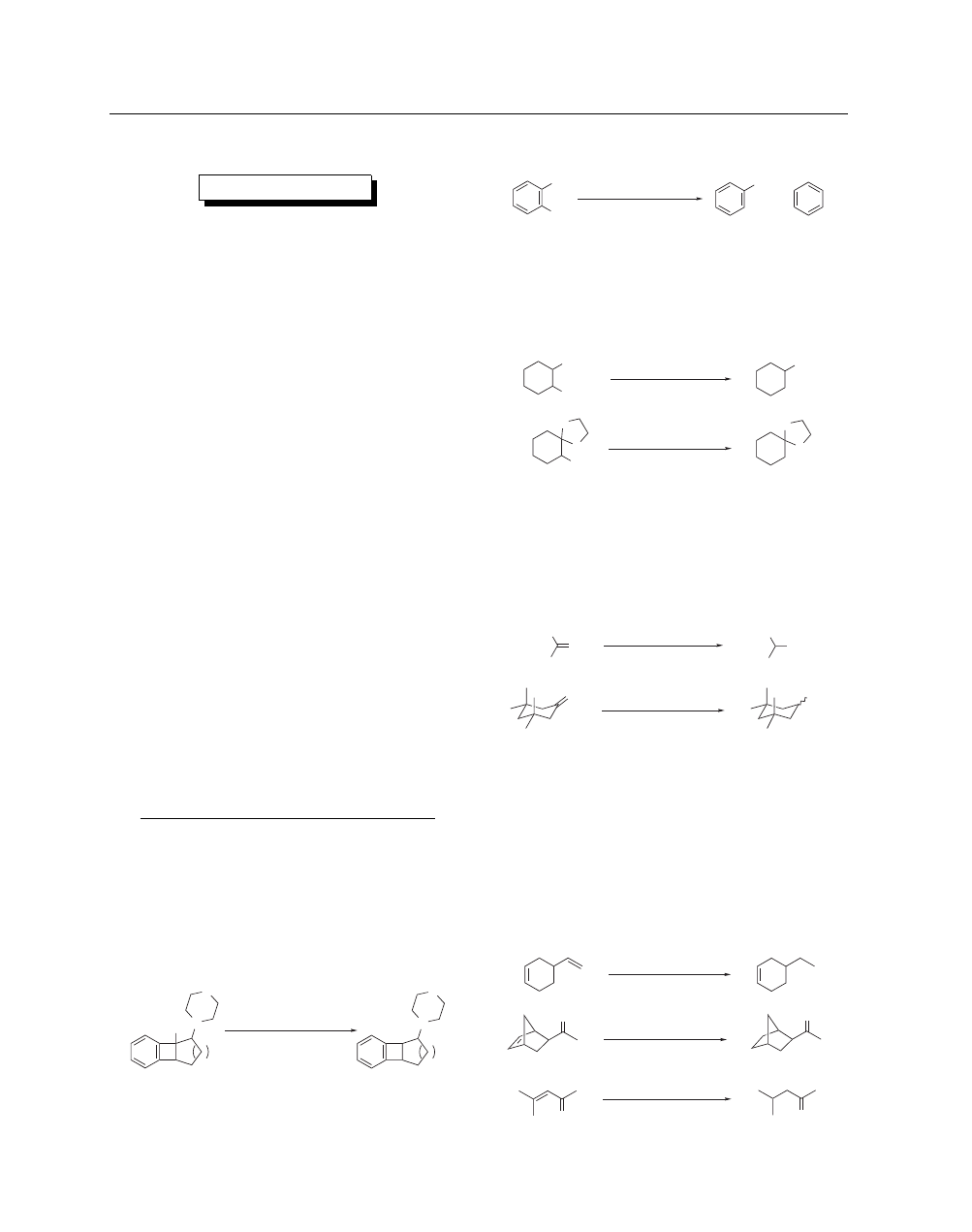
NICKEL COMPLEX REDUCING AGENTS
1
Nickel Complex Reducing Agents
1
NaH—t–C
5
H
11
ONa—Ni(OAc)
2
(NaH)
[7646-69-7]
HNa
(MW 24.00)
InChI = 1/Na.H/rHNa/h1H
InChIKey = MPMYQQHEHYDOCL-RVEWWXDUAC
(t-C
5
H
11
ONa)
[14593-46-5]
C
5
H
11
NaO
(MW 110.15)
InChI = 1/C5H11O.Na/c1-4-5(2,3)6;/h4H2,1-3H3;/q-1;+1
InChIKey = CGRKYEALWSRNJS-UHFFFAOYAC
(Ni(OAc)
2
·
4H
2
O)
[6018-89-9]
C
4
H
14
NiO
8
(MW 248.87)
InChI = 1/2C2H4O2.Ni.4H2O/c2*1-2(3)4;;;;;/h2*1H3,(H,3,4);;
4*1H2/q;;+2;;;;/p-2/f2C2H3O2.Ni.4H2O/q2*-1;m;;;;
InChIKey = OINIXPNQKAZCRL-YNARRYDOCG
(reduces many functional groups; couples organic halides; de-
sulfurizes organic substrates; source of hydrogenation catalyst
1
)
Alternate Name:
nickel-containing complex reducing agents
(NiCRAs).
