
Transport proteins control
Ion
In
Out
Potassium
140
mM
1 – 4.5
mM
Sodium
5 – 15
mM
145
mM
Magnesium
5
mM
1 – 2
mM
Calcium
>
0.5
M
2.5 – 5
mM
Chloride
4
mM
110
mM
Ionic composition of
intracellular fluid
osmolarity
Cell volume
Intracellular pH
Membrane potential
Ions gradients
Exchange of molecules
Passive flux down the gradient of chemical potential
The chemical potential is the
free energy per mole of
compound transported.
i
n
n
p
T
i
i
j
n
G
,
,
i
i
i
dn
dG
µ
0
, c
o
are the chemical potential and
concentration (1M) under standard
conditions.
]
[
]
[
ln
0
0
c
c
RT
A potential difference across a biological
membrane:
~
70 mV
The voltage gradient is
20,000,000
V/m
.
For e charged solute the electrochemical
potential is defined
zeF
c
c
RT
dn
dG
]
[
]
[
ln
~
0
0
o
and μ
o
are
for the standard
state.
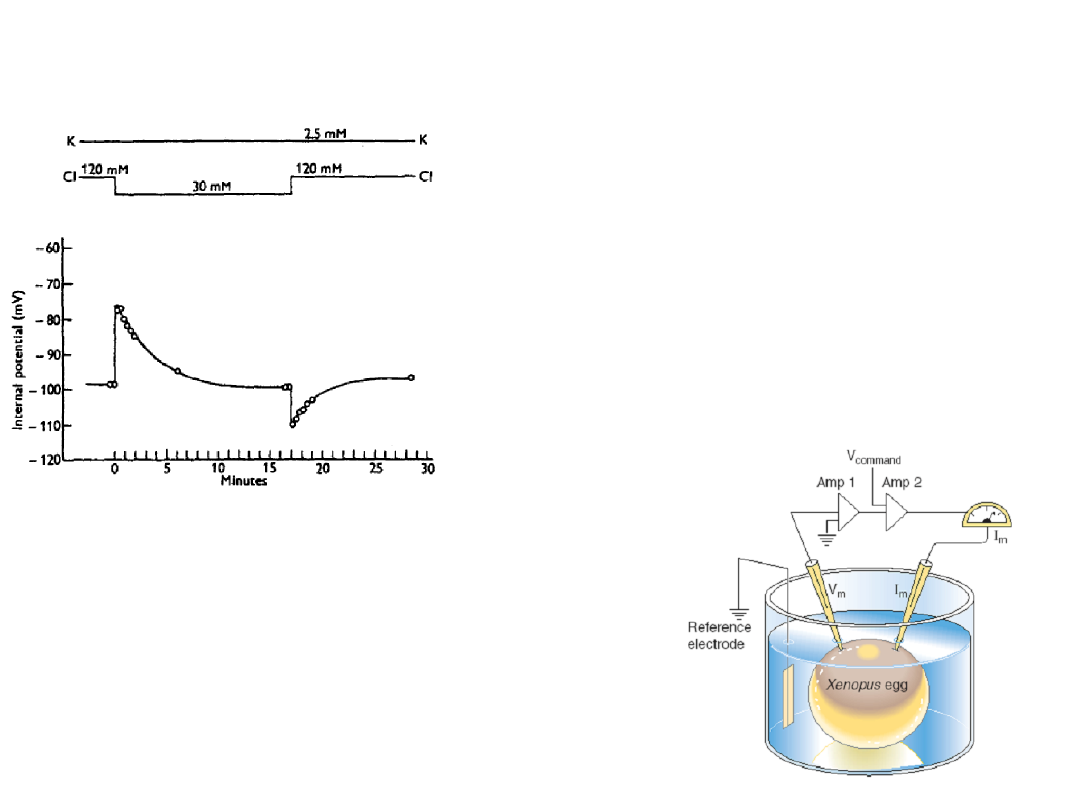
The effect of changes in
external chloride ion
concentration on the
membrane potential of an
isolated frog muscle fibre
(Hodgkin & Horowicz, 1959)
One electrode monitors
membrane potential (V
m
)
and the other passes enough
current (I
m
) through the
membrane to clamp V
m
to a
predetermined command
voltage (V
command
).
Something controls the membrane potential
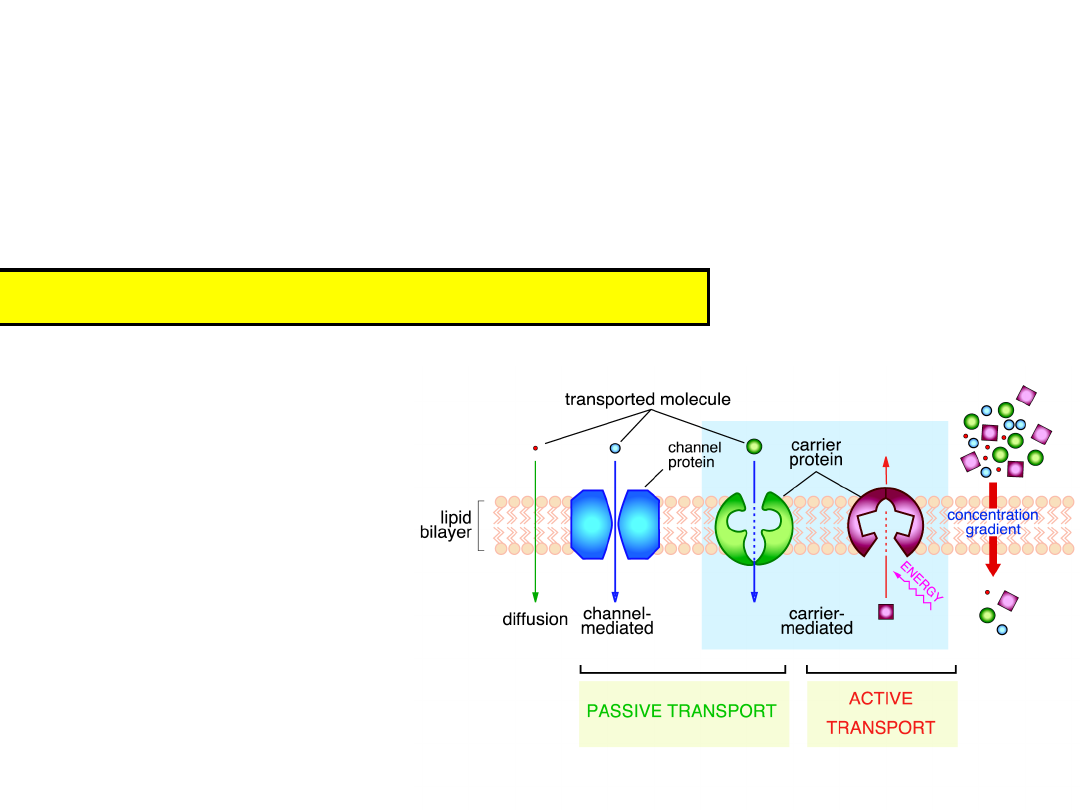
Movement of molecules across cell
membranes
3)
Facilitated diffusion (10
2
-10
4
ions/sec)
4) Active transport (1-1000 ions/sec)
5)
Bulk transport
A)
Exocytosis
B)
Endocytosis
1)
Diffusion through bilayer
2)
Difusion through a pore (10
7
-10
8
ions/sec)

Two requirements of membrane transport
Energy to move
substances
Route through the membrane
the lipid bilayer – nonspecific
facilitated by proteins –
specific
1.
Light
- powers
H
+
pumping
- bacteriorhodopsin
proteins undergo alternating cycles of
oxidation/reduction, which powers
H
+
pumping
2.
Electron transfer
(substrate oxidation)
during metabolism, electrons are passed along
the electron transport chain
3.
ATP
- large class of ATP-driven ion transporters.
Direct coupling of metabolism
to the transport process.
Inhibited by metabolic inhibitors such
as cyanide and dinitrophenol.
