
INVESTIGATING TRIBOCHEMICAL BEHAVIOR
OF NITRIDED DIE CASTING DIE SURFACES
V. Joshi, A. Srivastava and R. Shivpuri
The Ohio State University,
1971 Neil Avenue,
210 Baker Systems Columbus,
Ohio 43210,
USA
E. Rolinski
Advanced Heat Treat Corp.,
1625 Rose St.,
Monroe MI 48162,
USA
Abstract
A major reason for process down time and loss of casting quality in aluminum
die-casting is failure of the dies due to inadequate filling, soldering and ejec-
tion problems. The primary cause for the soldered metal is the tribochemical
interaction between the liquid aluminum alloy and the die steel surface which
results in intermetallic formation and adhesion. This paper investigates the
use of die surface nitriding in reducing this interaction. Cylindrical coupons
with various ion-nitriding case depths are dipped in hot liquid aluminum, kept
for predetermined time, cleaned and characterized for surface and substrate
changes. It is found that that nitriding not only reduces the intermetallic for-
mation at the surface but also its wettability that reduces the adhesive force
between of the soldered material and the treated surface. This probably is
due to the greater energy of the nitrided surface and due to the formation of
ferrous nitrocarbides that have lower reactivity with the molten aluminum
alloy.
Keywords:
Soldering, Ion Nitriding, Ejection forces, Corrosion, Intermetallic
961

962
6TH INTERNATIONAL TOOLING CONFERENCE
INTRODUCTION
In a die-casting process molten metal is injected at high speeds and pres-
sure into a die cavity often made of H-13 hot working die steel. Pressure is
maintained within this die until solidification has been completed. After the
solidification is completed, the dies are often sprayed with lubricants to cool
the die surface and to facilitate part removal. However, when the lubricant
layer washes out or is not properly applied, the molten cast metal comes in
contact with the die surface resulting in micro-welding or soldering. Due
to the high temperatures involved, elemental diffusion and transfer plays an
important role at the alloy-die steel interface. Molten aluminum alloy reacts
with the die steel forming complex aluminum-iron- silicon intermetallics and
resulting in soldering of the cast metal to the steel substrate [1]. Soldering
on a typical die-casting die is shown in Fig. 1.
Figure 1.
Typical Soldering on a die-casting die.
The economic significance of soldering to the competitiveness of the
die casting operation has resulted in many studies aimed at determining
the fundamental mechanisms behind its formation and growth. Early in
the 1970’s, Holz [2] classified soldering into impingement and deposition.
According to the authors, impingement soldering occurs when the metal
stream strikes the die surface with high velocity. It occurs in the vicinity of
the gates due to its need for high melt velocity. Deposition soldering, on
the other hand, is hindered by the washout action of the metal stream and
hence occurs at those regions of the die where the velocity is low during
cavity filling. In 1980, Naerheim and Hennie [3] observed that aluminum
was most likely to get stuck to those areas where the erosive action of the

Investigating Tribochemical Behavior of Nitrided Die Casting Die Surfaces
963
Figure 2.
Picture of the soldered intermetallic layers on a H13 pin.
liquid metal was adequate to remove surface coatings (oxides, lubricants)
from the die surface thus exposing the clean metal. The melt velocity in the
vicinity of the gates is high and the metal stream directly impinges on the
die surface. Another type of soldering was observed at regions where the
metal velocity was too low for washing action to occur.
