Technologia Chemiczna |
|
28.11.2007 |
grupa 5 zespół 5 |
Ćwiczenie nr 9 |
dr inż. Izabela Stępniak |
TEMAT: Kalorymetria - ciepło zobojętniania
CEL ĆWICZENIA
Celem ćwiczenia jest wyznaczenie entalpii zobojętniania kwasu zasadą.
POMIARY:
czas [s] |
1.1 |
1.2 |
2.1 |
2.2 |
3.1 |
3.2 |
1 |
11.677 |
11.416 |
11.413 |
11.397 |
11.390 |
11.389 |
2 |
11.677 |
11.415 |
11.412 |
11.399 |
11.390 |
11.387 |
3 |
11.677 |
11.417 |
11.414 |
11.398 |
11.389 |
11.388 |
4 |
11.677 |
11.415 |
11.413 |
11.397 |
11.389 |
11.387 |
5 |
11.677 |
11.415 |
11.413 |
11.399 |
11.389 |
11.387 |
6 |
11.676 |
11.415 |
11.413 |
11.397 |
11.389 |
11.387 |
7 |
11.677 |
11.414 |
11.412 |
11.397 |
11.389 |
11.389 |
8 |
11.677 |
11.415 |
11.412 |
11.397 |
11.388 |
11.387 |
9 |
11.676 |
11.415 |
11.411 |
11.397 |
11.388 |
11.387 |
10 |
11.676 |
11.415 |
11.412 |
11.397 |
11.389 |
11.386 |
11 |
11.676 |
11.415 |
11.412 |
11.397 |
11.388 |
11.387 |
12 |
11.676 |
11.417 |
11.411 |
11.397 |
11.389 |
11.387 |
13 |
11.676 |
11.416 |
11.412 |
11.397 |
11.389 |
11.386 |
14 |
11.676 |
11.415 |
11.412 |
11.397 |
11.388 |
11.387 |
15 |
11.676 |
11.415 |
