
H
2
(200 psi)
58%
Reductive Amination of Carbonyl Groups. Reactions of this
type can be accomplished by reduction of intermediate imines or
oximes.
33−35
Recently, Chan and co-workers found that Raney
nickel can be an efficient catalyst in the preparation of pheny-
lalanine. Treatment of sodium phenylpyruvate with either Am-
monia gas or aqueous ammonia solution in the presence of Raney
nickel under 200 psi pressure gave >98% of phenylalanine (eq 36).
Other α-keto esters such as 4-hydroxyphenylpyruvic acid, pyruvic
acid, and benzoyl acid also gave the corresponding amino acids in
excellent yield.
36
(35)
O
NH
2
Raney Ni, H
2
(100 atm), 70 °C
NH
3
, EtOH
61%
Raney Ni, H
2
, 80 °C
NH
2
OH•HCl, MeOH
80%
(78)
(79)
ONa
O
(80)
Ph
Raney Ni, H
2
(200 psi)
NH
2
ONa
O
(81)
Ph
(36)
O
NH
3
(or NH
4
OH), MeOH
>98%
Asymmetric Reduction. Recently, asymmetric synthesis has
become a center of attention for synthetic chemists. The use of
Raney nickel and tartaric acid was recently reported by Bartok.
Reduction of ketone (82) gave alcohols (83) and (84) as a 92:8
mixture in 70% chemical yield.
37
Takeshita et al.
9
also reported
an asymmetric reduction of β-keto ester (85) to give the corre-
sponding β-hydroxy ester (86) in 80% ee (eq 38). In addition, it
was found that enantioselectivities were improved by treatment
of the Raney nickel with ultrasound prior to use.
38
Blacklock
et al. reported an asymmetric reductive amination of α-keto ester
(88) in which they used the chiral amine (87) instead of a chiral
catalyst. The result, shown in eq 39, indicates that the amino ester
(89) was produced in 80% yield with 74% de.
39
EtO
2
C
O
EtO
2
C
OH
EtO
2
C
OH
(37)
Raney Ni, NaBr
+
(82)
(83)
(84)
92:8
(R,R)-C
4
H
6
O
6
70%
OMe
O
O
OMe
O
OH
(38)
W-1 Raney Ni, NaBr
(85)
(86) 86% ee
(R,R)
-C
4
H
6
O
6
87%
N
CO
2
–
O
H
3
N
CO
2
Et
O
N
CO
2
–
O
NH
CO
2
Et
N
CO
2
–
O
NH
+
(39)
CO
2
Et
Raney Ni
+
+
(87)
(88)
(89)
(90)
87:13
EtOH
70%
A list of General Abbreviations appears on the front Endpapers
RANEY NICKEL
5
First Update
Julia Haas
Array BioPharma, Boulder, CO, USA
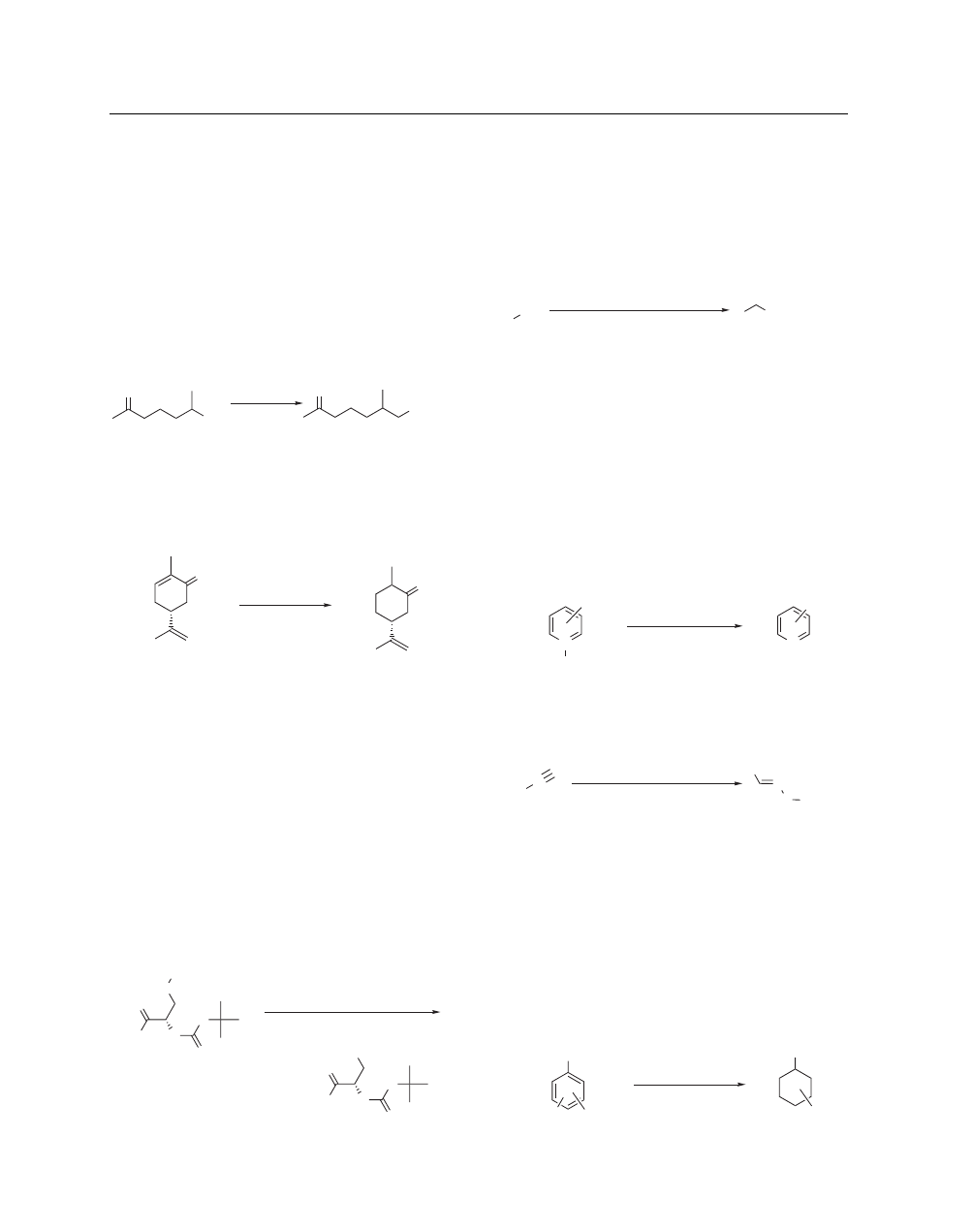
Selective Reduction of Functional Groups. New or impro-
ved conditions for the selective reduction of various functional
groups in the presence of others have recently been developed. To
discuss them in detail would be beyond the scope of this publi-
cation, but representative examples will be discussed. Raney Ni
in THF at room temperature can reduce aldehydes in the pres-
ence of ketones with high yields and selectivity.
40
An exam-
ple is shown in eq 40. While primary, aromatic, and secondary
aldehydes react under these conditions, tertiary aldehydes do not
react.
Raney Ni, THF
CHO
O
O
OH
(40)
rt, 92%
Raney Ni in THF, at room temperature or at 0
◦
C, selectively
reduces conjugated double bonds in the presence of isolated
olefins.
41