In early 1990s, Chu, Cheng and Shivpuri [4] proposed a three-stage sol-
dering model. They explained that soldering begins with the removal of the
oxide layer due to erosive action of the melt. The erosion of the die surface
exposes the clean die steel to the molten aluminum alloy, which results in
soldering. Metallographic analysis of the soldered areas reveals the presence
of intermetallic layers at the die steel and cast alloy A390 interface. Figure 2
is a photomicrograph of the soldered test coupon. Compositional analysis of
the soldered pins revealed the occurrence of multi-elemental diffusion when
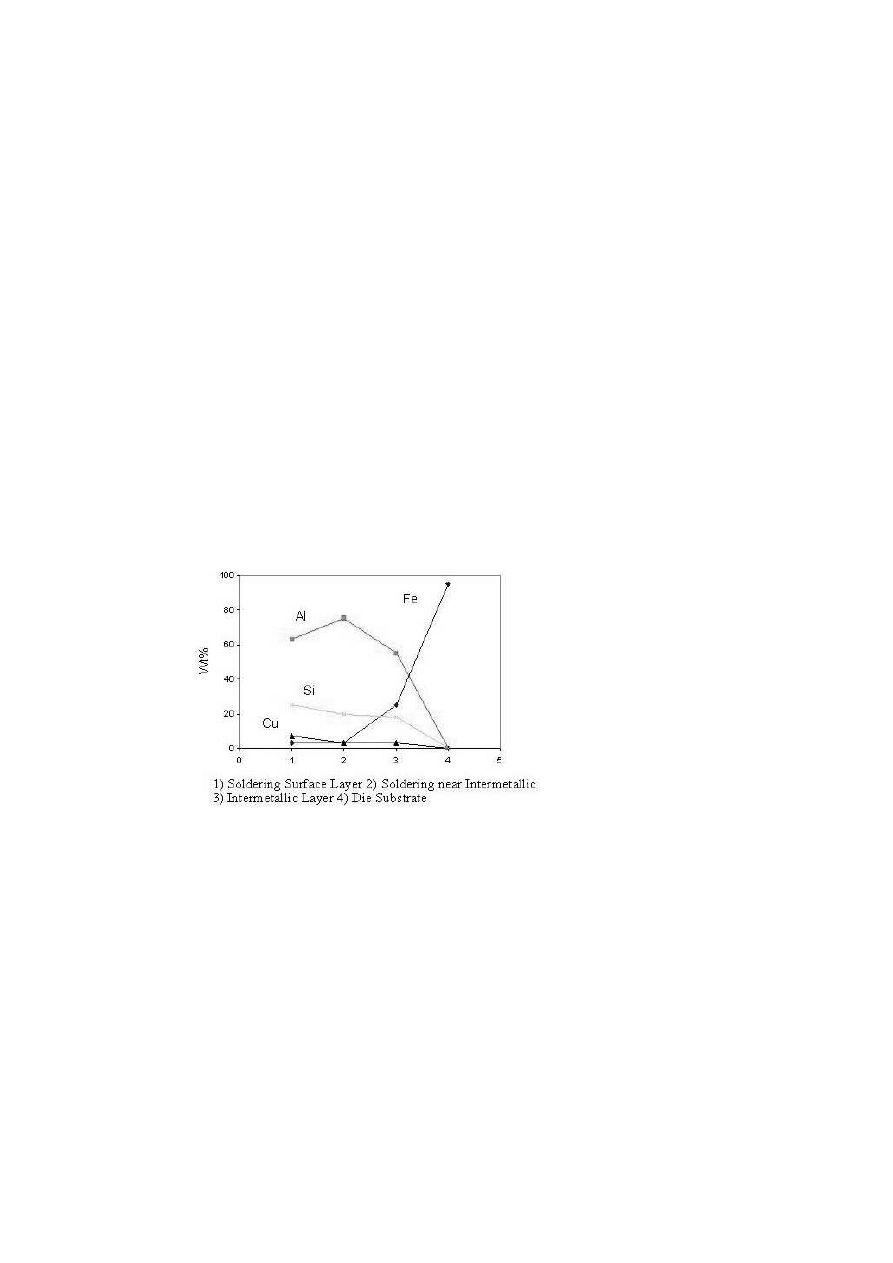
the cast aluminum alloy was in contact with the die steel. Figure 3 represents
the compositional profile of the soldered H13 pin. From Fig. 3, it can be
seen that the aluminum concentration decreases gradually from the soldered
layer towards the H13 substrate. The iron content gradually decreases from
the substrate towards the soldered layer. The drivers for this reaction are
the thermodynamics of intermetallic formation and the solubility of iron in
molten aluminum.