11.412 |
11.397 |
11.388 |
11.387 |
16 |
11.676 |
11.415 |
11.412 |
11.397 |
11.388 |
11.386 |
17 |
11.676 |
11.414 |
11.412 |
11.399 |
11.389 |
11.387 |
18 |
11.675 |
11.415 |
11.410 |
11.396 |
11.388 |
11.387 |
19 |
11.675 |
11.415 |
11.411 |
11.395 |
11.388 |
11.386 |
20 |
11.675 |
11.415 |
11.412 |
11.396 |
11.390 |
11.386 |
21 |
11.675 |
11.414 |
11.411 |
11.396 |
11.388 |
11.386 |
22 |
11.675 |
11.414 |
11.411 |
11.396 |
11.388 |
11.388 |
23 |
11.675 |
11.414 |
11.412 |
11.394 |
11.387 |
11.386 |
24 |
11.675 |
11.414 |
11.411 |
11.396 |
11.388 |
11.386 |
25 |
11.674 |
11.414 |
11.411 |
11.396 |
11.387 |
11.387 |
26 |
11.675 |
11.414 |
11.411 |
11.396 |
11.390 |
11.386 |
27 |
11.675 |
11.414 |
11.411 |
11.396 |
11.388 |
11.385 |
28 |
11.674 |
11.416 |
11.411 |
11.396 |
11.388 |
11.387 |
29 |
11.674 |
11.413 |
11.411 |
11.395 |
11.386 |
11.386 |
30 |
11.674 |
11.413 |
11.412 |
11.395 |
11.387 |
11.385 |
31 |
11.674 |
11.414 |
11.411 |
11.383 |
11.387 |
11.387 |
32 |
11.674 |
11.413 |
11.411 |
11.361 |
11.386 |
11.385 |
33 |
11.674 |
11.413 |
11.411 |
11.357 |
11.387 |
11.384 |
34 |
11.674 |
11.413 |
11.411 |