For example, the conjugated double bond in carvone
was selectively reduced under these conditions (eq 41). Under the
same conditions, acrylonitrile could be converted to propionitrile
without significant reduction of the cyano group (93% yield).
Raney Ni, THF
O
O
(41)
rt, 90%
The selective reduction of aromatic nitro groups in the pres-
ence of halides is possible using Raney Ni poisoned with thiourea
as the hydrogenation catalyst.
42
These conditions were used to
convert halogenonitrobenzenes to the corresponding halogenoani-
lines with high selectivity (>99%). The selective hydrogenation
of aromatic nitro groups in the presence of halides has also been
reported in ionic liquids.
43
Benzyl ethers can be cleaved efficiently under biphasic condi-
tions using Raney Ni in the presence of the phase transfer cat-
alyst Aliquat 336.
44
An example of this reaction is shown in
eq 42. While under traditional conditions, for example in alcohol
solvents, high hydrogen pressures are often needed for Raney-
Ni-promoted debenzylations, multiphase conditions allow for fast
reactions at atmospheric pressure. The hydrogenation of nitriles to
the corresponding amines in the presence of Boc-protected amines
has been reported using a mixture of Raney Ni and Pd/C.
45
Both
catalysts were needed in the reaction mixture for clean conversion.
Raney Ni, A 336, KOH (aq), H
2
(1 atm)
HN
O
O
HO
O
O
Bn
HN
HO
O
HO
O
O
(42)
isooctane, 50
°C, 100%
Catalytic Hydrogen Transfer Reactions. Mebane et al. have
reported the transfer hydrogenation of nitriles to amines using
refluxing isopropanol as the stochiometric reducing agent.
46
After hydrolysis of the intermediate dimethyl imines, the corres-
ponding amines could be obtained in excellent yields (eq 43).
Although excess Raney Ni was required, the catalyst could be
recycled several times. The authors have also used the system
Raney Ni/isopropanol for the reduction of alcohols
47
and for the
deiodination of iodolactones.
48
1. Raney Ni, KOH, i-PrOH, reflux
R
NH
2
⋅HCl
R
CN
(43)
2. HCl (aq), then NaOH, then HCl (ether)
88–97% overall
Phase transfer hydrogenations using hydrazinium monofor-
mate as the reducing agent together with Raney Ni are also
very interesting.
49
The reactions proceed at room temperature in
methanol, and the yields are good for both the reduction of ni-
tro groups (75–94%) and the reduction of nitriles (72–80%). The
reduction of azo compounds to hydrazo compounds and ani-
lines using Raney Ni/hydrazinium monoformate has recently been
reported.
50
Azo compounds can also be reduced using Raney
Ni/ammonium formate.
51
Balicki et al. have reduced pyridine-
N
-oxides to the corresponding pyridines in good yields using
ammonium formate as the catalytic hydrogen transfer agent
(eq 44).
52
Various functional groups were tolerated in this
reaction including nitro groups, nitriles, halides, and ketones.