Active
transport
S
1
S
2
ATP
ADP + Pi
Side 1
Side 2
Active
Transport
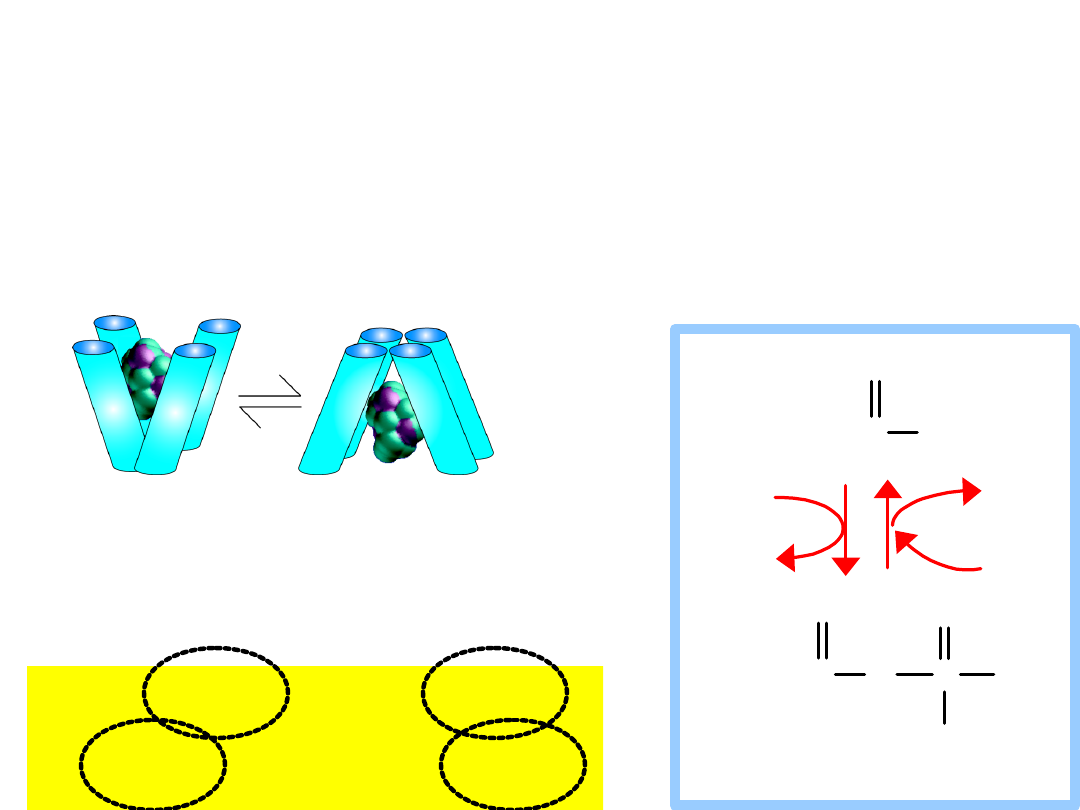
ATP activates the protein by giving up a
phosphate
Transport protein must be activated
Primery active transport
(direct energy utilizing active transport)
Na
+
/K
+
-ATPase Ca
2+
-
ATPase
H
+
-ATPase
H
+
/K
+
-
ATPase
Binding of ATP changes protein shape and affinity
for solute
(a gene family exhibiting
sequence homology)
P-Class Pumps
ATP
C
O
O
P
O-
O-
O
C
O
OH
ADP
Enzyme-
Enzyme-
P
i
H
2
O
P-class ion pumps
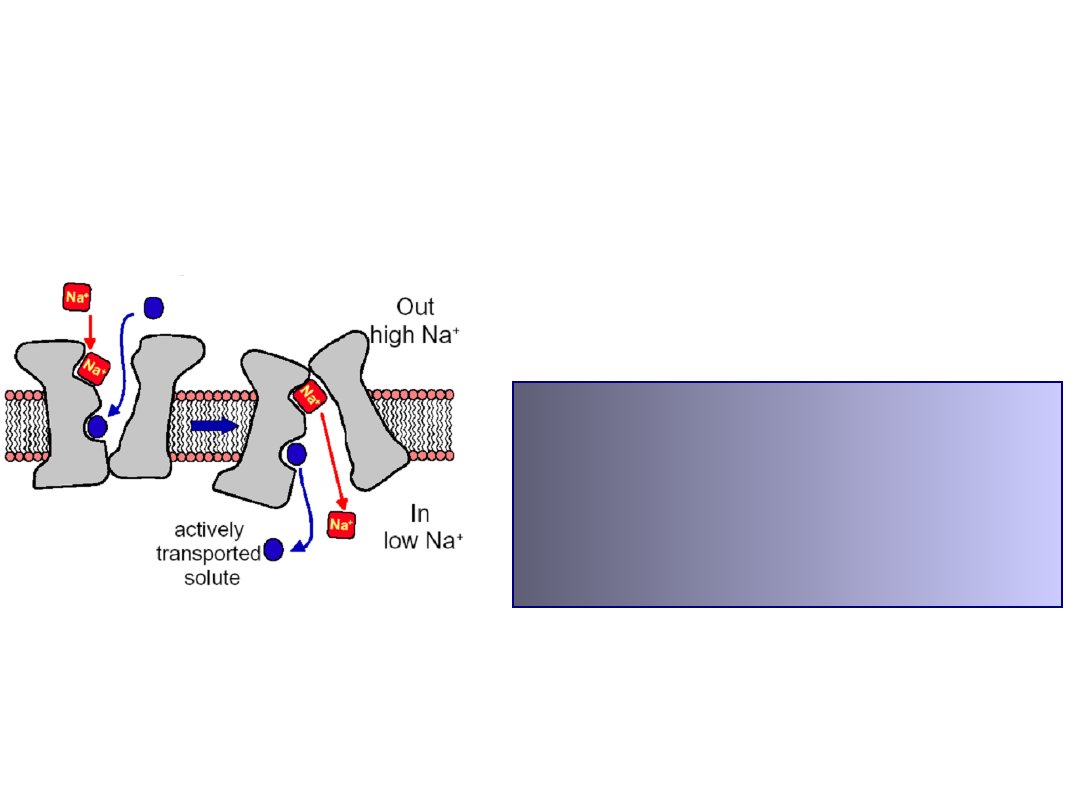
Secondary Active Transport
(Coupled Transport)
Driven by chemical or electrochemical gradients
Expenditure of metabolic energy is
INDIRECTLY coupled to
translocation.
Uniport
– transport of a single solute driven only by ΔΨ
B
z
S
S
Z
O
Z
I
]
[
]
[
log
10
2.3RT/F = 59 mV
B at
25
0
C
Nerst equation
S
+z
Et the equilibrium
0
~
~
Z
Z
S
H
S
n
G
n is the number of moles of H
+
that would
have to move down the
~
J
gradient to
generate the accumulation.
pH
RT
F
H
3
.
2
~
zF
S
S
RT
Z
O
Z
I
S
Z
]
[
]
[
log
3
.
2
~
10
0
)
3
.
2
(
]
[
]
[
log
3
.
2
~
~
10
pH
RT
F
n
zF
S
S
RT
G
Z
O
Z
I
H
S
S
Z
Z
B
z
n
pH
n
S
S
Z
O
Z
I
)
(
]
[
]
[
log
10
2.3RT/F = 59 mV
B at
25
0
C
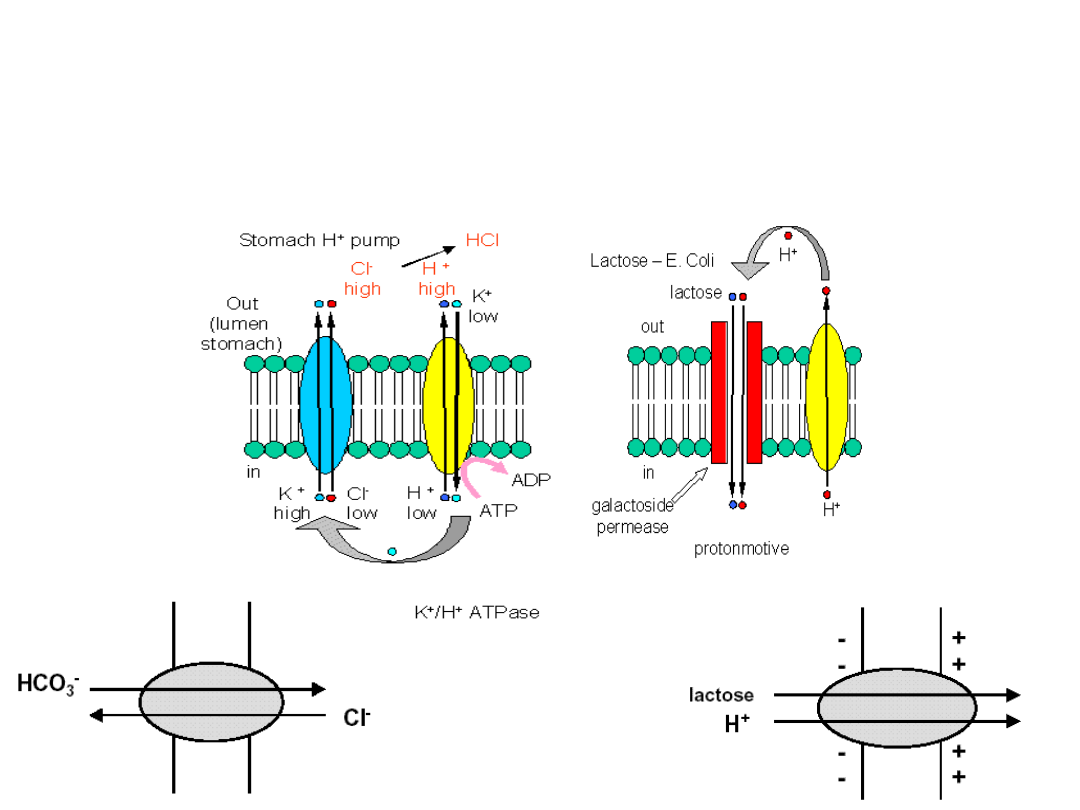
Symport
(cotransport) amino acids
and sugers
S
+z
H
+
Antiport
(countertransport)
restricted to ions
If n = z, then the charge
movement would be neutral and
has no effect.
pH
n
S
S
Z
O
Z
I
10
log
Combining
~
H
and
~
S+Z
pH
n
Z
z
n
S
S
Z
O
Z
I
)
(
log
10
n is the number of moles of
H
+
that would have to move
againsty the
~
J
gradient
to
generate
the
accumulation.
zF
S
S
RT
Z
I
Z
S
Z
0
10
log
3
.
2
~
pH
RT
F
H
3
.