Physical Data:
black solid of uncertain structure containing Ni
0
species.
2
Solubility:
insol organic solvents.
Preparative Methods: t
-C
5
H
11
ONa–NiCRA (x.y.z) is conven-
iently prepared by the reaction of t-C
5
H
11
OH (y equiv) with
a mixture of Sodium Hydride (x + y equiv) and Nickel(II) Ac-
etate (z equiv) in THF or DME. Commercial NaH (55–60% in
oil) is used after three washings with anhydrous THF or DME
under N
2
. t-C
5
H
11
OH is distilled from sodium. Ni(OAc)
2
is
dried in vacuo (15 mmHg) for 16 h at 120–130
◦
C. Note: t-
C
5
H
11
ONa–NiCRA (x.y.z) means a reagent obtained from x,y,
and z equivalents of NaH, t-C
5
H
11
ONa, and Ni(OAc)
2
, respec-
tively.
Handling, Storage, and Precautions:
NiCRAs are nonpyrophoric
but are handled and stored under inert atmosphere (N
2
or, better,
Ar). Hydrolysis must be performed by slow addition of cold
water or EtOH to destroy the hydride.
Reductions. t-C
5
H
11
ONa–NiCRAs are significantly less ba-
sic than NaH and t-C
5
H
11
ONa. t-C
5
H
11
ONa–NiCRA (4.2.1) re-
duces under mild conditions and in good yields aryl halides, in-
cluding aryl fluorides,
3
vinyl, allyl, benzyl, and alkyl halides (I
>
Br > Cl; primary ≥ secondary ≥ tertiary)
4
and monoreduces
gem
-dihalocyclopropanes.
5
It thus allows the removal of a ter-
tiary hydroxy group (eq 1) while all other classical methods were
unsuccessful.
6
N
O
OH
n
N
O
n
= 2, 3
1. SOCl
2
2. t-C
5
H
11
ONa–NiCRA (4.2.1)
THF, 63 °C
n
(1)
45%
NiCRAs are sensitive to the nature and structure of the halides
and allow selective reductions of polyhalogenated substrates, as
exemplified in eq 2.
5
t
-C
5
H
11
ONa–NiCRA (4.2.1)
(2)
Cl
Br
Cl
+
96%
traces
DME, 65 °C
Except with substrates very sensitive to bases, chemoselective
reduction of halides may be easily performed in the presence of
alkoxy, acetal, hydroxy, OTHP, carboxy, alkoxycarbonyl, keto,
and cyano groups.
5
No elimination takes place with 1,2-alkoxy
halides (eqs 3 and 4).
t
-C
5
H
11
ONa–NiCRA (4.2.1)
DME, 65 °C
(3)
OMe
Br
OMe
90%
t
-C
5
H
11
ONa–NiCRA (4.2.1)
DME, 65 °C
(4)
Br
O
O
O
O
100%
NiCRAs are much more powerful than ZnCRAs (see Zinc
Complex Reducing Agents) in the reduction of halides. t-C
5
H
11
ONa–NiCRA (4.1.1) reduces all classes of ketones or aldehydes
and is not very sensitive to steric hindrance.
7,8
The efficiency of
the reduction may be increased by the addition of electrophilic
salts such as Magnesium Bromide or Lithium Chloride (eqs 5
and 6).
(5)
O
t
-Bu
t
-Bu
t
-Bu
t
-Bu
t
-C
5
H
11
ONa–NiCRA (4.1.1)
MgBr
2
, THF, 63 °C
OH
~100%
t
-C
5
H
11
ONa–NiCRA (4.1.1)
MgBr
2
, THF, 63 °C
(6)
O
OH
85%
Under appropriate conditions, t-C
5
H
11
ONa–NiCRAs easily
epimerize alcohols.