HCO
2
NH
4
, Raney Ni
N
O
R
N
R
(44)
25–50
°C, 62–91%
Raney Ni/H
2
PO
4
has been used to convert nitriles to to-
sylhydrazines in one step.
53
Both aromatic and aliphatic nitriles
were used, and with one exception the reported yields are good.
NaH
2
PO
2
, Raney Ni, NH
2
NHTs, AcOH
R
C
N
R
N
HN Ts
(45)
H
2
O, pyridine, rt, 20–96%
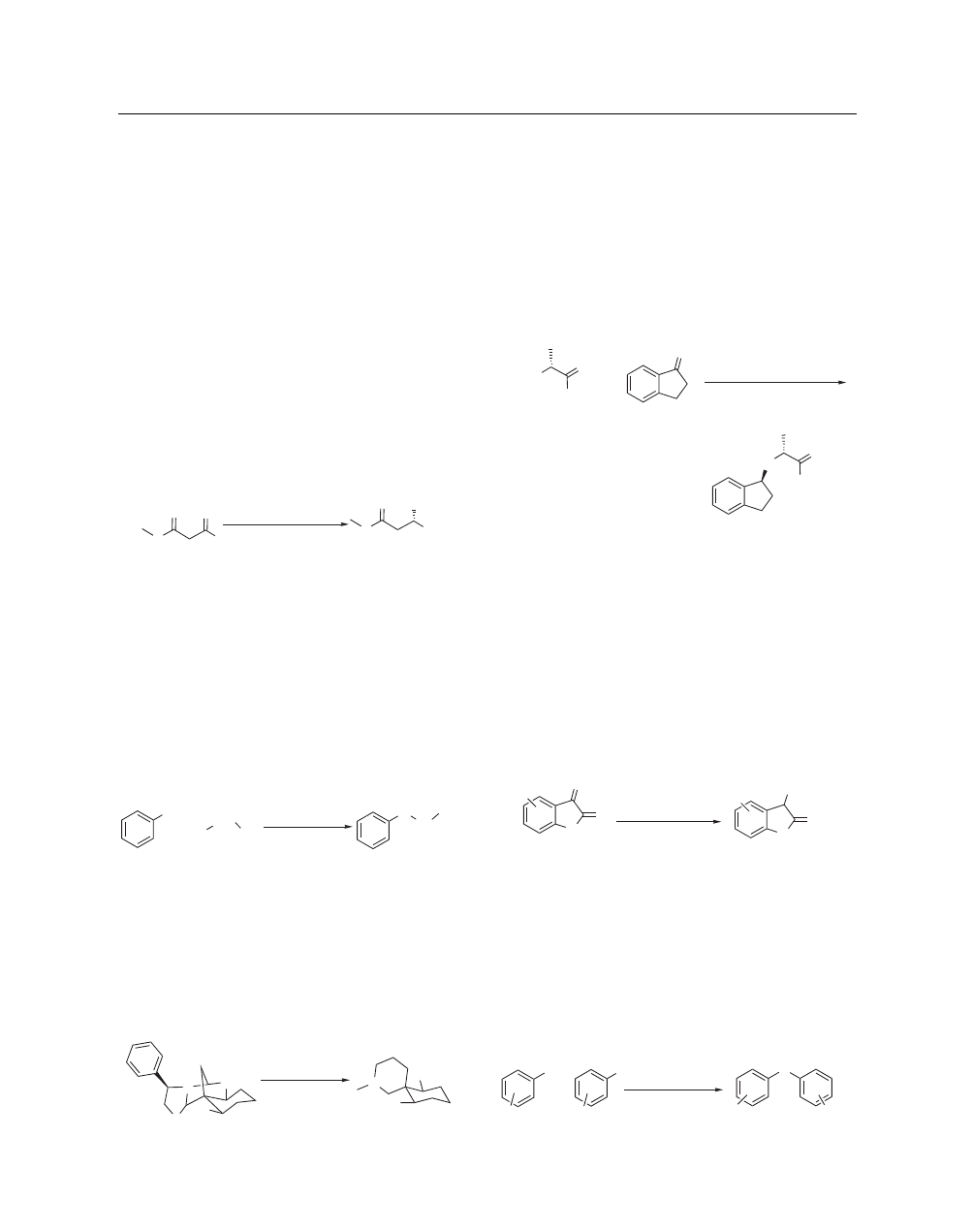
Reduction of Aromatic Rings. Tsukinoki et al. have shown
that activated aromatic groups can be reduced to the correspond-
ing saturated compounds using Raney Ni-Al alloy in aq KOH
at 90
◦
C.
54
Using these conditions, phenol could be reduced to
cyclohexanol in 93% yield. In a different publication, the authors
have reported that ring halogens [see eq 46 (X = Br or Cl)] can
promote the reduction of phenols to the corresponding cyclohex-
anols under mild conditions.
55
The halogens are removed during
the reaction. Bromides and chlorides appear to promote this
reaction equally well. Under the same conditions, phenol does
not react.
Raney Ni, aq Ba(OH)
2
OH
X
R
OH
R
(46)
60
°C, 42–93%
Avoid Skin Contact with All Reagents
6
RANEY NICKEL
Improved Reaction Conditions for Raney Ni Reductions.
