MERCURY(II) CHLORIDE–SILVER(I) NITRITE
1
Mercury(II) Chloride–Silver(I) Nitrite
HgCl
2
AgNO
2
(HgCl
2
)
[7487-94-7]
Cl
2
Hg
(MW 271.49)
InChI = 1/2ClH.Hg/h2*1H;/q;;+2/p-2/f2Cl.Hg/h2*1h;/q2*-1;m
InChIKey = LWJROJCJINYWOX-ZZJRNXLTCY
(AgNO
2
)
[7783-99-5]
AgNO
2
(MW 153.88)
InChI = 1/Ag.HNO2/c;2-1-3/h;(H,2,3)/q+1;/p-1/fAg.NO2/qm;-1
InChIKey = KKKDGYXNGYJJRX-QGMACRPXCX
(nitromercuration
1
–
4
and
synthesis
of
nitroalkenes
from
alkenes
4
,
5
)
Form Supplied in:
white, odorless needles or powder (both
reagents).
Handling, Storage, and Precautions:
acute poison. Exposure to
all mercury compounds is to be strictly avoided. Releases toxic
Hg fumes when heated to decomposition. Protect from light.
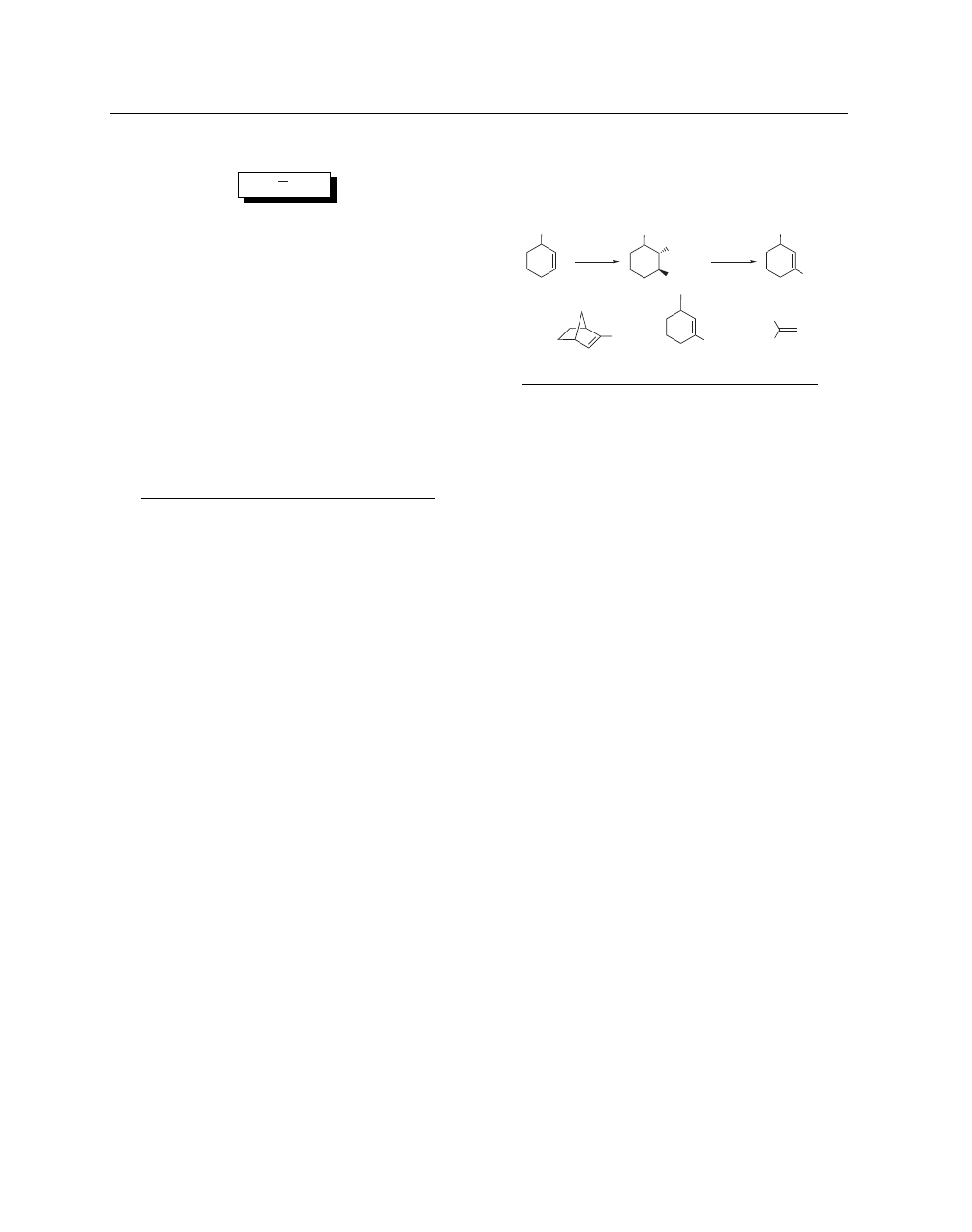
Mixing Mercury(II) Chloride and Silver(I) Nitrite generates
Hg(NO
2
)
2
in situ, which reacts with alkenes to give nitromercu-
ration products with high regio- and stereoselectivity (eq 1).
1
–
3
The reaction with norbornene occurs without rearrangement,
indicating that carbocationic character in the transition state is
little developed. On treatment with a base (NaOH or R
3
N), the
primary adducts undergo an elimination reaction to afford
nitroalkenes (eq 1).
4
This two-step transformation represents a
general route to nitroalkenes
4
and has been employed in the
synthesis of 3-nitrocycloalkenones
5
and (E)-2-nitrovinyltri-
methylsilane.
6