2
~
S
+z
H
+
The consequence of the
transfer of charged
malecules
Electrogenic
Electroneutral
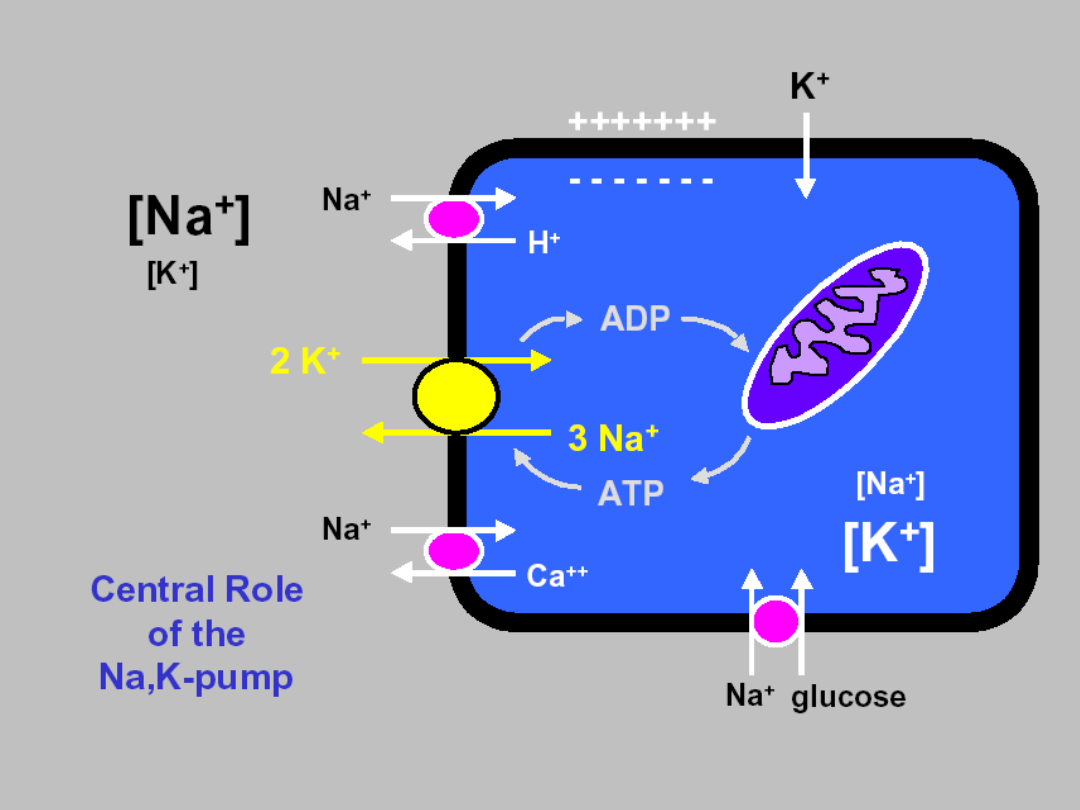
Master pump !!!

The master pump concept
Creates transmembrane gradient of a
selected ion.
The electrochemical potential energy is
stored only across the membrane in which
the pump is located.
Other ions and molecules are transported
across the membrane by coupling their
movement to the movement of the selected
ion.
Ion gradients generally store smaller
packets of energy than ATP - coupled
transporters (increased efficiency).
Coupling transport to a single master pump
serve a
control function
.

Atributes of a master pump
High efficiency
Low dissipation
(leakage current)
is
the reason that pumps almost exclusively
transport
the
relatively
impermeant
inorganic cations.
High capacity
– the ion gradient involve
concentrations that are relatively large
compared to the concentrations of the
compounds that are to be transported.
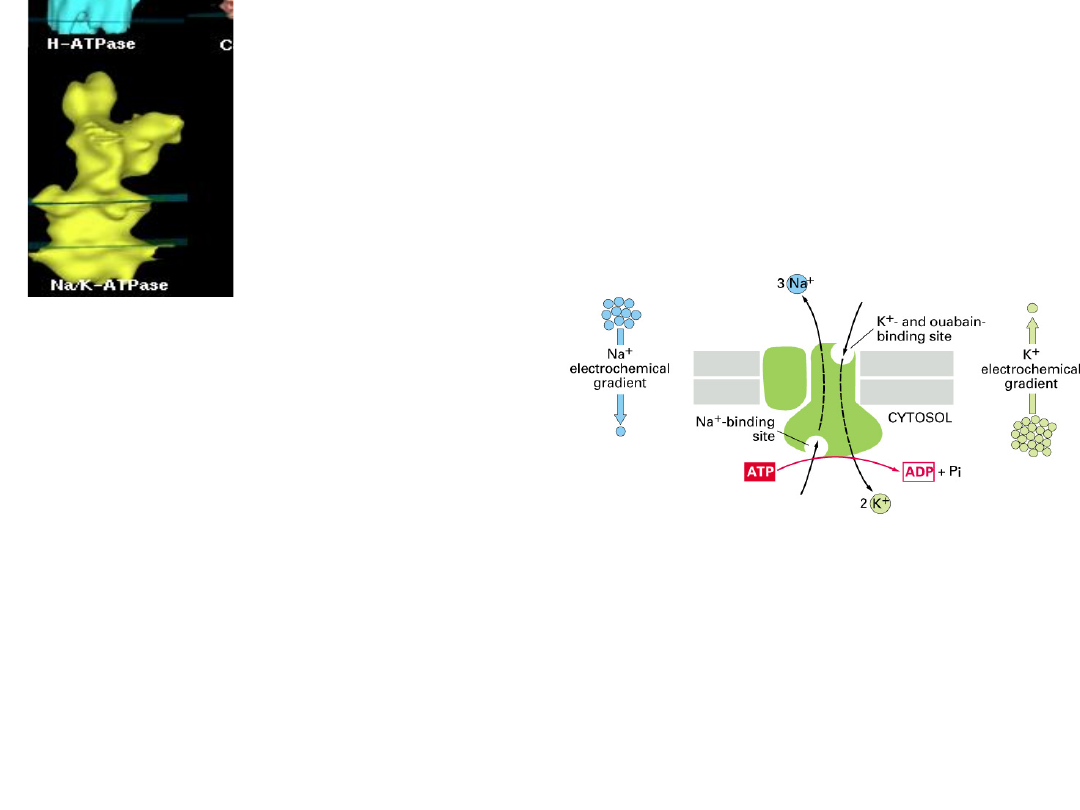
Cytosol
David Stokes,
Univ. Virginia
Na
+
,K
+
-ATPase
Abundance reflects
importance
– Erythrocyte = 20-30 copies
– Heart cell or neuron > 100,000
copies
Substrates
– 1 ATP
(intracellular)
– 3 Na
+
per cycle
- obligatory, no other ion can
substitute.
– 2 K
+
per cycle
- an extracellular cation is
obligatory, but K
+
and Rb
+
both work well.
– Other monovalent ions have finite but low
activity (NH
4
+
> Cs
+
> Li
+
)
Pump Activity is Electrogenic
•
Maintenance of high intracellular K
+
needed
for optimal intracellular enzyme activity.
Na
+
,K
+
-ATPase Functions
•
Maintenece of osmotic stability and cell volume.
•
Restoration of potentials in
excitable cells.
•
Generates anergy for transport in the form of Na
+
gradient.
•
Generation of heat
20% of body heat
in mammals is from
the basal activity of
Na
+
,K
+
-ATPase.
> 30% of metabolic
energy in resting
mammals is consumed
by Na
+
,K
+
-ATPase.
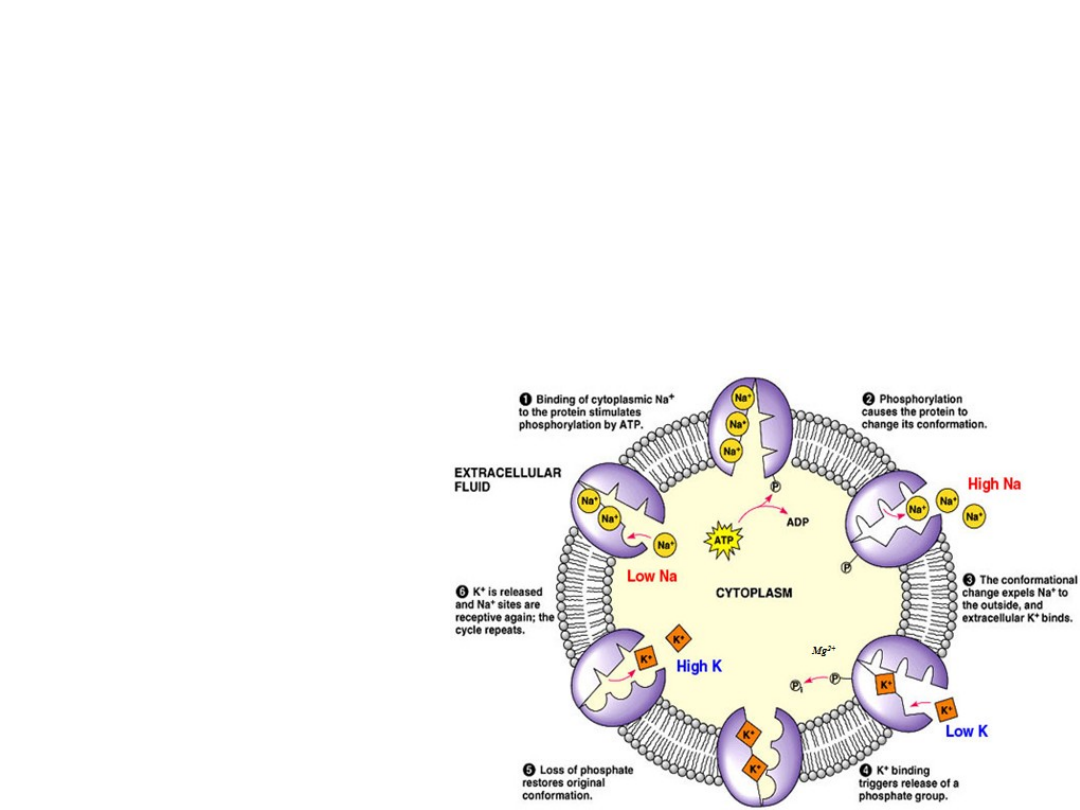
Na
+
and K
+
bind to separate sites.