Several groups have recently reported the positive effect of
ultrasonication on hydrogenation reactions with Raney Ni.
56,57
Ultrasound can afford faster reaction rates
58
and prevent deacti-
vation of the catalyst.
59
It can also promote higher yields and better
recyclability of the catalyst. Both aqueous ammonium chloride
60
and sulfuric acid
61
appear to improve certain Raney Ni-promoted
reductions, for example, the reduction of nitro groups and benzyl
halides. Isopropanol as the reaction solvent has been reported to
have advantages over other solvents including ethanol or metha-
nol.
62
A microwave-assisted reaction using Raney Ni/ammonium
vanadate as the reducing agent has been reported.
63
Enantiodifferentiating Hydrogenations of β
β
β-Keto Esters.
Enantiodifferentiating hydrogenation reaction of β-keto esters
with tartaric acid-modified Raney Ni (TA-MRNi) have been
known since the 1980s.
64
For alkyl ketones, the conditions have
recently been optimized to achieve enantiomeric excess of up to
98% (eq 47).
65
For aromatic ketones, the reaction is not as se-
lective with optical yields (OY) of 46–72%, depending on the
electronic nature of the aromatic group.
66
TA-MRNi, H
2
, THF/AcOH
O
R
O
O
OH
R
O
O
(47)
R = Alkyl: 82–98% ee
R = Aryl: 46–72% OY
60–100
°C
Reductive Amination Reactions. Raney Ni-promoted reduc-
tive amination reactions are well known.
64
Because Raney Ni can
reduce various functional groups to the corresponding amines, and
it can also promote the oxidation of alcohols to aldehydes and
ketones, functional precursors such as nitro groups
67
and
nitriles
68,69
can be utilized instead of the traditionally used amines,
and alcohols can be used in place of the carbonyl compound. For
example, Zhou et al. have reported the reaction of nitroaromatics
with primary alcohols to form the corresponding N-alkyl anilines
in good yields (eq 48).
70
Raney Ni, H
2
, EtOH
R
CH
2
H
N
CH
2
R
NO
2
+
(48)
140
°C, 83–90%
OH
The authors report that secondary alcohols and methanol gave
only low yields of the corresponding alkylation products under
these conditions. However, depending on the reaction conditions,
efficient methylation reactions or secondary alcohol alkylations
71
of amines can be achieved. For example, Francois et al. have re-
ported a one-pot benzyl group cleavage/methylation reaction us-
ing Raney Ni/MeOH (eq 49).
72
Raney Ni-promoted reductive am-
inations of α-ketocarboxylic acids have been used for the synthesis
of amino acids in excellent yields.
73
Raney Ni
MeOH, reflux
N
OH
R
O
O
R
N
(49)
91% for R = SO
2
Tol
Raney-Ni-promoted reductive alkylation reactions can show
high diastereoselectivity when chiral amines are used. Chiral
benzylamine derivatives like 1-phenylethylamine
74,75
and phenyl-
glycine amide
76
have been used as chiral auxiliaries that were
subsequently removed to reveal chiral amines. For example,
Uiterweerd et al. have used this method to synthesize (S)-1-amino-
indane with high enantiomeric excess (eq 50). Compared to other
transition metal catalysts like Pd/C, Raney Ni may show higher
diastereoselectivity in this type of reaction.
76
A disadvantage of
using Raney Ni can be the high catalyst loading required to achieve
complete conversion in a reasonable timeframe.
HN
O
NH
2
98% ee
(single diastereomer)
1. p-TsOH·H
2
O
i-PrOAC, reflux, 90%
2. Raney Ni, H
2
(3.5 bars)
i-PrOAC, 40 °C
H
2
N
O
NH
2
O
+
(50)
3. recrystallization of HCl salt
84%
3-Alkylation of Oxindole. The 3-alkylation of oxindole with
alcohols can be achieved in high yields using Raney Ni (eq 51).
77
This interesting reaction is believed to proceed via oxidation of
the alcohol, condensation of the formed carbonyl compound with
the oxindole, and reduction of the intermediate enone. The alcohol
is also used as the reaction solvent. Both primary and secondary
alcohols form the corresponding products in good yields, and diols
can also be used in this reaction. Isatins (X = O) can be used as
oxindole precursors and reduced to the corresponding oxindoles
in situ when the reaction is run under a hydrogen atmosphere.
78
R-OH, Raney Ni (H
2
)
150–220
°C, 69–98%
N
H
O
X
N
H
O
R
Y
Y
(51)
X = H
2
, O
Cross-coupling Reactions. Raney Ni-Al alloy efficiently pro-
motes Ullman-type cross-coupling reactions between phenols
and aryl halides (eq 52).
79
These reaction conditions provide an
interesting alternative to the usually used procedures because they
are fairly mild, the catalyst is not moisture- or air sensitive, and
the cheap base K
2
CO
3
can be used instead of Cs
2
CO
3
. The yields
are generally good for aryl bromides and iodides, and even ortho-
substituents are tolerated on both the phenol and the aryl halide.