However, better yields are usually obtained if
AgNO
2
/I
2
is used instead.
7
R
HgCl
2
NO
2
AgNO
2
R
HgNO
2
NO
2
NO
2
NaOH
H
2
O
O
2
N
Bu
R
NO
2
(1)
77%
80%
71%
1.
(a) Larock, R. C., Angew. Chem., Int. Ed. Engl. 1978, 17, 27. (b) Larock,
R. C., Tetrahedron 1982, 38, 1713. (c) Larock, R. C. Organomercury
Compounds in Organic Synthesis
; Springer: Berlin, 1985. (d) Larock,
R. C. Solvomercuration/Demercuration Reactions in Organic Synthesis;
Springer: Berlin, 1986.
2.
Bachman, G. B.; Whitehouse, M. L., J. Org. Chem. 1967, 32, 2303.
3.
Bloodworth, A. J.; Griffin, I. M., J. Chem. Soc., Perkin Trans. 1 1975,
695.
4.
Corey, E. J.; Estreicher, H., J. Am. Chem. Soc. 1978, 100, 6294.
5.
Vankar, Y. D.; Bawa, A., Synth. Commun. 1985, 15, 1253.
6.
Ali, S. M.; Matsuda, Y.; Tanimoto, S., Synthesis 1988, 805.
7.
Sy, W.-W.; By, A. W., Tetrahedron Lett. 1985, 26, 1193.
Pavel Ko˘covský
University of Leicester, Leicester, UK
Avoid Skin Contact with All Reagents
Wyszukiwarka
Podobne podstrony:
mercury II chloride eros rm031
copper II chloride eros rc214
palladium II chloride eros rp007
mercury II nitrate eros rm037
mercury II sulfate eros rm044
iron II chloride eros ri055
copper II chloride eros rc214
vanadium II chloride eros rv002
benzyl chloride eros rb050
oxalyl chloride eros ro015
lithium chloride eros rl076
phenylzinc chloride eros rp148
iron III chloride eros ri054
więcej podobnych podstron