3685665732
WYKŁADY PLENARNE I ZAPROSZONE
CONQUERING ONE OF THE LAST BASTIONS IN ORGANIC SYNTHESES — CATALYTIC ASYMMETRIC C-C BOND FORMATION IN >99% EE VIA ZACA-PD OR CU-CATALYZED CROSS-COUPLING
Ei-ichi Negishi*. Shiqing Xu, Akimichi Oda, and Yohei Matsueda
H. C. Brown Laboratories of Chemistry, Purdue University, West Lafayette, IN, USA E-mail: negishi@purdue.edu_
Zr-catalyzed asymmetric carboalumination of alkenes (ZACA reaction) is a catalytic asymmetric C-C bond forming reaction, which has been used to significantly modernize and improve syntheses of natural products including deoxypolypropionates, isoprenoids, and other heterocyclic compounds of biological and medicinal interest.1'1 Some key features of the ZACA reaction include (i) catalytic asymmetric C-C bond formation, (ii) use of alkene substances of one-point-binding without requiring any other functional groups and, (iii) many potential transformations of the initially formed alkylalane intermediates.
Here we will present the recent advances in ZACA reaction for highly enantioselective syntheses of various /?- and more-remotely chiral 1-alkanols of >99% ee.121 ZACA-in situ iodinolysis of allyl alcohol and ZACA-in situ oxidation of TBS-protected co-alkene-l-ols protocols were applied to synthesis of both (R)- and (S)-difunctional intermediates 1 and 3 in 80-90% ee, which were further purifted to provide enantiomerically pure (>99% ee) compounds 1-4 by lipase-catalyzed acetylation. These functionally rich intermediates serve as very useful synthons for the construction of a broad array of chiral compounds including those of isotopomers in excellent enantiomeric purity (>99% ee) by introducing various carbon groups via Cu- or Pd-catalyzed cross-coupling without epimerization.
|
(1)(-)*ZACA (SI"1’ i93* |
, |
|
RŁA^oh | |
|
>99% ee (B), hydrolysis |
'>99%ee |
|
MMbJ | |
Scheme 1. Strategy for the synthesis of 2-alkyl-1-alkanols of of >99% ee.
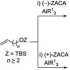
R^I^^^OZJ
|
) Oj |
<ii,=™m,,Z6d |
Rl^on | |
|
i( ImM “on |
(R|-3, >99% ee |
fili) TBAF desilylalion |
R^ai-^A^-OZ
|
AcO^J^OZ |
asr.™. | |
|
(SH. *99% «9 |
>99% ee |
of >99% ee.
Scheme 2. Strategy for the synthesis of y-
[2] (a) Xu, S.; Lee, C.-T.; Wang, G.; Negishi, E. Chem. Asian J. 2013, 8, 1829. (b) Xu, S.; Oda, A.; Kamada, H.; Negishi, E. Proc. Natl. Acad. Sci. USA 2014, 111,8368.
54
(a) Kondakov, D. Y.; Negishi, E. J. Am. Chem. Soc. 1995, 117, 10771. (b) Kondakov, D. Y.; Negishi, E. J. Am. Chem. Soc. 1996, 118, 1577. (c) Negishi, E. Angew. Chem. Int. Ed. 2011, 50,6738. (d) Xu, S.; Negishi, E. Heterocycles 2014, 88, 845.
Wyszukiwarka
Podobne podstrony:
calibre cover BEYOND THE PALE BOOK ONE OF THE LAST RUNĘ
BEYOND THE PALE BOOK ONE OF THE LAST RUNĘ MARK ANTHONY ©MMIJjlf tiRflO
image002 ROGER ZELA2NY WAS ONE OF THE BRłGHTEST UGHTS IN SCIENCE FłCTION AND FANTASY...FANS OF HIS
ABOUT THE AUTHOR Carlton Mellick III is one of the leading authors in the new Bizarro genre uprising
The town has many historie features, including: • One of the largest marketplaces
Nadachowski A., Madeyska T., 1995. Evolution of vertebrate and plant communities of the Last Glacłat
Moreover, one of the hijras’ favorite in-group insults is the term bhosri vald Vagina-owner’, an epi
ABOUT THE AUTHOR Carlton Mellick III is one of the lcading authors in thc ncw Bizarro genre uprising
The first reaction (1.) involved blocking one of the reactive positions in 2,2’-bithiophene via intr
O THE TURĘ Irwestments include three fields of activity and are considered as one of the most s
Our environment. Ali of us realize, that contamination of the eiwironment is one of the main problem
The Nobel Prize in Chemistry 2005"for the development of the metathesis method in organie
WYKŁADY PLENARNE I ZAPROSZONESP-WZ1 MACROMOLECULAR CRYSTALLOGRAPHY IN THE YEAR OF CRYSTALLOGRAPHY Ma
więcej podobnych podstron