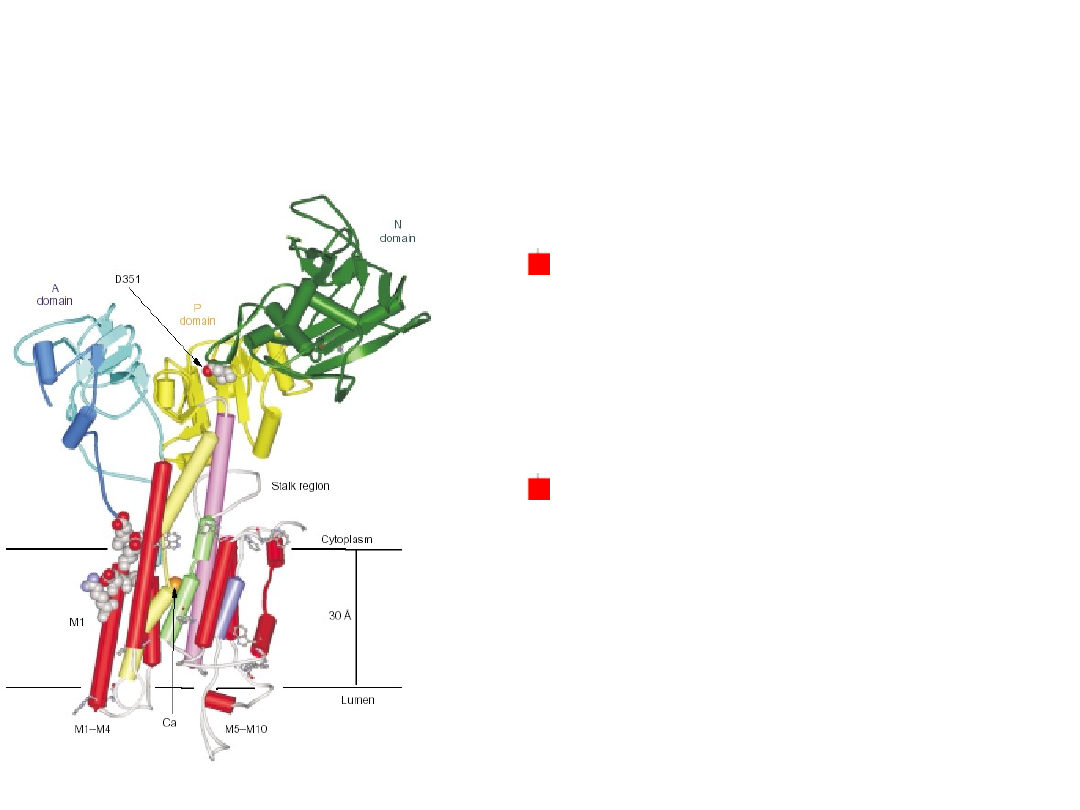
When the transported
substrate
serves
a
regulatory function, then it
may be desirable to control
its
concentration
separately.
A transport system might not be
coupled to the master pump
When the transport
system has a high capacity
itself, it may adversely
affect the ion gradients
established by the master
pump.
Ca
2+
-ATPase
Integration of a transport systems !!!
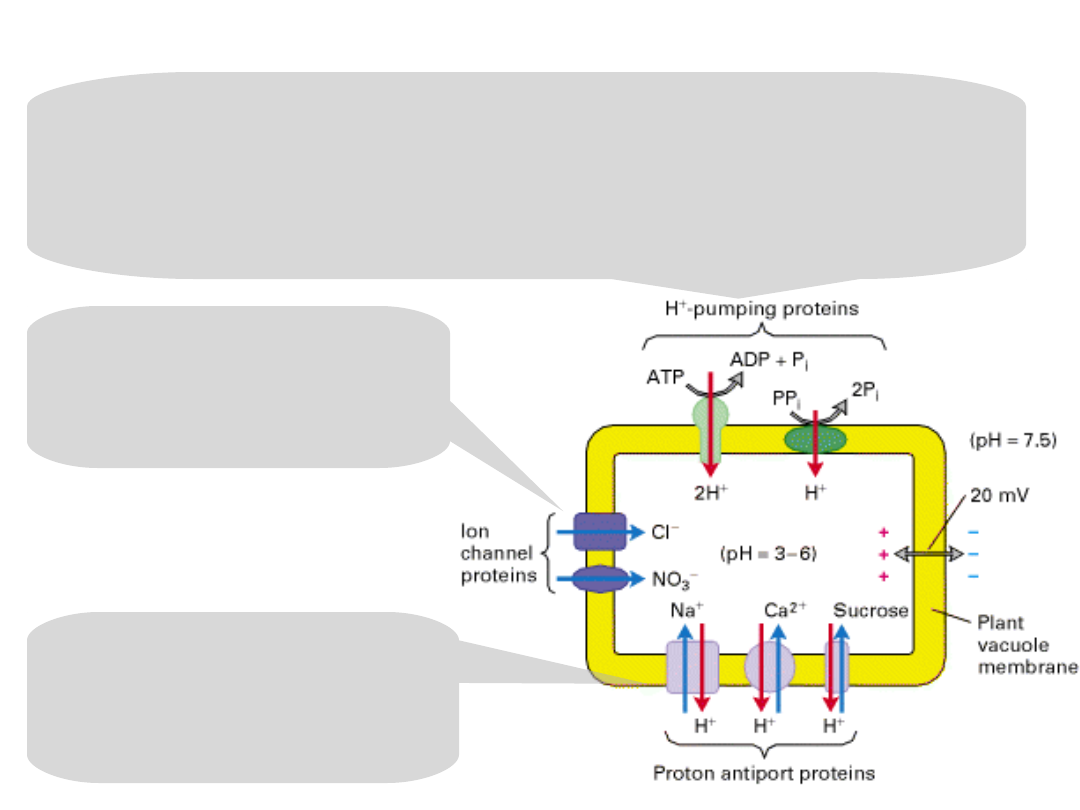
Accumulation of ions and sucrose in the plant vacuole.
Two types of proton pumps:
V-class H
+
ATPase
a pyrophosphate-hydrolyzing proton pump
They generate a lowered luminal pH and an inside-
positive electric potential – the inward pumping of
H
+
ions.
The inside-positive
potential powers the
movement of Cl
−
and
NO
3
−
from the cytosol
through separate
channels.
Proton antiporters,
powered by the H
+
gradient, accumulate
Na
+
, Ca
2+
, and sucrose
inside the vacuole.