11.357 |
11.388 |
11.385 |
35 |
11.673 |
11.413 |
11.411 |
11.357 |
11.387 |
11.385 |
36 |
11.673 |
11.414 |
11.412 |
11.358 |
11.387 |
11.385 |
37 |
11.673 |
11.413 |
11.410 |
11.359 |
11.387 |
11.385 |
38 |
11.672 |
11.413 |
11.411 |
11.358 |
11.387 |
11.384 |
39 |
11.672 |
11.413 |
11.412 |
11.358 |
11.386 |
11.384 |
40 |
11.673 |
11.413 |
11.410 |
11.358 |
11.386 |
11.385 |
41 |
11.672 |
11.413 |
11.410 |
11.358 |
11.387 |
11.384 |
42 |
11.672 |
11.413 |
11.409 |
11.357 |
11.386 |
11.384 |
43 |
11.673 |
11.413 |
11.410 |
11.358 |
11.387 |
11.384 |
44 |
11.672 |
11.413 |
11.409 |
11.358 |
11.386 |
11.384 |
45 |
11.672 |
11.408 |
11.410 |
11.358 |
11.386 |
11.381 |
46 |
11.672 |
11.405 |
11.410 |
11.357 |
11.386 |
11.379 |
47 |
11.672 |
11.395 |
11.410 |
11.359 |
11.387 |
11.377 |
48 |
11.671 |
11.392 |
11.410 |
11.358 |
11.386 |
11.377 |
49 |
11.672 |
11.391 |
11.410 |
11.357 |
11.385 |
11.374 |
50 |
11.671 |
11.387 |
11.410 |
11.358 |
11.386 |
11.370 |
51 |
11.671 |
11.387 |
11.392 |
11.358 |
11.386 |
11.356 |
52 |
11.669 |
11.387 |
11.375 |
|
11.386 |
11.354 |
53 |
11.652 |
11.388 |
11.374 |
|
11.386 |