9
This property may be used to reduce ke-
tones with a very high selectivity for the most stable alcohol. In
such reactions t-C
5
H
11
ONa may be advantageously replaced by
the sodium salt of 2,5-dimethyl-2,5-hexanediol.
8b
In the presence of Chlorotrimethylsilane (1 equiv), t-C
5
H
11
ONa–NiCRA (5.2.1) (or 5.1.1) (t-C
5
H
11
ONa–NiCRASi) very ef-
ficiently reduces carbon–carbon double bonds. The reagent is sen-
sitive to steric hindrance as well as to electronic effects and allows
the selective reduction of dienes, unsaturated ketones, esters, and
acids.
10
The high selectivity is illustrated in eqs 7–9.
t
-C
5
H
11
ONa–NiCRA (5.2.1)
1 equiv TMSCl, THF, 45 °C
(7)
98%
t
-C
5
H
11
ONa–NiCRA (5.1.1)
1 equiv TMSCl, THF, 45 °C
(8)
O
O
99%
t
-C
5
H
11
ONa–NiCRA (5.1.1)
1 equiv TMSCl, THF, 45 °C
(9)
O
O
97%
Avoid Skin Contact with All Reagents
2
NICKEL COMPLEX REDUCING AGENTS
t
-C
5
H
11
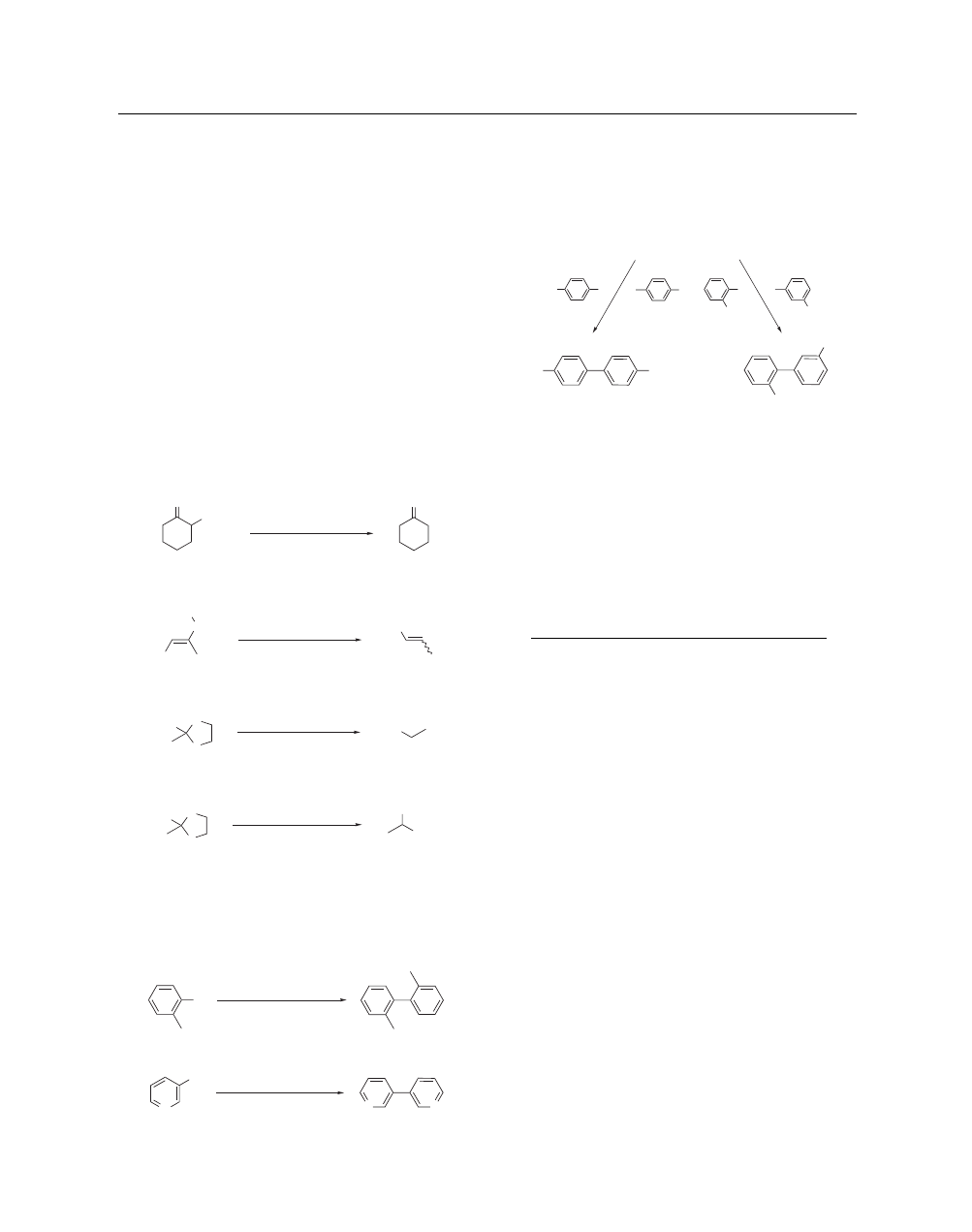
ONa–NiCRA (4.1.1) also selectively reduces a number
of α,β-unsaturated ketones, although sometimes less efficiently
than t-C
5
H
11
ONa–NiCRASi.