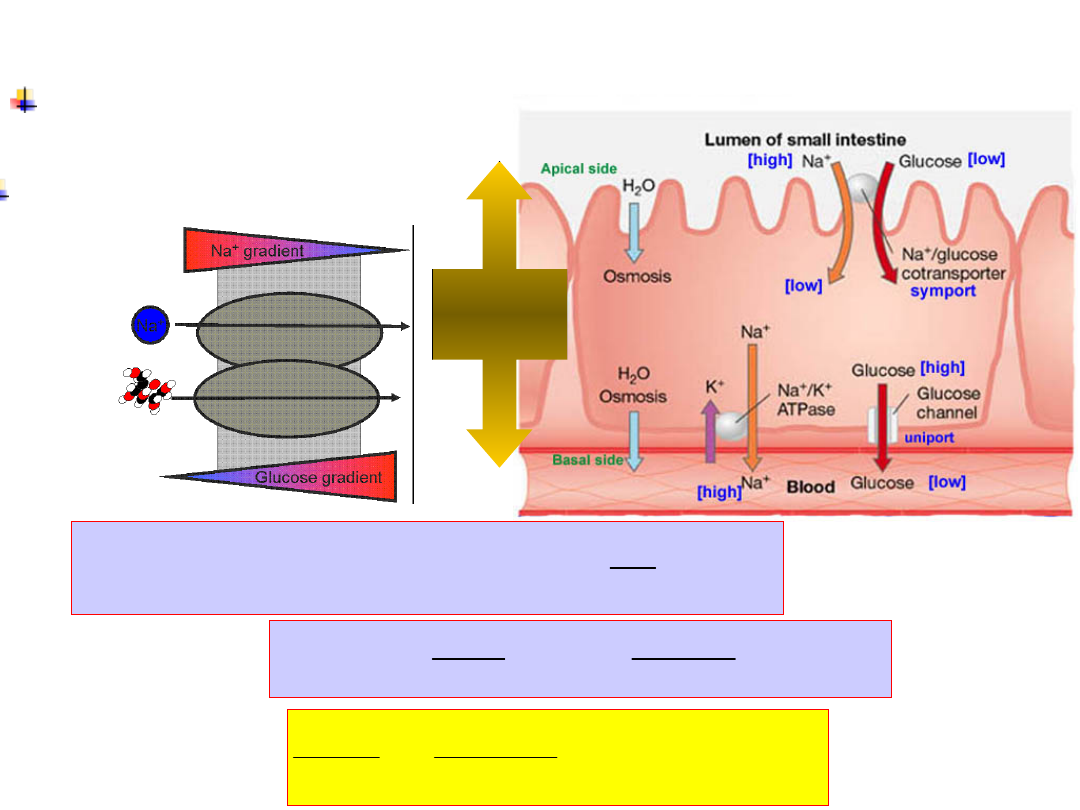
Na
+
/glucose
cotransporter
Glucose
transport
requires Na
+
gradient
Coupling is 2:1
i
m
i
out
in
i
i
out
i
in
i
i
i
i
i
F
z
C
C
RT
n
n
n
G
ln
)
(
At
equilibrium:
0
]
[
]
[
ln
]
[
]
[
ln
m
out
in
out
in
F
Na
Na
RT
G
G
RT
G
)
/
exp(
]
[
]
[
]
[
]
[
RT
F
Na
Na
G
G
m
in
out
out
in
High
C[glucose]
Low
Low
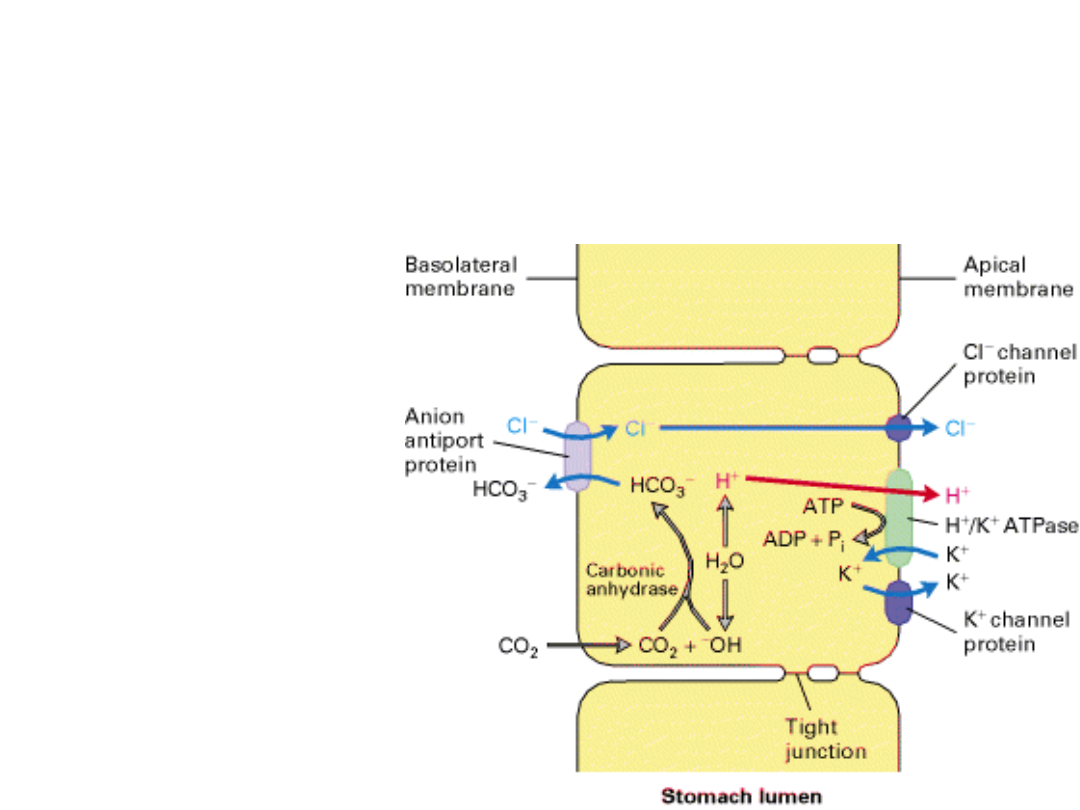
The neutral
pH and
electroneutrali
ty of the
cytosol is
continuously
maintained.
Acidification of the
stomach lumen
The role of H
+
/K
+
ATPase
This is the largest
concentration
gradient across a
membrane in
eukaryotic
organisms!
Ion channels are
enzymes that catalyze
the flow of ions across
cell membranes
causing picoamp
current.
The catalytic rate is on the order of 10
7
per second.
ions/s
10
C
10
1.6
ion
1
s
C
10
A
10
1pA
7
19
12
12
How much is a picoamp of current?
Channels
(
gated pore)
secondary active transport
Counter-transport
:
Na
+
/H
+
,
HCO
3
/Cl
,
K
+
/H
+
,
Ca
2+
/H
+
, Na
+
/Ca
2+
Co-transport:
Na
+
/glucose, Na
+
/amino acid,
Na
+
/K
+
/Cl
-
•
Cystic
fibrosis
•
Epilepsy
•
Diabetes
•
Migraines
•
Neuro-toxins
Channels
malfuncti
on

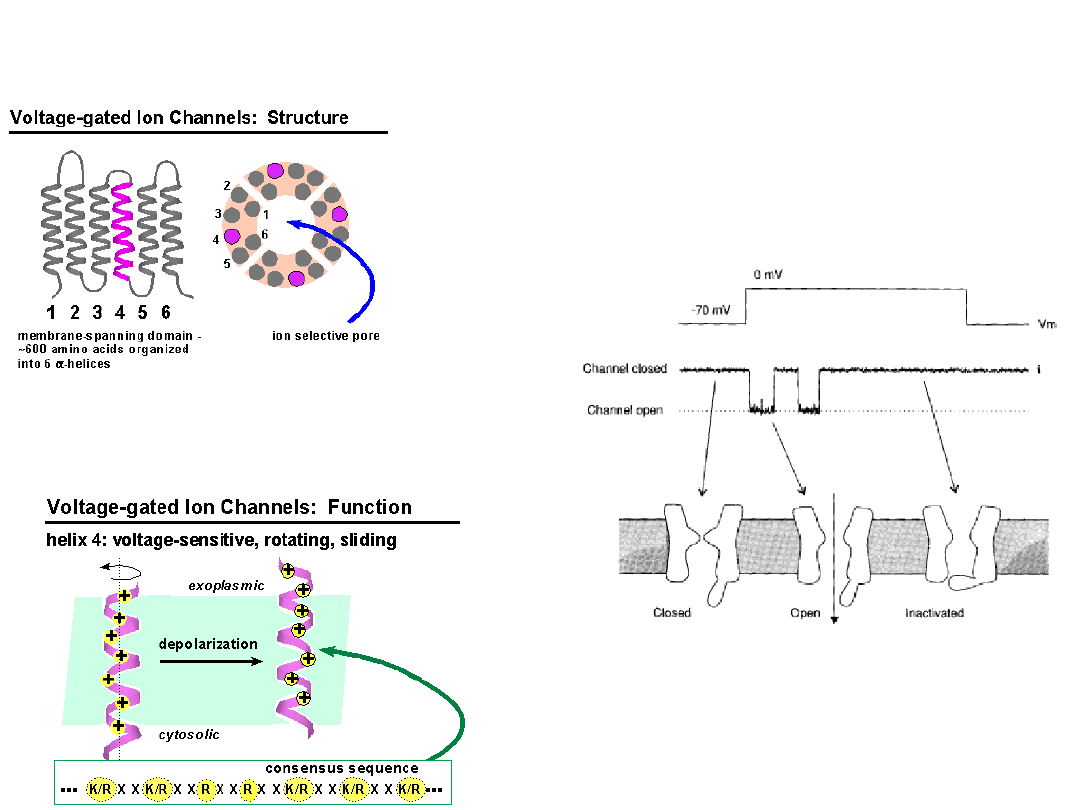
Properties of Ion Channels
Membrane-spanning protein
Hydrophilic ion conductive pathway
water-filled
traversing ion must lose hydration shell
Gating
Mechanical gating
(MscL)
Voltage gated channels
Ligand-gated channels
Both voltage and ligand gating
Selective
size
charge
charge distribution
Voltage Gated Sodium
Channel
Voltage-dependent
gating
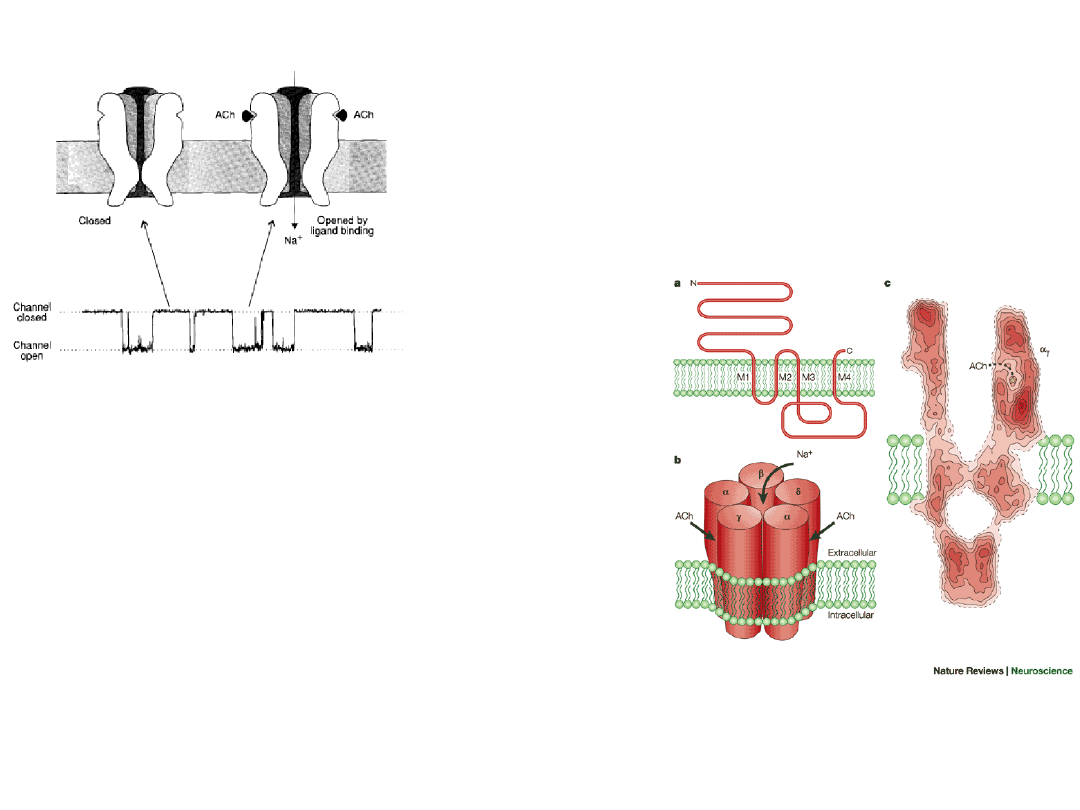
Receives acetyl choline
released from the
presynaptic cleft and
reinitiates an action
potential by allowing Na
+
and K
+
ions to pass through
the channel
Ligand-gated channel –
acetyl choline
gated channel
1.
