100450
4,280,957
1
IMIDAZODIAZEPINKS AND PROCESSES THEREFOR
This is a continuation. of application Ser. No. 663,660 filed Mar. 4, 1978 now abandoned which is a CIP of Ser. No. 602,691, filed Aug. 7, 1975, now abandoned which is a CIP of Ser. No. 504,924, filed Sept. 11, 1974,
now abandoned.
DESCRIPTION OF THE INVENT!ON
This invention relates to the pharmacologicaHy ac-tivc imidazo[l,S*a] [l,4Jdia2epine compounds series. The Chemical structure of these compounds may bc depicted by the following formula:
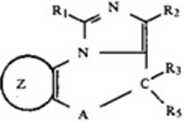
wherein A is
NC»N/';
I
R*
Ri is selected from the group consisting of hydrogen, lower alkyl, hydroay lower alkyl, acyloxy Iower alkyj, phenyl, alkoxy lower alkyl. halo lower alkyl, amino lower alkyl, substituted amino lower alkyl, substituted phenyl, pyridyl, aralkyl and the groups
I
RCO
wherein R is hydrogen or lower alkyl; and ROOC wherc R is lower alkyl; Ri is selected from the group consisting of hydrogen, lower alkyl, hydroxy lower alkyl, acyloxy lower alkyl, alkoxy lower alkyl, halo lower alkyl, amino lower alkyl, amino, cyano. cyano lower alkyl, substituted amino, chloro, bromo or iodo, substituted amino lower alkyl, the group ROOC wherc R is hydrogen or lower alkyl, the grcup
2
(where R, R’ are individually hydrogen, lower alkyl. hydroxy lower alkyl, lower alkcnyl, aryl or together R and R' form a part of a hctcrocyclic ring and the group (CH2)„NRR' where R and R‘ are hydrogen, lower alkyl, hydroxy lower alkyl, lower alkcnyl or together R and R' form a part of a heterocyclic ring and n is 1 to 4); the group
I ^R"
—connC
^R,J
where R10, R11, and RI2arc hydrogen arc hydrogen or lower alkyl and the group (CH^NR^r*4 where n is 1 to 4 and R:3, R14 arc hydrogen, lower alkyl, hydroxy lower alkyl, lower alkcnyl or R,3and R14 together form part of a heterocyclic ring, with the limitalion that where R10, RH or R12 is a basie sidc chain, then the remaining substituents arc hydrogen or lower alkyl; Rj is selected frora the group consisting of hydrogen and lower alkyl; Rj is selected from the group consisting of alkanoyloxy. hydroxy or hydrogen; R4 is selected from the group consisting of hydrogen, halogen, nitro, cyano, trifluoromethyl, lower alkyl. substituted amino, amino, hydroxy lower alkyl and lower alkanoyl; Rt is selected from the group consisting of phenyl, mono-substituted phenyl, di-substituted phenyl, pyridyl and mono-subslituted pyridyl; and
€C
is selected from the group consisting of
OC.TJC ^
wherein X it chłonne broroinc. iodine or hydrogen
T

wherein X n chlo- wherofl T
fine, brcmine. » hydrogen
iodine or or lower
hydrogen »lkyl
I
RCO
where R is hydrogen or lower alkyl, or derivatives thereof, i.e.. the group
I
R—C=N-R'
wherein R' is hydrogen, lower alkyl, hydroxy, phenyl, alkoxy, amino, mono or di-alkylamino and arylamino and R is hydrogen or lower alkyl, the group
-CON^
R’
and the pharmaccutically acceptable salts thereof.
Various analogous compounds derived from the above compounds, together with various novcl inter-mediates leading to the abovc compounds, are also considcrcd to be within the scopc of the invcntion and exhibit pharmacological activity per se or arc useful intermediates to pharmacologically aclivc compounds.
Analogs of the above compounds which form a part of this invention include compounds of the formula
Wyszukiwarka
Podobne podstrony:
image002 “There has never been a collection like this before” James Blish. . . and there hasn’t. Thi
hit and run Assassination! This is an Instant Attack to Destroy any Personality, at any time. It doe
Name: Number: Datę: 1- Match each picturc to its namc and paint thcm. This is a pink dress. This is
31667 WELCOME KIDS 2 ANGIELSKI ZESZYT ĆWICZEŃ 05 O Look, read and match. 1 Oscar
ape 049 •19 THE SHOULDEK. Front the front horizonlal, swing arms to position shown. and return. This
Project objectives Please, notice that this is a summary of the whole project and the scope of PHD s
jff 026 USING OPPONENT S STRENGTH This is a demonstration of another important prin-ciple underlyin
node list Latest NewsNews _ No news is TTJHB uood news. fl
International Studenta and Visitors Office (ISVO) is a part of International Relations Office of Gda
132 T. Wilgat et al. cesspools and primitive toilets are a threat to groundwaters and the lakes. The
PG009 18 Grammar This is my luggage. korę w a watakushi no nimotsu desu This wal n
2. Pm sorry, but that’s out of the ąuestion. Na pewno nie będzie: This is out of the question 6. Add
screenshot 3 <7—wp-html-compression no compression—> This is a bunch of content that will not
Replica. This is a copy of a cluster partition. Each node in a node group Stores a replica. Also som
A PRESENTS CHRISTMAS Admit it -this is one of the most disturbing things youVe ever seen.
CSG312 Unit 2 5Interrogative and Mlainaton WordsInterrogative Words and Expressions Asking questions
więcej podobnych podstron