OH
R
1
X
R
2
+
Raney Ni-Al alloy
CuI, K
2
CO
3
, dioxane
(52)
O
R
1
R
2
100
°C, 32–99%
X = I, Br, Cl
A list of General Abbreviations appears on the front Endpapers
RANEY NICKEL
7
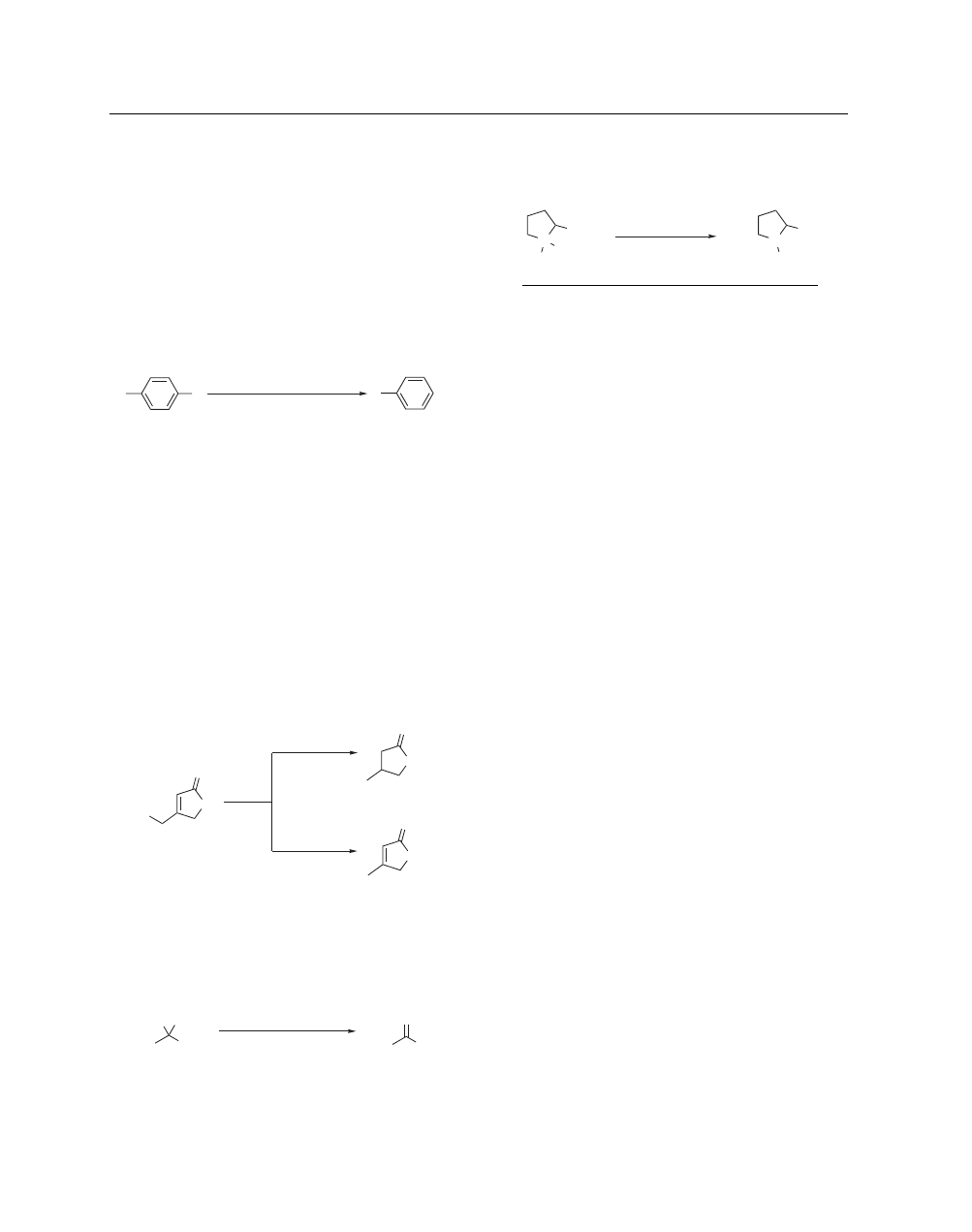
Hydrodehalogenation Reactions.
Although Raney Ni-
mediated dehalogenation reactions are mostly used for the detoxi-
fication of environmental pollutants like PCBs, under certain
circumstances they can also be of value to synthetic chemists.
For example, a dechlorination reaction has been applied to the
total synthesis of gabapentin, an anti-epileptic drug.
80
The most
commonly used procedures use a mixture of an organic solvent
and aq KOH in the presence of the phase transfer catalyst Aliquat
336 (A 336). Mild reaction conditions such as catalytic Raney Ni,
50
◦
C and 1 atm H
2
are often sufficient. In the absence of A 336,
no reaction takes place for most substrates under these conditions.
Aromatic bromides can be reduced in the presence of chlorides
(eq 53),
81
and the selective deiodination of 1-bromo-3-fluoro-4-
iodobenzene has also been reported.
81
Raney Ni, A 336, KOH (aq), H
2
(1 atm)
Cl
Br
Cl
(53)
isooctane, 50
°C, 80%
The outcome of Raney Ni-mediated dehalogenations can de-
pend strongly on the reaction conditions. Different products may
be formed depending on the base,
82
or the pH of the reaction
mixture. For example, 4-bromobenzyl bromide can be reduced
to 4-bromo toluene under acidic conditions, while under basic
conditions debromination on the aromatic ring competes with
benzylic debromination.