Five membrane spanning
subunits [
2
] all similar.
2.
An allosteric protein (three
conformations; open, closed, and
inactive).
3.
Acetylcholine binding promotes opening the closed channel
4.
Open channels allow Na
+
but not
Cl
–
to pass.
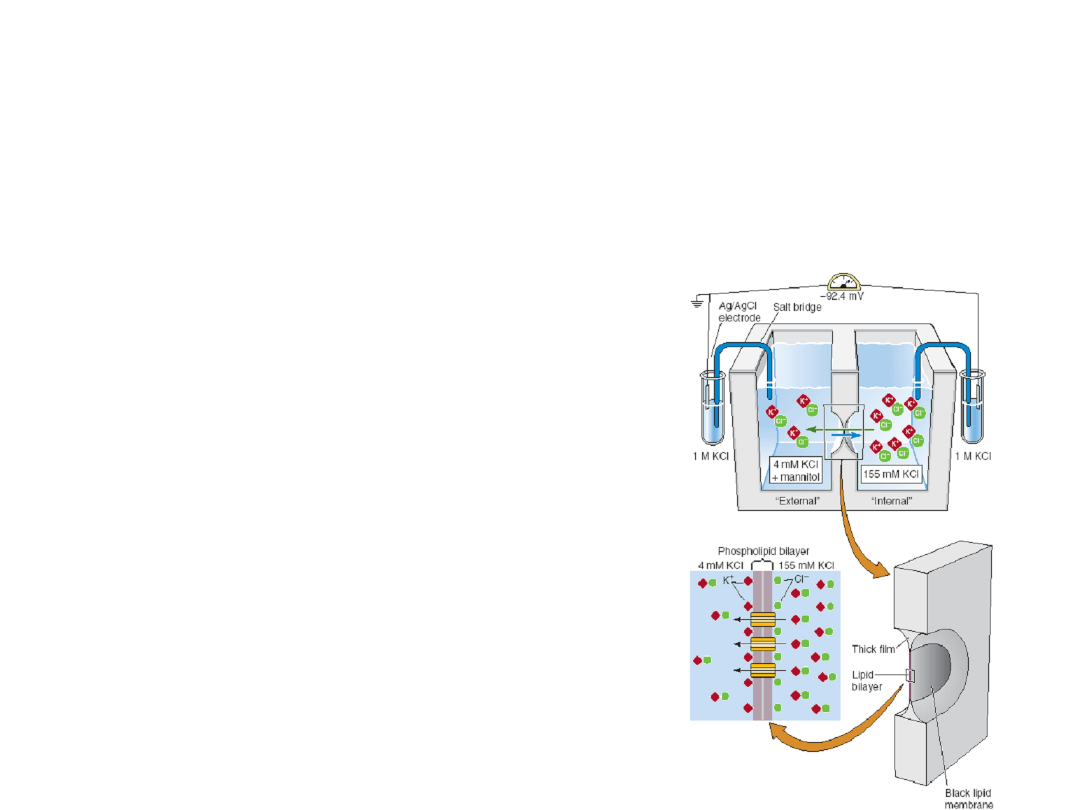
Methods for Studying Ion Channels
Biochemistry
– agonist, antagonist or drug
binding
– isolation and purification
– reconstitution
– radioactive ion flux
Molecular
biology
– sequencing,
cloning,
mutagenesis
Structural biology
– microscopy, crystallography,
NMR, ...
Electrophysiology
– tissue slice
– extracellular recording
– intracellular recording
– whole-cell recording
– single channel
recording
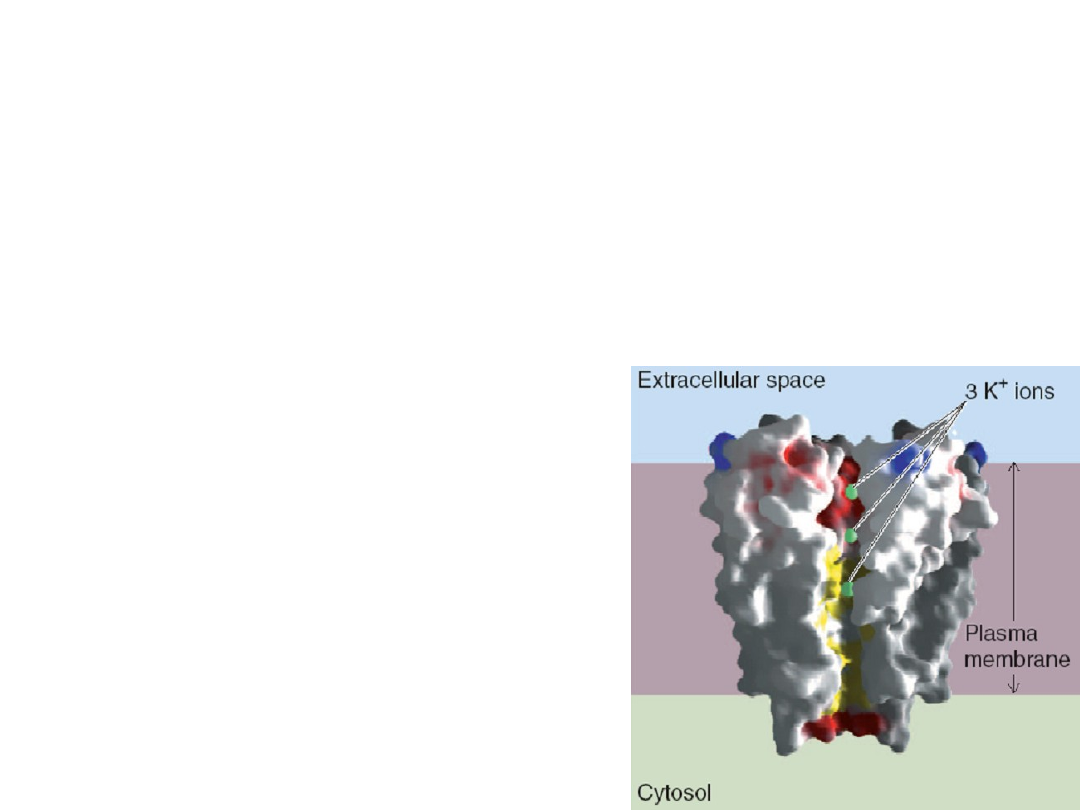
Voltage-gated potassium channels
Membrane voltage determines
whether channels are open – provide
a way for the membrane voltage to
feed back onto itself.
It has a diffusion rate of
10
8
ions per second.
One K
+
ion is dehydrated,
transfered, and rehydrated
every 10 ns.
Roux & McKinnon, Science (1999)
w
m
r
Q
E
1
1
2
1
2
Hydrophobic barrier
Born-Formula
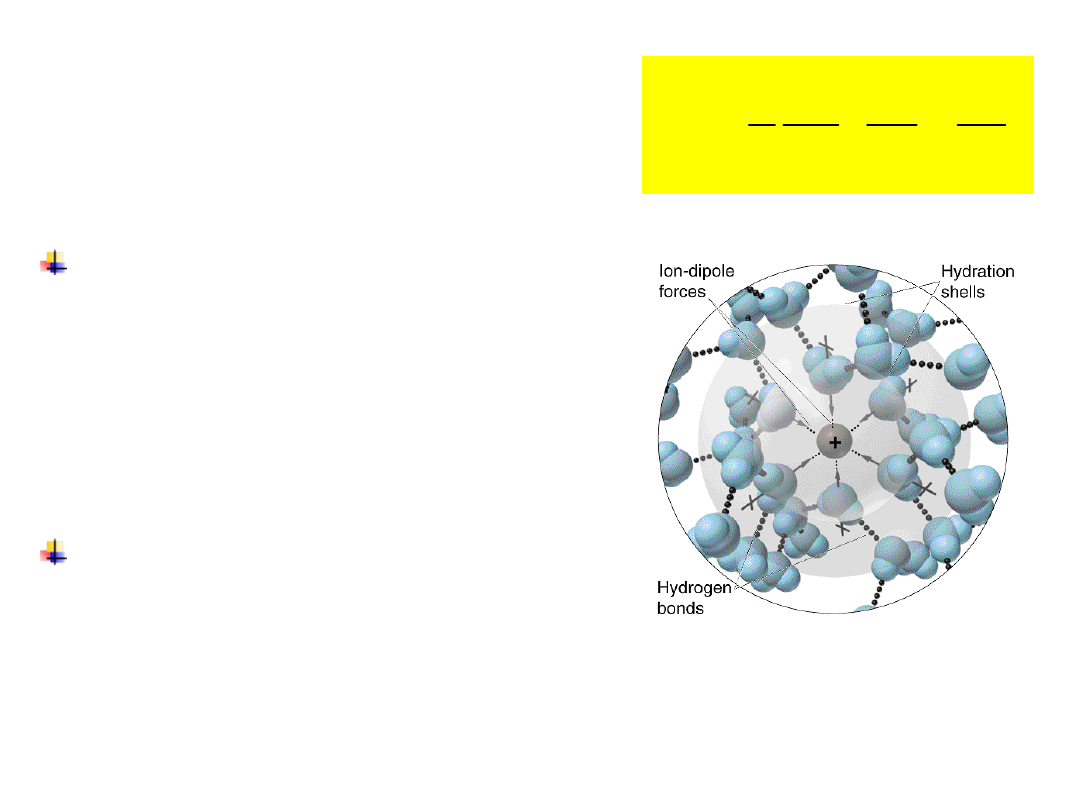
There are about 7 water
molecules in the first
hydration
shell
of
potassium ion.
