skanowanie0033
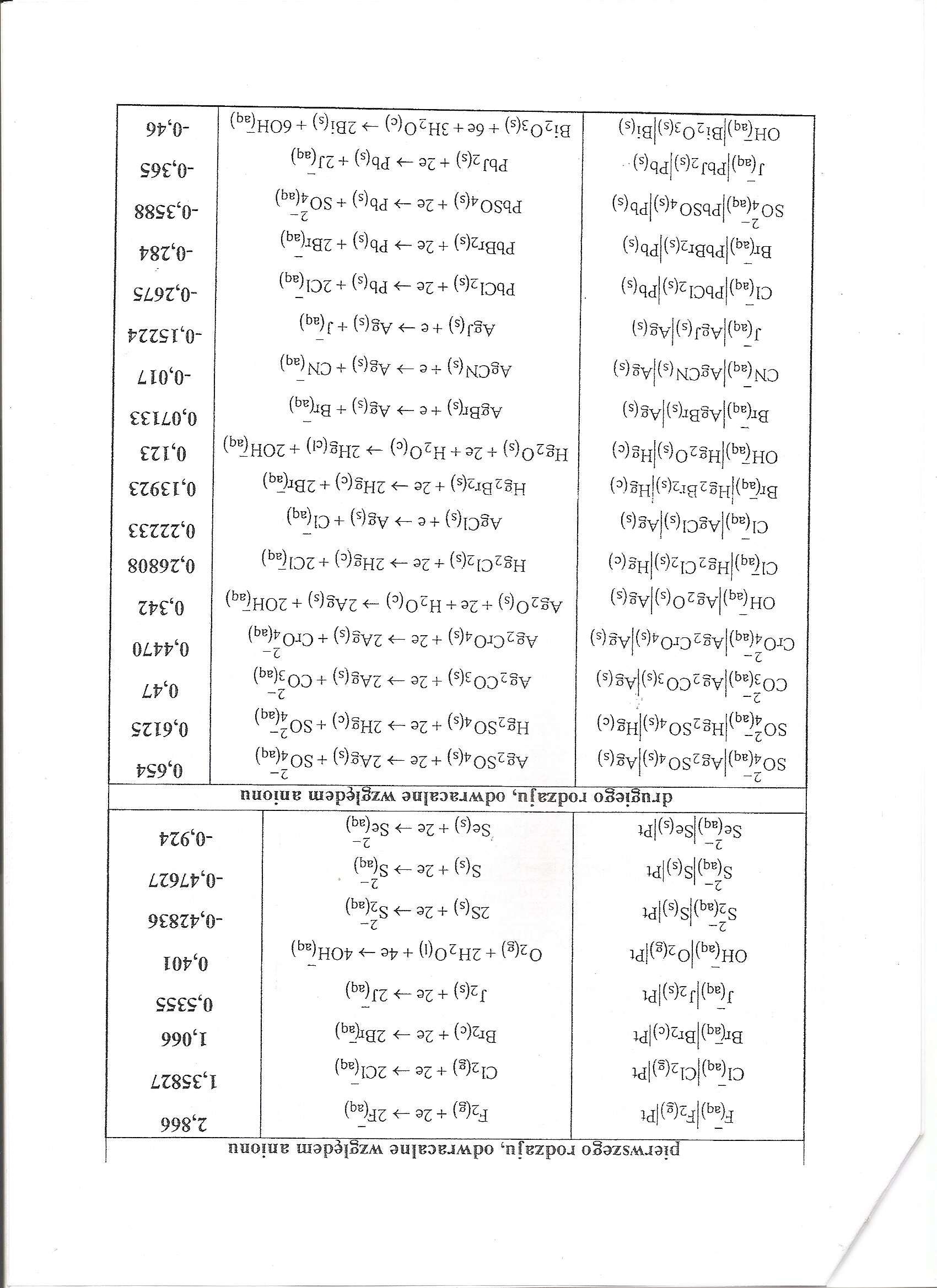
|
pierwszego rodzaju, odwracalne względem anionu 1 | |||
|
F(aq)|F2(g)[Pt |
F2(g)+2e^2F(aq) |
2,866 | |
|
Cl(aq)|d2(g)|Pt |
a2(g) ■+ 2e —> 2Clfaq) |
1,35827 | |
|
®r(aq)|®r2(c)|^ |
Br2(c) + 2e -> 2BĄq) |
1,066 | |
|
J(aq)|J2(s)lPt |
J2(s)+2e->2J(aq) |
0,5355 | |
|
oH(;q)|o2(g)|pt |
°2(g) + 2h20(i) + 4e -> 40H^q) |
0,401 | |
|
S2(aq)jS(s)|Pt S{aq)|S(s)lPt |
2S(s)+2e->S2(aq) S(s) + 2e -> S(aq) |
0,42836 0,47627 | |
|
Se(aq)|Se(s)jPt |
2- S®(s) + —> Se^aqj |
-0,924 | |
|
drugiego rodzaju, odwracalne względem anionu | |||
|
S04(aq)|Ag2S04(s)|Ag(s) |
2- Ag2S04(s) + 2e -»■ 2Ag(s) + SC>4(aq) |
0,654 | |
|
S°4(aq)|H§2S°4(s)|Hg(c) |
Hg2S04(s) + 2e —>■ 2Hg(c) + SO^ |
0,6125 | |
|
C03(aq)|Ag2C03(s)|Ag(s) |
Ag2C03(s) + 2e -» 2Ag(s) + C03(aq) |
0,47 | |
|
Cr04(aq)|Ag2 Cr04(s)|Ag(s) |
2- Ag2Cr04(s) +2e -> 2Ag(s) + Cr04(aq) |
0,4470 | |
|
0H(aq)jAg20(s)jAg(s) |
Ag20(s) + 2e + H20(c) 2Ag(s) + |
0,342 | |
|
Cl(aq)|Hg2Cl2(s)|Hg(c) |
Hg2a2(s) +2e ->• 2Hg(c) +2CJ(aq) |
0,26808 | |
|
Cl (aq)jAgCI (s) j^-S (s) |
AgCl(s) + e -> Ag(s) + Cl(aq) |
0,22233 | |
|
Br(Iq)jHg2Br2(s)|Hg(c) |
Hg2Br2(s) +2e -> 2Hg(c) + 2Br(aq) |
0,13923 | |
|
0Hfaq)jHg20(s)|Hg(c) |
Hg20(s) +2e + H20(c) -> 2Hg(c|) +2OH(aq) |
0,123 | |
|
Br(aq)|AgBr(s)|Ag(s) |
AgBr(s) +e —> Ag(s) + Bq(aq) |