Arai et al showed the potentials benefits of surface modifications in reduc-
ing soldering, is early as 1970s [5]. Systematic investigation of surface mod-
964
6TH INTERNATIONAL TOOLING CONFERENCE
ification technology for preventing reaction and dissolution using controlled
laboratory tests was undertaken at the Ohio State University [4, 6, 7, 8, 10].
In this, the thermodynamics of the intermetallic formation during soldering
was analyzed, and pack cementation technique was used to chromize the die
steel (H-13) substrate to prevent the interface reaction. While high temper-
ature chrome-carbide (Cr
x
C
y
) layer did significantly reduce the soldering
and dissolution tendency of the interface, significant decarburization and
tempering of the steel substrate was observed.
In this paper, the ion nitriding process is investigated for its potential for
preventing the intermetallic formation and adhesion of the cast metal to the
die steel surface. Ion (plasma) nitriding introduces high-energy ions into the
substrate at high temperatures. This thermally driven reaction results in a
compound layer to form at the die steel surface followed by a diffusion zone
of nitrogen case. As the specimen cools down from the nitriding temperature,
a compressive residual stress state develops at the surface. This altered state
of the surface may result in reduced wettability and interface reactions. These
are investigated through controlled hot dip (immersion) and ejection tests on
steel samples nitrided with different case depths.
Figure 3.
Compositional profile of a soldered pin.

Investigating Tribochemical Behavior of Nitrided Die Casting Die Surfaces
965
ION NITRIDING PROCEDURE
Ion nitriding is an extension of the conventional nitriding process using
the plasma-discharge physics. In vacuum, high-voltage electrical energy is
used to form plasma through which nitrogen ions are accelerated to impinge
on the workpiece. This ion bombardment not only cleans the workpiece sur-
face but also heats up the surface and provides active nitrogen for nitriding.
The parts to be nitrided are cleaned, loaded into the vacuum chamber and
secured. The chamber is evacuated by means of a roughing pump so that the
pressure is reduced to a level of 0.05 to 0.1 torr (mm of mercury). The initial
air and any contaminants are removed from the chamber. Resistance heaters
or cathode shields are used to bring the load to nitriding temperatures (375–
650℃) before the glow discharge. While heating, the pressure is increased
so that the glow stream does not get too thick and cause localized overheat-
ing. After the workload is heated to the desired temperature, process gas is
introduced into the chamber at a flow rate determined by the load surface
area. Pressure is maintained between 1–10 torr range. The process gas is
normally a mixture of nitrogen, hydrogen and sometimes, small quantities
of methane. In the presence of this process gas, this workload is maintained
at a high negative DC potential (500–1000 volts) with respect to the vessel.
Under the influence of this high voltage, the nitrogen gas is dissociated, ion-
ized and accelerated towards the workload (cathode). Upon impact with the
workpiece, the kinetic energy of the nitrogen ion is converted to heat and
which brings the load to the nitriding temperature. The glow discharge sur-
rounding the negatively charged workpiece forms at voltages of 200–1000
volts with gas pressures of 1–10 torr [9].
During the glow discharge process, different species and iron atoms from
the workpiece combine with the nitrogen as it diffuses into the material,
forming a hardened surface and case. A uniform glow discharge is necessary
for uniform case depth. After the nitriding cycle, cooling is achieved by back
filling the chamber with nitrogen or other inert gas and re- circulating the
gas through a cooling device.