83
When the Raney Ni is used in cat-
alytic amounts, H
2
is most commonly used as the stoichiometric
reducing agent but NaBH
4
84
can also be used. Isopropanol has
been reported as the stoichiometric reducing agent in the deio-
dination of iodolactones.
48
Excess Raney Ni in THF efficiently
reduces aliphatic halides.
85
Aromatic and vinylic halides do not
react under these conditions, and various other functionalities
are tolerated including esters, nitriles, and sulfones. Bromides
(but not chlorides) can be reduced in the presence of ketones,
and double bonds may be conserved depending on the reaction
conditions (eq 54).
Raney Ni, THF
Raney Ni, THF
O
O
Br
O
O
O
O
(54)
rt, 5 min, 80%
rt, 60 min, 90%
Synthesis of Aldehydes and Ketones. Secondary alcohols
can be oxidized to the corresponding ketones using catalytic
Raney Ni, aluminum isopropoxide, and alumina (eq 55).
86
The
reported yields are good, and the catalyst can be reused. The oxida-
tion of an allylic alcohol to the corresponding saturated aldehyde
was also reported.
Raney Ni, Al(Oi-Pr)
3
, Al
2
O
3
R
1
R
2
OH
H
R
1
R
2
O
(55)
120–170
°C, 52–98%
Cleavage of Borane-amine Complexes.
Raney Ni can
catalyze the methanolysis of borane-amine complexes (eq 56).
87
This reaction is especially of interest for acid-sensitive substrates
where an acid-catalyzed cleavage of the borane-amine complexes
is not viable. Good yields are generally obtained in this reac-
tion, and various acid sensitive functional groups are tolerated, for
example, tert-butyl esters and MOM ethers.
Raney Ni, MeOH, rt
N
H
3
B
R
2
R
1
N
R
2
R
1
(56)
95–96%
1.
(a) Hauptmann, H.; Walter, W. F., Chem. Rev. 1962, 62, 347. (b) Caubere,
P.; Coutrot, P., Comprehensive Organic Synthesis 1991, 8, 835. (c)
Billica, H. R.; Adkins, H., Org. Synth., Coll. Vol. 1955, 3, 176.
2.
Troin, Y.; Diez, A.; Bettiol, J. L.; Rubiralta, M. Grierson, D. S.; Husson,
H.-P., Heterocycles 1991, 32, 663.
3.
Graham, A. R.; Millidge, A. F.; Young, D. P., J. Chem. Soc. 1954, 2180.
4.
Fujisawa, T.; Mobele, B. I.; Shimizu, M., Tetrahedron Lett. 1992, 33,
5567.
5.
Lottaz, P. A.; Edward, T. R. G.; Mentha, Y. G.; Burger, U., Tetrahedron
Lett. 1993
, 34, 639.
6.
Ohta, S.; Yamamoto, T.; Kawasaki, I.; Yamashita, M.; Katsuma, H.;
Nasako, R.; Kobayashi, K. Ogawa, K., Chem. Pharm. Bull. 1992, 40,
2681.
7.
Tietze, L. F.; Hartfiel, U.; Hubsch, T.; Voss, E.; Bogdanowicz-Szwod,
K.; Wichmann, J., Liebigs Ann. Chem. 1991, 275.
8.
Högberg, H. E.; Hedenström, E.; Fägerhag, J.; Servi, S., J. Org. Chem.
1992, 57, 2052.
9.
Takeshita, M.; Tsuge, A.; Tashiro, M., Chem. Ber. 1991, 124, 411.
10.
Kast, J.; Hoch, M.; Schmidt, R. R., Liebigs Ann. Chem. 1991, 481.
11.
Sadanandan, E. V.; Srinivasan, P. C., Synthesis 1992, 648.
12.
Cox, P. J.; Persad, A.; Simpkins, N. S., Synlett 1992, 197.
13.
Carrington, H. C.; Vasey, C. H.; Waring, W. S., J. Chem. Soc. 1953, 3105.
14.
Kung, P. P.; Jones, R. A., Tetrahedron Lett. 1991, 32, 3919.
15.
Coscia, A. T.; Dickerman, S. C., J. Am. Chem. Soc. 1959, 81, 3098.
16.
Bourdon, R., Bull. Soc. Claim. Fr. 1958, 722.
17.
Liu, H.-J.; Luo, W., Can. J. Chem. 1992, 70, 128.
18.
Behren, C.; Egholm, M.; Buchardt, O., Synthesis 1992, 1235.
19.
Ishibashi, H; So, T. S.; Okochi, K.; Sato, T.; Nakamura, N.; Nakatani,
H.; Ikeda, M., J. Org. Chem. 1991, 56, 95.
20.
Ojima, I.; Zhao, M.; Yamato, T.; Nakahashi, K., J. Org. Chem. 1991, 56,
5263.
21.
Krafft, M. E.; Crooks, W. J., III; Zorc, B.; Milczanowski, S. E., J. Org.
Chem. 1988
, 53, 3158.
22.