0,07133 | |
|
CN[a^[AgCN(s)jAg(s) |
AgCN(s) + e -> Ag(s) + CN(aq) |
-0,017 | |
|
•J(aq)|AgJ(s)|Ag(s) |
AgJ(s)+e->Ag(s)+i(aq) |
-0,15224 | |
|
cl(aq)|PbCl2(s)|Pb(s) |
^bCł2(s) + 2e —> Pb(s) + 2CI(aq) |
-0,2675 | |
|
Bl(aq)|PbBr2(s)Jpb(s) |
PbBr2(s) + 2e-> Pb(s) + 2Br(aq) |
-0,284 | |
|
S04(aq)|PbS04(s)|pb(s) |
2- PbS04(s) + 2e —> Pb(s) +s04(aq) |
-0,3588 | |
|
J(aq)|PbJ2(s)|Pb(s).- OH(-aq)jBi203(s)|Bi(s) |
PbJ2(s) + 2e —>■ Pb(s) +2J(aq) Bi2°3(s) + 6e + 3H20(c) 2Bi(s) + ÓOH(aq) | |
-0,365 -0,46 | |
Wyszukiwarka
Podobne podstrony:
arkusz2 9 pierwszego rodzaju, odwracalne względem anionu FM
P1020329 • elektrody trzeciego rodzaju, odwracalne względem wspólnego kationu, zbu
23699 skanuj0037 (111) Pod względem typologicznym pierwszy rodzaj zapisu obu głosek (/ i //) należy
Slajd7 (30) Klastry - rodzaje (2/2) Ze względu na rodzaj świadczonych usług, klastry dzieli się na (
skanowanie0013 (102) Praca w terenie Względne zaangażowanie: 1 Względne niezaangażowanie: subiekt
Pierwszy rodzaj aktywności to aktywność fizyczna, nie mająca nic wspólnego aktywnością matematyczną.
S6300979 99 Przykłady Z równości tych wynika, że funkcja g ma w punkcie *o * 2 nieciągłość pierwszeg
str127 (4) § 2. FUNKCJA BESSELA 127 Definicja 3. Funkcją Bessela pierwszego rodzaju o wskaźniku v na
więcej podobnych podstron