11.354 |
54 |
11.645 |
11.388 |
11.372 |
|
11.386 |
11.354 |
55 |
11.643 |
11.387 |
11.372 |
|
11.385 |
11.354 |
56 |
11.642 |
11.388 |
11.373 |
|
11.384 |
11.355 |
57 |
11.642 |
11.389 |
11.373 |
|
11.383 |
11.355 |
58 |
11.642 |
11.386 |
11.372 |
|
11.380 |
11.355 |
59 |
11.642 |
11.387 |
11.373 |
|
11.377 |
11.355 |
60 |
11.642 |
11.388 |
11.372 |
|
11.365 |
11.354 |
61 |
11.642 |
11.387 |
11.373 |
|
11.352 |
11.354 |
62 |
11.642 |
11.387 |
11.373 |
|
11.354 |
11.352 |
63 |
11.642 |
11.387 |
11.373 |
|
11.353 |
11.352 |
64 |
11.642 |
11.386 |
11.373 |
|
11.355 |
11.352 |
65 |
11.642 |
11.387 |
11.374 |
|
11.352 |
11.352 |
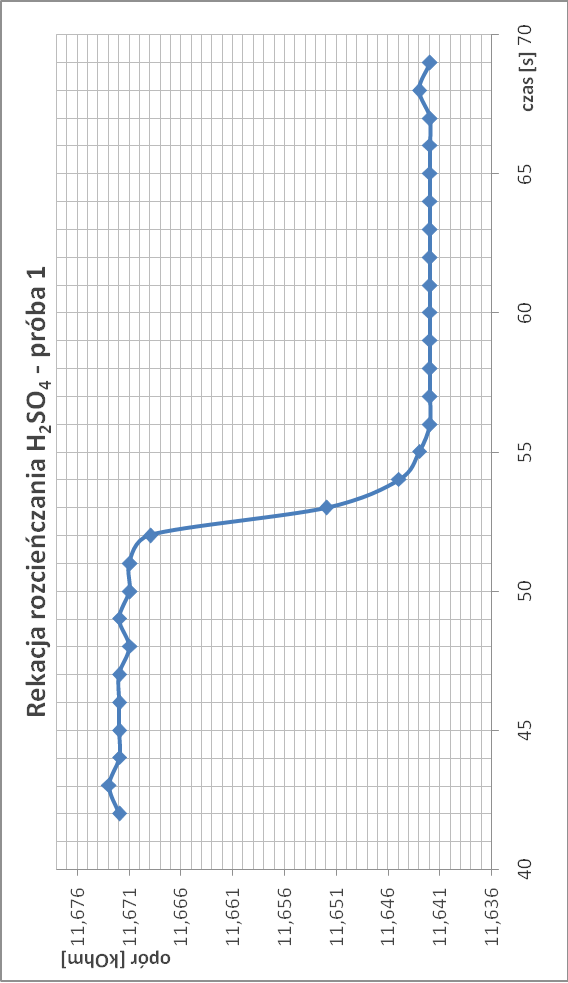
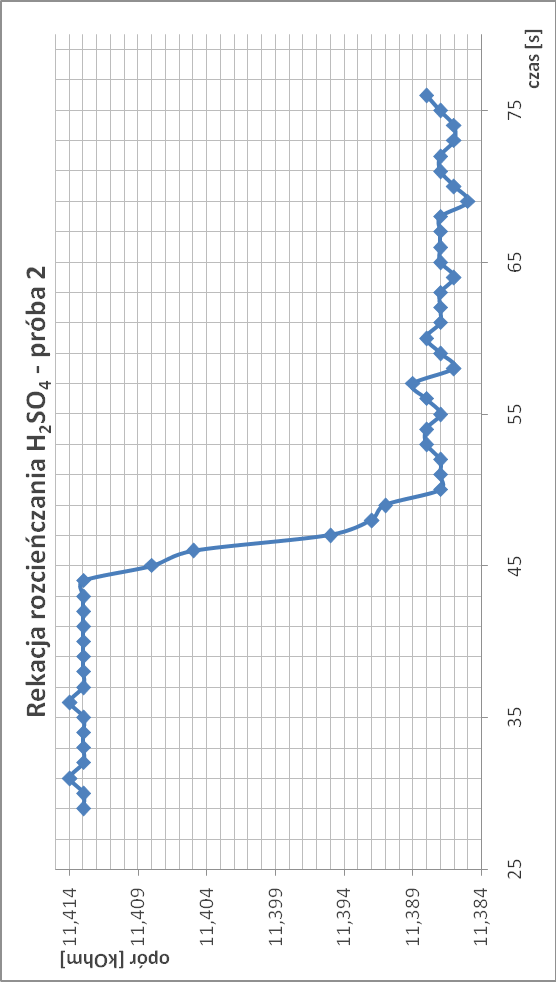
Rozcieńczanie kwasu siarkowego:
∆T1.1 = 1/11,642 - 1/11,671 = 2,13*10-4
∆T1.2 = 1/11,387 - 1/11,413 = 2,00*10-4
∆T1 = 2,065*10-4
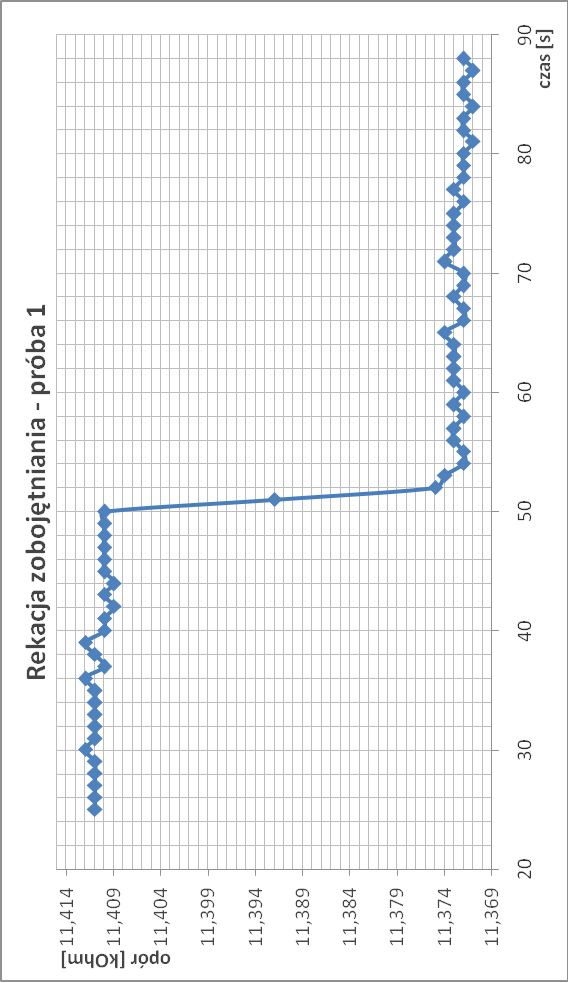
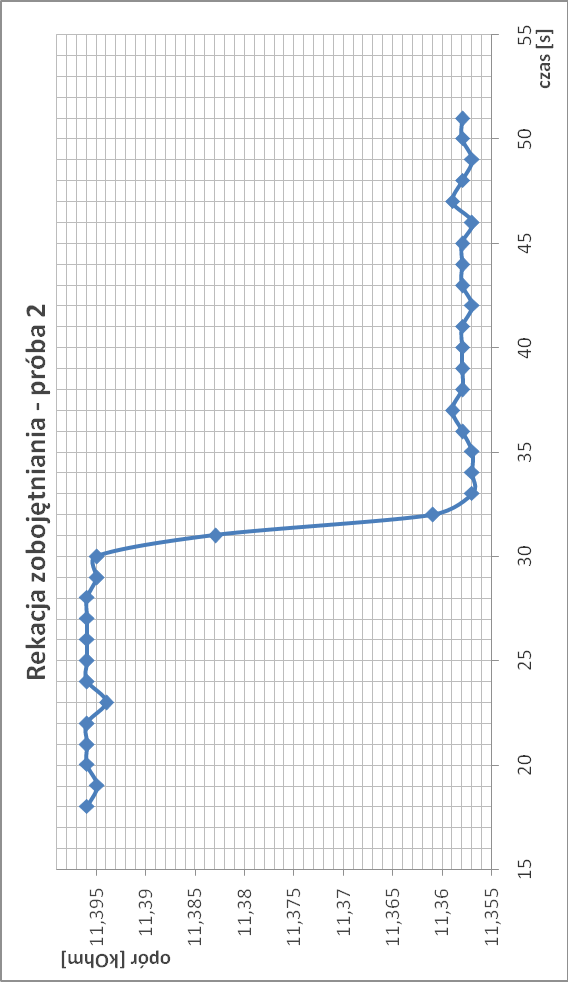
Zobojętnianie zasady sodowej kwasem siarkowym:
∆T2.1 = 1/11,373 - 1/11,411 = 2,93*10-4
∆T2.2 = 1/11,358 - 1,11,396 = 2,94*10-4
∆T2 = 2,935*10-4
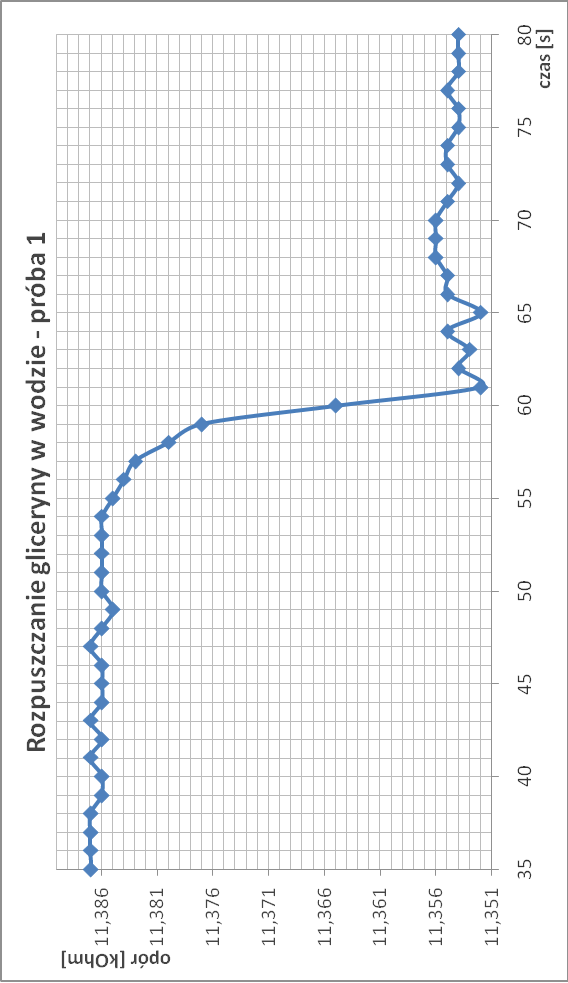