Aoyagi, Y.; Maeda, A.; Inoue, M.; Shiraishi, M.; Sakakibara, Y.; Fukui,
Y.; Ohta, A.; Kajii, K.; Kodama, Y., Heterocycles 1991, 32, 735.
23.
Alexakis, A.; Lensen, N.; Mangeney, P., Tetrahedron Lett. 1991, 32,
1171.
24.
Alexakis, A.; Lensen, N.; Mangeney, P., Synlett 1992, 3, 625.
25.
Zimmer, R.; Collas, M.; Roth, M.; Reissig, H. U., Liebigs Ann. Chem.
1992, 709.
26.
Purrington, S. T.; Sheu, K.-W., Tetrahedron Lett. 1992, 33, 3289.
27.
Lunn, G.; Sansone, E. B., J. Org. Chem. 1986, 51, 513.
28.
Soukup, M.; Widmer, E., Tetrahedron Lett. 1991, 32, 4117.
29.
Florey, K.; Restivo, A. R., J. Org. Chem. 1957, 22, 406.
30.
Wiseman, G. E.; Gould, E. S., J. Am. Chem. Soc. 1954, 76, 1706.
31.
Wiseman, G. E.; Gould, E. S., J. Am. Chem. Soc. 1955, 77, 1061.
32.
Backvall, J. E.; Bergman, J.; Engman, L., J. Org. Chem. 1983, 48, 3918.
33.
Freifelder, M.; Smart, W. D.; Stone, G. R., J. Org. Chem. 1962, 27, 2209.
Avoid Skin Contact with All Reagents

8
RANEY NICKEL
34.
Botta, M.; De Angelis, F.; Gambacorta, A.; Labbiento, L.; Nicoletti, R.,
J. Org. Chem. 1985
, 50, 1916.
35.
Graham, S. H.; Williams, A. J. S., J. Chem. Soc. (C) 1966, 655.
36.
Chan, A. S. C.; Lin, Y.-C.; Chen, C.-C. Personal communication.
37.
Wittmann, G.; Gondos, G.; Bartok, M., Helv. Chim. Acta 1990, 73, 635.
38.
Tai, A.; Kikukawa, T.; Sugimura, T.; Inone, Y. D.; Osawa, T.; Fujii, S.,
J. Chem. Soc. (C) 1991
, 795.
39.
Blacklock, T. J.; Shuman, R. F.; Butcher, J. W.; Shearin, W. E., Jr.;
Budavari, J.; Grenda, V. J., J. Org. Chem. 1988, 53, 836.
40.
Barrero, A. F.; Alvarez-Manzaneda, E. J.; Chahboun, R.; Meneses, R.,
Synlett 2000
, 197.
41.
Barrero, A. F.; Alvarez-Manzaneda, E. J.; Chahboun, R.; Meneses, R.,
Synlett 1999
, 1663.
42.
Cordier, G.; Grosselin, J. M.; Bailliard, R. M., Catalysis of Organic
Reactions, Chemical Industries (Dekker) 1994
, 53, 103.
43.
Xu, D.-Q.; Hu, Z.-Y.; Li, W.-W.; Luo, S.-P.; Xu, Z.-Y., Journal of
Molecular Catalysis A: Chemical 2005
, 235, 137.
44.
Perosa, A.; Tundo, P.; Zinovyev, S., Green Chemistry 2002, 4, 492.
45.
Klenke, B; Gilbert, I. H., J. Org. Chem. 2001, 66, 2480.
46.
Mebane, R. C.; Jensen, D. R.; Rickerd, K. R.; Gross, B. H., Synth.
Commun. 2003
, 33, 3373.
47.
Gross, B. H.; Mebane, R. C.; Armstrong, D. L., Applied Catalysis, A:
General 2001
, 219, 281.
48.
Mebane, R. C.; Grimes, K. D.; Jenkins, S. R.; Deardorff, J. D.; Gross,
B. H., Synth. Commun. 2002, 32, 2049.
49.
Gowda, S.; Gowda, D. C., Tetrahedron 2002, 58, 2211.
50.
Prasad, H. S.; Gowda, S.; Gowda, D. C., Synth. Commun. 2004, 34, 1.
51.
Gowda, D. C.; Gowda, S.; Abiraj, K., Indian Journal of Chemistry,
Section B 2003
, 42B, 1774.
52.
Balicki, R.; Maciejewski, G., Synth. Commun. 2002, 32, 1681.
53.
Toth, M.; Somsak, L., Tetrahedron Lett. 2001, 42, 2723.
54.
Tsukinoki, T.; Kanda, T.; Liu, G.-B.; Tsuzuki, H.; Tashiro, M.,
Tetrahedron Lett. 2000
, 41, 5865.
55.
Tsukinoki, T.; Kakinami, T.; Iida, Y.; Ueno, M.; Ueno, Y.; Mashimo,
T.; Tsuzuki, H.; Tashiro, M., J. Chem. Soc., Chem. Commun. 1995,
209.
56.
Wang, H.; Lian, H.; Chen, J.; Pan, Y.; Shi, Y., Synth. Commun. 1999, 29,
129.
57.
Heropoulos, G. A.; Georgakopoulos, S.; Steele, B. R., Tetrahedron Lett.