Each water molecule
stabilizes
the
ion
by
approximately 24 kT.

Voltage-gated K
+
channels
mediate outward K
+
currents during nerve action
potentials.
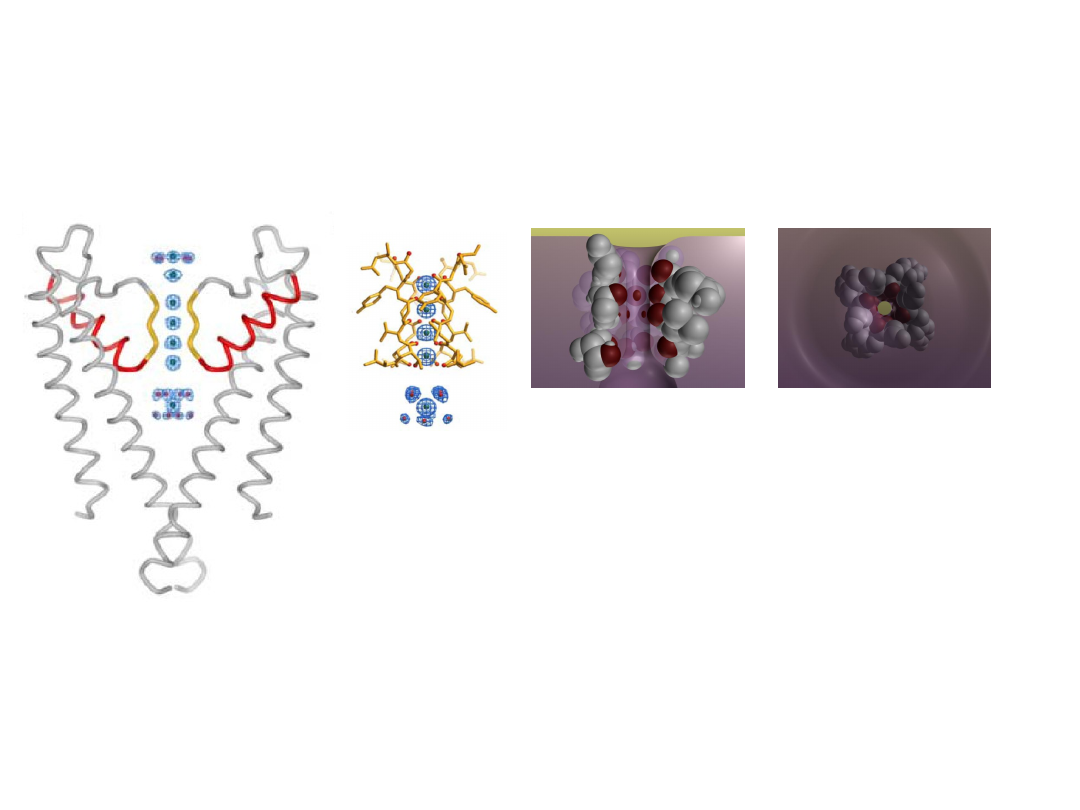
Selectivity filter
K
+
ions encounter four
layers of carbonyl oxygen
atoms & a layer of threonine
hydroxyl oxygen atoms.
Four K
+
ion binding sites.
K
+
is surrounded by eight oxygen atoms from
the protein
- four ‘above’ and four ‘below’.
- very similar to water molecules around
hydrated K
+
.
C=O atoms of the protein backbone
form selectivity filter (4
Tyr-Val-Gly-
Tyr-Gly
).
The sequence is conserved in all K
+
-
channels.
Zhou et al. Nature (2001)
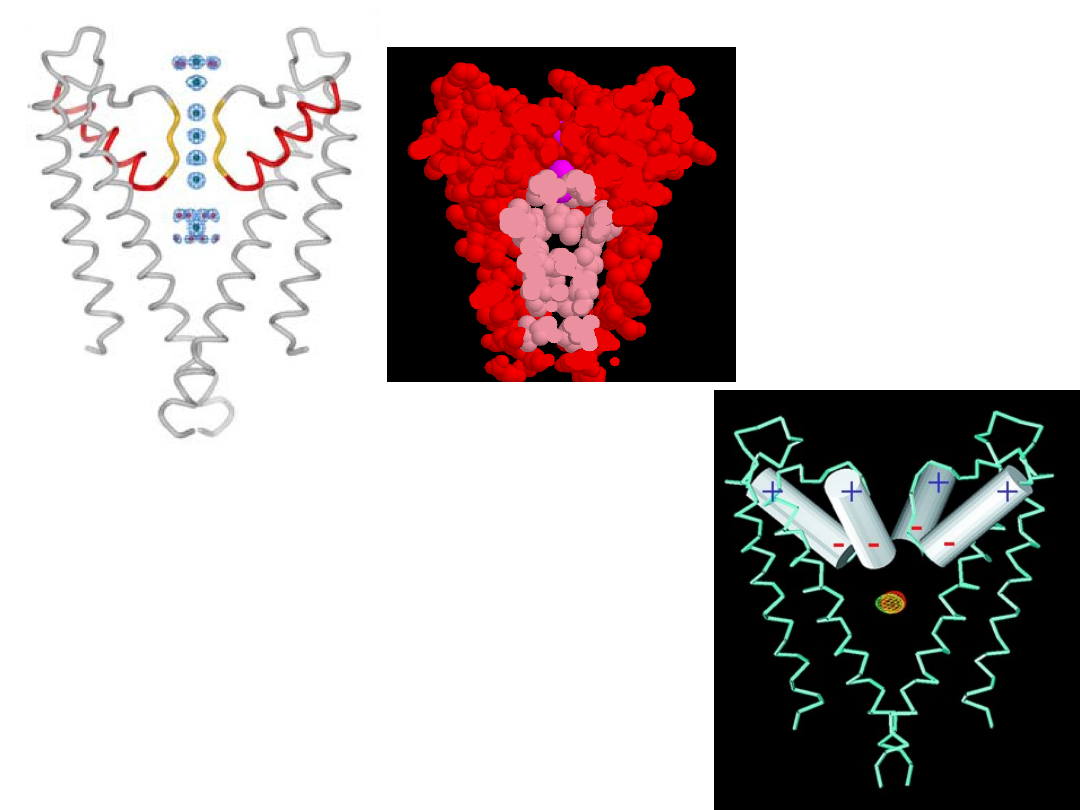
The inner
pore is lined
with
hydrophobic
residues.
Central cavity contains
K
+
ion that is surrounded
by 8 water molecules
Helices represent
dipoles which attract
cations
Why does the ion coordination required for high
selectivity not cause the ions to bind too tightly
& prevent rapid diffusion through the pore?
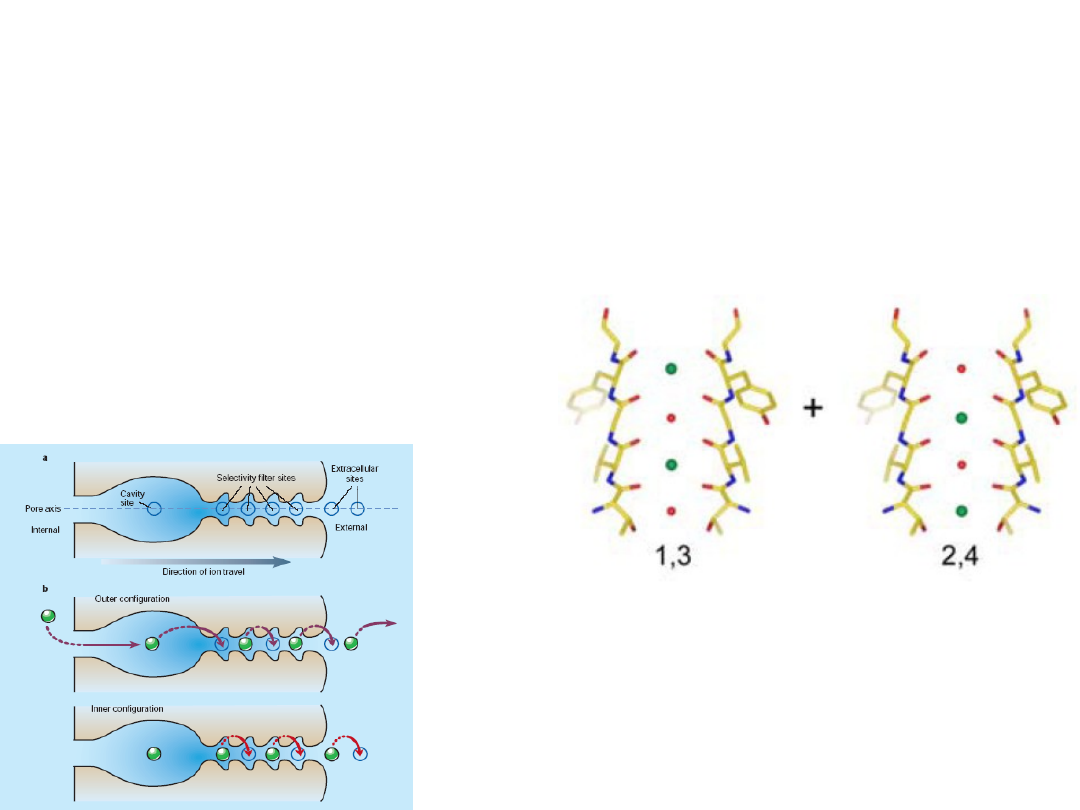
An ion enters the queue
from one side of the
filter while a diferent ion
exits from the opposite
side.