11
In the reduction of unsaturated
carbonyl substrates, NiCRAs are complementary to ZnCRAs,
which only reduce the carbonyl groups.
t
-C
5
H
11
ONa–NiCRA (4.2.1) regioselectively reduces epoxi-
des,
12
with the major or only product formed coming from the
regioselective attack on the most hindered carbon. This regiose-
lectivity is opposite to that observed with ZnCRAs.
Finally, anilines are easily prepared from the corresponding ni-
tro derivatives using t-C
5
H
11
ONa–NiCRA (7.1.1) as the reducing
agent.
13
Reductive Desulfurization. t-C
5
H
11
ONa–NiCRAs as such
or in the presence of a ligand (t-C
5
H
11
ONa–NiCRALs) very
efficiently and chemoselectively desulfurize thiols, thioethers,
dithioacetals, sulfoxides, and sulfones.
14
Ketones, esters, and
carbon–carbon double bonds are tolerated (eqs 10–12). Under ap-
propriate conditions, monodesulfurization of dithioacetals may be
easily performed (eq 13).
t
-C
5
H
11
ONa–NiCRA (5.2.1)
THF, 63 °C
Ni/S = 20
(10)
O
SMe
O
90%
(11)
SO
2
Ph
Ph
Ph
t
-C
5
H
11
ONa–NiCRA (2.2.1)
DME, 65 °C
Ni/S = 10
96%
t
-C
5
H
11
ONa–NiCRA (5.2.1)
THF, 63 °C
Ni/S = 30
(12)
S
S
Ph
Ph
100%
t
-C
5
H
11
ONa–NiCRA (4.2.1)
2 equiv bipy, DME, 65 °C
Ni/S = 20
(13)
S
S
Ph
SEt
Ph
90%
Coupling Reactions.
t
-C
5
H
11
ONa–NiCRALs very effi-
ciently couple aryl as well as heteroaryl halides (eqs 14 and 15).
15
t
-C
5
H
11
ONa–NiCRA (4.2.1)
2 equiv bipy, THF, 1 equiv KI
63 °C
(14)
Cl
2
90%
t
-C
5
H
11
ONa–NiCRA (4.2.1)
4 equiv Ph
3
P, DME, 63 °C
(15)
N
Cl
N
N
2
88%
Unsymmetrical cross-couplings may be performed in fair to
good yields simply by addition of two aryl halides to the reagent
(eq 16).
MeO
Br
Cl
CF
3
CF
3
MeO
Br
OMe
Cl
F
OMe
73%
F
t
-C
5
H
11
ONa–NiCRA (4.2.1)
2 equiv bipy, 1 equiv KI
THF, C
6
H
6
, 63 °C
(16)
62%
Hydrogenation Catalyst.
A nonpyrophoric hydrogenation
catalyst, referred to as Nic, may be easily obtained from t-C
5
H
11
ONa–NiCRA by simple addition of a catalytic amount of the
reagent to the reaction medium when the solvent is protic. In apro-
tic solvent, NaH is neutralized with t-C
5
H
11
OH before use. If
necessary, soluble alkoxide is removed by washing with EtOH
(Nic
w
). Nic is very efficient in atmospheric hydrogenation of
carbon–carbon double bonds, ketones, and aldehydes and in the
selective partial hydrogenation of carbon–carbon triple bonds.
16
Related
Reagents. Sodium
Hydride–Nickel(II) Acetate–
Sodium t-Pentoxide.
1.
Caubère, P., Top. Curr. Chem. 1978, 73, 50; Angew. Chem., Int. Ed. Engl.
1983, 22, 599; Pure Appl. Chem. 1985, 57, 1875 Rev. Heteroatom Chem.
1991, 4, 78.
2.