2005, 46, 2469.
58.
Disselkamp, R. S.; Hart, T. R.; Williams, A. M.; White, J. F.; Peden, C.
H. F., Ultrasonics Sonochemistry 2004, 12, 319.
59.
Mikkola, J.-P.; Salmi, T., Chemical Engineering Science 1999, 54, 1583.
60.
Bhaumik, K.; Akamanchi, K. G., Can. J. Chem. 2003, 81, 197.
61.
Okimoto, M.; Takahashi, Y.; Nagata, Y.; Satoh, M.; Sueda, S.;
Yamashina, T., Bull. Chem. Soc. Jpn. 2004, 77, 1405.
62.
Regla, I.; Reyes, A.; Korber, C.; Demare, P.; Estrada, O.; Juaristi, E.,
Synth. Commun. 1997
, 27, 817.
63.
Dave, M. A.; Prabhu, P. J., Asian Journal of Chemistry 2002, 540.
64.
See also previous part of this article.
65.
Sugimura, T., Catalysis Surveys from Japan 1999, 3, 37.
66.
Nakagawa, S.; Tai, A.; Okuyama, T.; Sugimura, T., Topics in Catalysis
2000, 13, 187.
67.
Zhou, X.; Wu, Z.; Li, L.; Wang, G.; Li, J., Dyes and Pigments 1998, 36,
365.
68.
Herkes, F. E., Chemical Industries (Dekker) 2003, 89, 429.
69.
Dallons, J. L.; Van Gysel, A.; Jannes, G., Catalysis of Organic Reactions,
Chemical Industries (Dekker) 1992
, 47, 93.
70.
Zhou, X.; Wu, Z.; Lin, L.; Wang, G., Dyes and Pigments 1998, 402,
205.
71.
Botta, M.; De Angelis, F.; Gambacorta, A.; Labbiento, L.; Nicoletti, R.,
J. Org. Chem. 1985
, 50, 1916.
72.
Francois, D.; Lallemand, M.-C.; Selkti, M.; Tomas, A.; Kunesch, N.;
Husson, H.-P., Angew. Chem., Int. Ed. 1998, 37, 104.
73.
Chan, A. S. C.; Chen, C-C.; Lin, Y-C., Applied Catalysis, A: General
1994, 119, L1.
74.
Lauktien, G.; Volk, F.-J.; Frahm, A. W., Tetrahedron: Asymmetry 1997,
8
, 3457.
75.
Schlichter, W. H.; Frahm, A. W., Archiv der Pharmazie 1993, 326,
429.
76.
Uiterweerd, P. G. H.; van der Sluis, M.; Kaptein, B.; de Lange, B.;
Kellogg, R. M.; Broxterman, Q. B., Tetrahedron: Asymmetry 2003, 14,
3479.
77.
Volk, B.; Mezei, T.; Simig, G., Synthesis 2002, 595.
78.
Volk, B.; Simig, G., Eur. J. Org. Chem. 2003, 3991.
79.
Xu, L.-W.; Xia, C.-G.; Li, J.-W. Hu, X.-X., Synlett 2003, 2071.
80.
Cagnoli, R. Ghelfi, F. Pagnoni, U. M.; Parsons, A. F.; Schenetti, L.,
Tetrahedron 2003
, 59, 9951.
81.
Marques, C. A.; Rogozhnikova, O.; Selva, M.; Tundo, P., Journal of
Molecular Catalysis A: Chemical 1995
, 96, 301.
82.
Liu, G.-B.; Tsukinoki, T.; Kanda, T.; Mitoma, Y.; Tashiro, M.,
Tetrahedron Lett. 1998
, 39, 5991.
83.
Evdokimova, G.; Zinovyev, S.; Perosa, A.; Tundo, P., Applied Catalysis,
A: General 2004
, 271, 129.
84.
Roy, H. M.; Wai, C. M.; Yuan, T.; Kim, J.-K.; Marshall, W. D., Applied
Catalysis, A: General 2004
, 271, 137.
85.
Barrero, A. F.; Alvarez-Manzaneda, E. J.; Chahboun, R.; Meneses, R.;
Romera, J. L., Synlett 2001, 485.
86.
Tarta, I. C.; Silberg, I. A.; Vlassa, M.; Oprean, I., Central European
Journal of Chemistry 2004
, 2, 214.
87.
Couturier, M.; Tucker, J. L.; Andresen, B. M.; Dube, P.; Negri, J. T., Org.
Lett. 2001
, 3, 465.
A list of General Abbreviations appears on the front Endpapers
Wyszukiwarka
Podobne podstrony:
nickel catalysts heterogeneous eros rn011
nickel in charcoal eros rn00732
nickel boride eros rn008
nickel complex reducing agents eros rn013s
benzyl chloride eros rb050
hydrobromic acid eros rh031
chloroform eros rc105
magnesium eros rm001
oxalyl chloride eros ro015
potassium permanganate eros rp244
peracetic acid eros rp034
p toluenesulfonic acid eros rt134
hexamethylenetetramine eros rh019
copper II chloride eros rc214
glyoxylic acid eros rg009
Nickelback Lullaby
więcej podobnych podstron