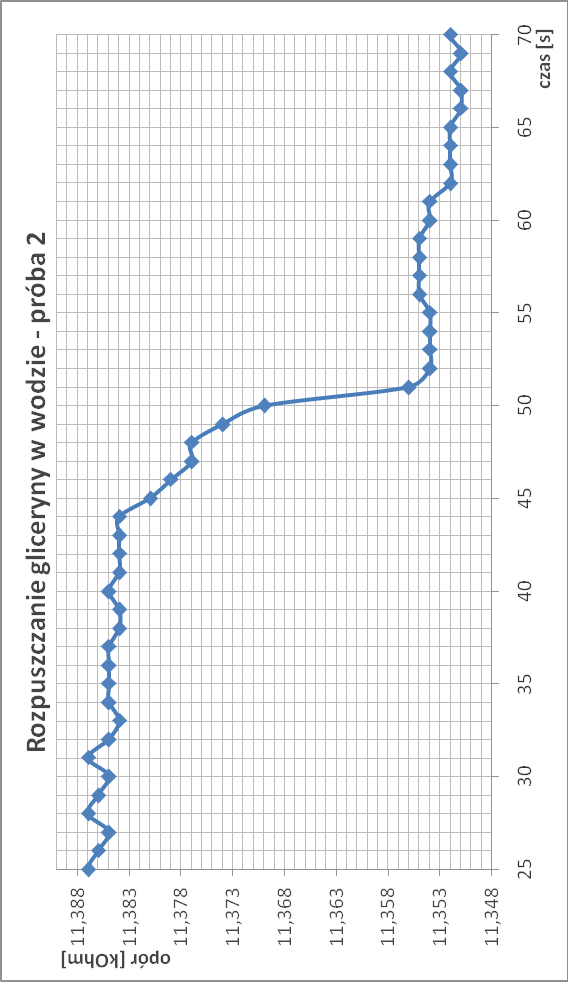
Rozpuszczanie gliceryny w wodzie:
∆T3.1 = 2,50*10-4
∆T3.2 = 2,48*10-4
∆T3 = 2,48*10-4
Obliczanie liczby moli kwasu siarkowego użytego w reakcji zobojętniania:
v = 0,05ml
c = 3mol/dm3
3 mole - 1000ml
x moli - 0,05ml x = 1,5 * 10-4 moli kwasu siarkowego
Obliczanie liczby moli gliceryny:
v = 0,25ml
d = 1,264 g/cm3
Mgliceryny = 92g
1,264g - 1ml
x g - 0,25ml x = 0,316g
1 mol gliceryny - 93g
y moli - 0,316g y = 0,0034moli
Obliczenie molowej entalpii zobojętniania:
∆H1M = ![]()
∆H1M = ![]()
= - 65,12 kJ/mol
Wnioski:
Dla reakcji rozcieńczania kwasu siarkowego oraz zobojętniania zasady kwasem temperatura układów rośnie i ciepło jest oddawane do otoczenia co świadczy że są to reakcje egzotermiczne. Entalpia zobojętnienia z mojego doświadczenia wynosi - 65,12 kJ/mol co jest wynikiem różnym od wartości podanej w skrypcie która wynosi 65,6kJ/mol. Błąd którego nie mogę się doszukać prawdopodobnie występuje w obliczeniach ponieważ wartości są zbliżone do siebie i różnią się tylko znakiem matematycznym.
Wyszukiwarka
Podobne podstrony:
2358
2358
2358
2358
2358
2358
2358
więcej podobnych podstron