Brunet, J. J.; Besozzi, D.; Courtois, A.; Caubère, P., J. Am. Chem. Soc.
1982, 104, 7130.
3.
Guillaumet, G.; Mordenti, L.; Caubère, P., J. Organomet. Chem. 1975,
92
, 43 (Chem. Abstr. 1975, 83, 131 349g).
4.
Vanderesse, R.; Brunet, J. J.; Caubère, P., J. Org. Chem. 1981, 46, 1270.
5.
Guillaumet, G.; Mordenti, L.; Caubère, P., J. Organomet. Chem. 1975,
102
, 353 (Chem. Abstr. 1976, 84, 73 704b).
6.
Jamart-Grégoire, B.; Fort, Y.; Zouaoui, M. A.; Caubère, P., Synth.
Commun. 1993
, 23, 885.
7.
Brunet, J. J.; Mordenti, L.; Caubère, P., J. Org. Chem. 1978, 43, 4804.
8.
(a) Feghouli, A.; Fort, Y.; Vanderesse, R.; Caubère, P., Tetrahedron Lett.
1988, 29, 1379. (b) Fort, Y.; Feghouli, A.; Vanderesse, R.; Caubère, P.,
J. Org. Chem. 1990
, 55, 5911.
9.
Feghouli, G.; Vanderesse, R.; Fort, Y.; Caubère, P., Tetrahedron Lett.
1988, 29, 1383. Vanderesse, R.; Feghouli, G.; Fort, Y.; Caubère, P., J.
Org. Chem. 1990
, 55, 5916.
10.
Fort, Y.; Vanderesse, R.; Caubère, P., Tetrahedron Lett. 1986, 27, 5487.
11.
Mordenti, L.; Brunet, J. J.; Caubère, P., J. Org. Chem. 1979, 44, 2203.
12.
Fort, Y.; Vanderesse, R.; Caubère, P., Tetrahedron Lett. 1985, 26, 3111.
13.
Feghouli, G.; Vanderesse, R.; Fort, Y.; Caubère, P., J. Chem. Soc., Perkin
Trans. 1 1989
, 2069.
14.
Becker, S.; Fort, Y.; Vanderesse, R.; Caubère, P., Tetrahedron Lett. 1988,
29
, 2963. Becker, S.; Fort, Y.; Vanderesse, R.; Caubère, P., J. Org. Chem.
1989, 54, 4848. Becker, S.; Fort, Y.; Caubère, P., J. Org. Chem. 1990,
55
, 6194.
A list of General Abbreviations appears on the front Endpapers

NICKEL COMPLEX REDUCING AGENTS
3
15.
Vanderesse, R.; Brunet, J. J.; Caubère, P., J. Organomet. Chem. 1984,
264
, 263. Vanderesse, R.; Fort, Y.; Becker, S.; Caubère, P., Tetrahedron
Lett. 1986
, 27, 3517. Vanderesse, R.; Lourak, M.; Fort, Y.; Caubère,
P., Tetrahedron Lett. 1986, 27, 5483. Lourak, M. Thesis, University
of Nancy I, 1990. Lourak, M.; Vanderesse, R.; Fort, Y.; Caubère, P.,
Tetrahedron Lett. 1988
, 29, 545. Lourak, M.; Vanderesse, R.; Fort, Y.;
Caubère, P., J. Org. Chem. 1989, 54, 4840. Lourak, M.; Vanderesse, R.;
Fort, Y.; Caubère, P., J. Org. Chem. 1989, 54, 4844.
16.
Brunet, J. J.; Gallois, P.; Caubère, P., J. Org. Chem. 1980, 45,
1937. Gallois, P.; Brunet, J. J.; Caubère, P., J. Org. Chem. 1980, 45,
1946.
Paul Caubère
University of Nancy I, Nancy, France
Avoid Skin Contact with All Reagents
Wyszukiwarka
Podobne podstrony:
The Notion of Complete Reducibility in Group Theory [lectures] J Serre (1998) WW
nickel catalysts heterogeneous eros rn011
nickel in charcoal eros rn00732
nickel boride eros rn008
raney nickel eros rr001
lecture3 complexity introduction
L 3 Complex functions and Polynomials
benzyl chloride eros rb050
hydrobromic acid eros rh031
chloroform eros rc105
magnesium eros rm001
Complexes
LES PRONOMS COMPLEMENT
oxalyl chloride eros ro015
Complete Timeline of Darkest Powers Stories 2011 04 13
501 Sentence Completion Questions
więcej podobnych podstron