Selectivity filter contains more than one ion –
repulsion between closely spaced ions will helps
overcome the intrinsic binding site affnity.
On average, two K
+
ions
present at a given time
separated by one water
molecule.
The Val and Tyr hold the
selectivity filter at a certain
diameter by hydrogen bonding
with the inner helix.
They form hydrogen bonds
which acts as tight springs that
will not allow the pore to
collapse.
The "springs" prevent the selectivity
filter from interacting with cations
smaller than K
+
.
Radius(Å) 1.33 1.48 1.69 0.95
0.60
Ion
K
+
~ Rb
+
> Cs
+
>> Na
+
> Li
+
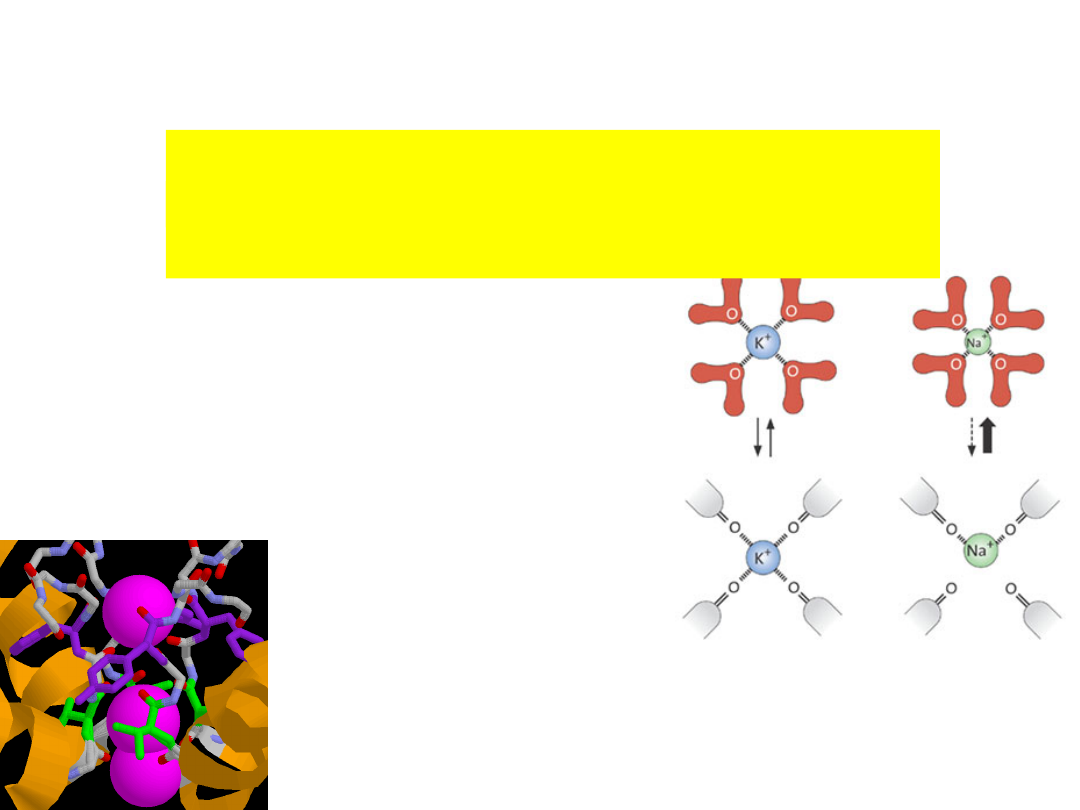
The selectivity is based on the size
difference between K
+
and Na
+
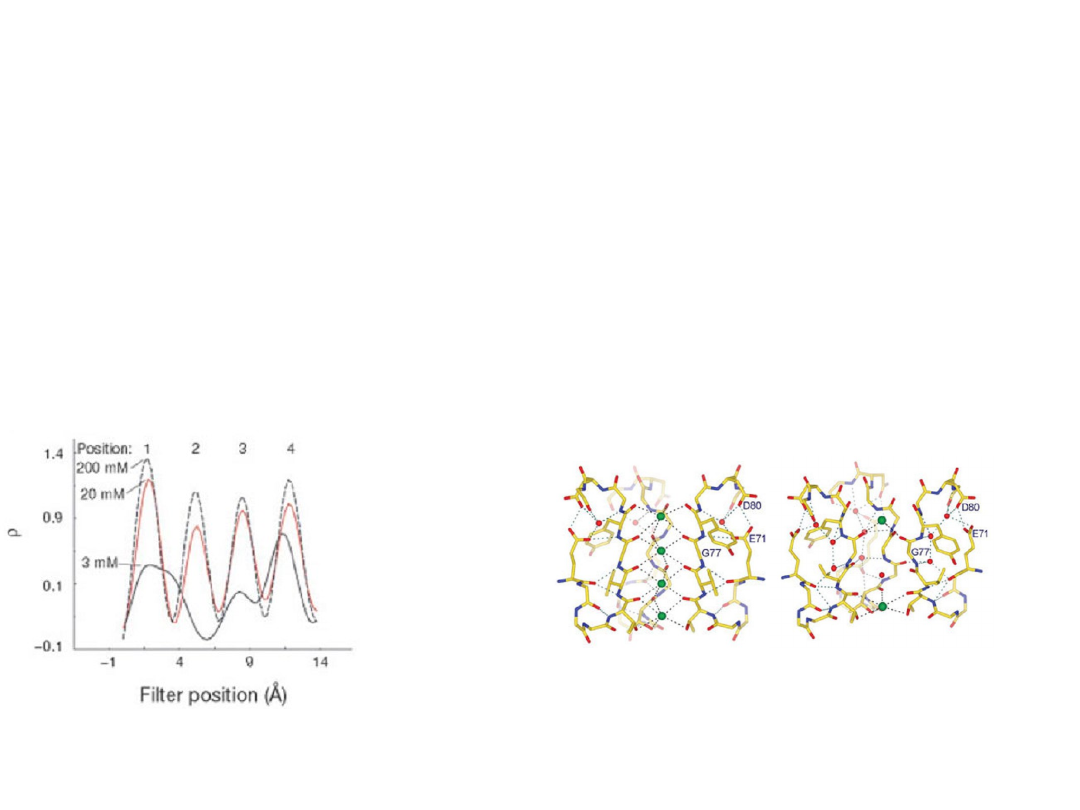
The conductive conformation of the filter requires the two K
+
.
Entry of the second K+ ion induces a conformational change.
A simple thermodynamic consequence
Some fraction of the ion binding energy is used
to change the filter’s structure.
Consequently ions bind less tightly than if a
conformational change did not occur.
Weak binding is a prerequisite for high conduction rates.
KcsA: crystal structures at high and
low K
+
concentration.
Zhou et al. Nature
(2001)
Transfer is isoenergetic
conductivity close to
diffusion limit.
Document Outline
- Slide 1
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
- Slide 19
- Slide 20
- Slide 21
- Slide 22
- Slide 23
- Slide 24
- Slide 25
- Slide 26
- Slide 27
- Slide 28
- Slide 29
- Slide 30
- Slide 31
- Slide 32
- Slide 33
- Slide 34
- Slide 35
Wyszukiwarka
Podobne podstrony:
Wyklad 09 2006
biofizyka wyklad 04
biofizyka 11 09 10
MN energetyka zadania od wykładowcy 09-05-14, STARE, Metody Numeryczne, Część wykładowa Sem IV
wykład 2 - 09.10.2008, FARMACJA, ROK 5, TPL 3, Zachomikowane
miernictwo wyklad 09, INNE MATERIAŁY
Metodyka WF studia I stopnia wyklad 09
Kopia Wyklad 2 09 03 2012 dla studenta
msg ce wyklad 09
FINANSE PRZEDSIĘBIORSTW WYKŁAD 5 (09 12 2012)
Metrologia Wykład) 09 14
wyklady 9 09 2012 r
Biofizyka pytania z kola, Biotechnologia PWR, Semestr 5, Biofizyka - Wykład, Biofizyka - materiały
Asembler wykład 09-10-2000
biofiz, Wykład V, Wykład V
więcej podobnych podstron