netter47
MUSCLE PHYSIOLOGY
Skeletal Muscle: Excitation>Contraction Coupling III
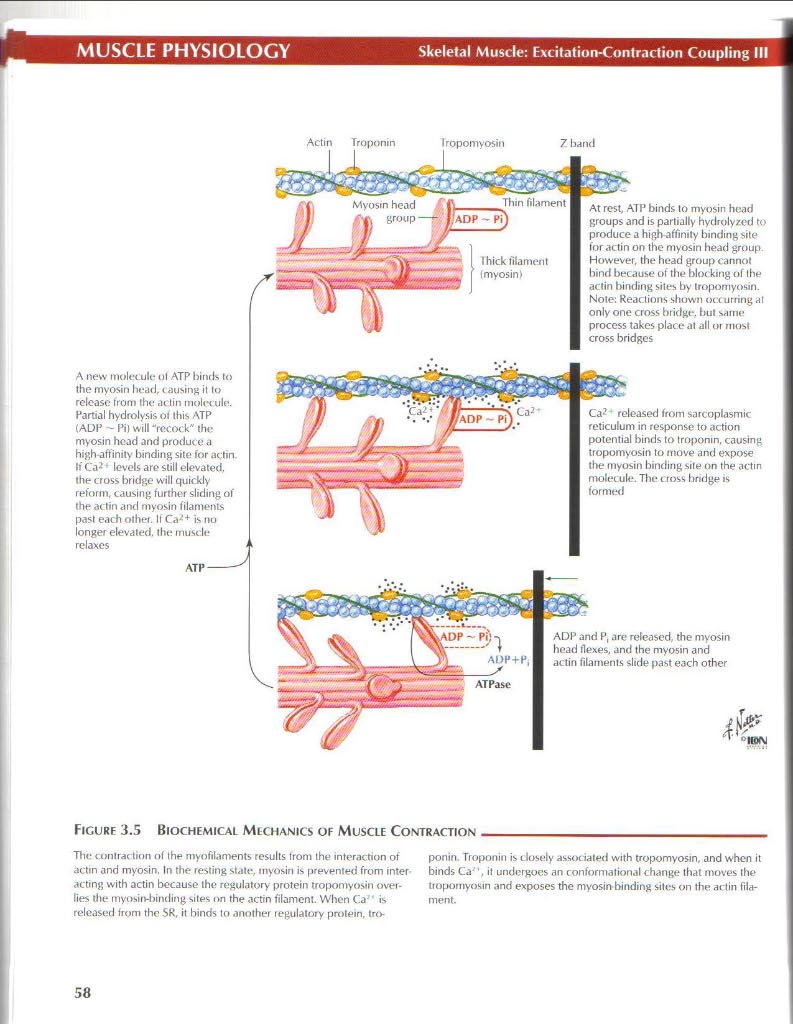
Actin Troponin Tropomyosin
7 band

At rest, ATP binds to myosin head groups and is partially hydrolyzed to producc a high-affinily binding >ite for actin on the myosin head group However, the hoad group cannot bind because ot the biot king of the actin binding sites by tropomyosin. Notę: Reaclions shown oc:curring al only one cross hridge. tiul same process takes place at uli or most cross bridges
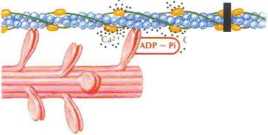
Ca? released from sarcoplasmic reticulum in response to action potential binds to troponin, causing tropomyosin to move and expose the myosin binding site on the actin molecule. The cross bridge is formed
A new molecule ul ATP binds to the myosin head, causing it to release trom the actin inolet ule. Partral hydrolysis ot this ATP (ADP - Pil will "rccock" the myosin head and producc a high-affinity binding site for actin. If Ca2 • levcls are still elevated. the cross bridgc will ąuiddy reform, causing further sliding of the actin and myosin filaments past each ofher. II Ca2+ is no longer elevated. thc muscle relaxes


ADP and P, are released, the myosin head flexes, and the myosin and actin filaments slide past each other
Ficure 3.5 Biochemical Mechanics of Muscle Contraction
The contrac tion of the myofilaments results trom the interaction of actin and myosin. In the resting stale, myosin is prevented from inter acting with actin because the regulatory protein tropomyosin over-lies tlu* myosin-l)inding sites on the actin filament. When Ca'' is released from thc SR, it binds to another regulatory protein, troponin. Troponin is dosely associaled with tropomyosin, and when it binds Ca , it undergocs an conformational change that moves the tropomyosin and exposes the myosin binding sites on the artin fila-ment.
58
Wyszukiwarka
Podobne podstrony:
netter45 MUSCLE PHYSIOLOGYSkeletal Mustlc: Excitation-Contracti»n Coupling I Thin Electric impulse
netter46 MUSCLE PHYSIOLOGYSielrtal Musde: Excitation-Contraction Ciiupling II During musde contracti
53514 netter51 MUSCLE PHYSIOLOGYSmooth Muscle: Excitation-€ontraction Coupling Cuntracliun Cyde Figu
78877 netter48 MUSCLE PHYSIOLOGY>*rietal Muscle: Length-Tension Relationship »ariation in Size o
28771 netter122 EsophagusGASTROINTESTINAL PHYSIOLOGY Window cul in longiludinal muscle Orcular muscu
więcej podobnych podstron