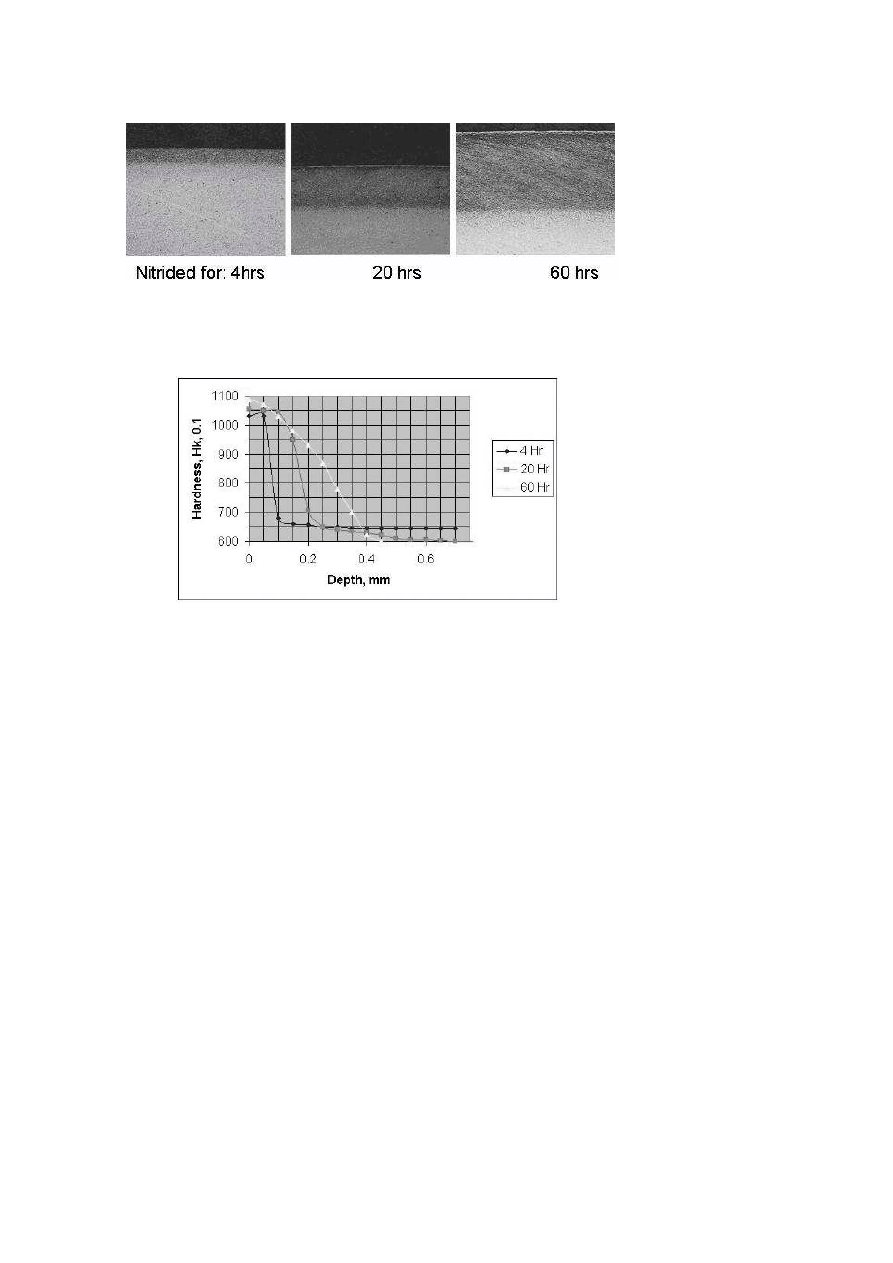
Figures 4 and 5 show the microstructures and hardness profiles from the
pins nitrided in three batches. First batch was 60 hrs, the second 4 hrs and
the third one 20 hrs long. Final temperature was 500–550℃. gas composition
20% nitrogen + 80% hydrogen. The case was defined as: 50 HK 0.1 above
the core hardness. The total case depth for 4 hrs nitriding was 0.099 mm,
966
6TH INTERNATIONAL TOOLING CONFERENCE
Figure 4.
Microstructure of Nitrided samples after 4hrs, 20 hrs and 60 hrs of Nitriding
respectively.
Figure 5.
Hardness profiles for the as received Nitrided samples.
for 20 hrs nitriding was 0.256 hrs and for 60 hrs nitriding are 0.391 mm.
The microstructure of nitrided surface shows a single layer of Fe
4
N and a
diffusion zone of nitride containing tempered martensite. The thickness of
compound zone for 4 hr nitriding was 2 to 4.5 µm, for 20 hr nitriding was
3.4 to 5.6 µm and for 60 hr nitriding was 5.6 to 6.7 µm.
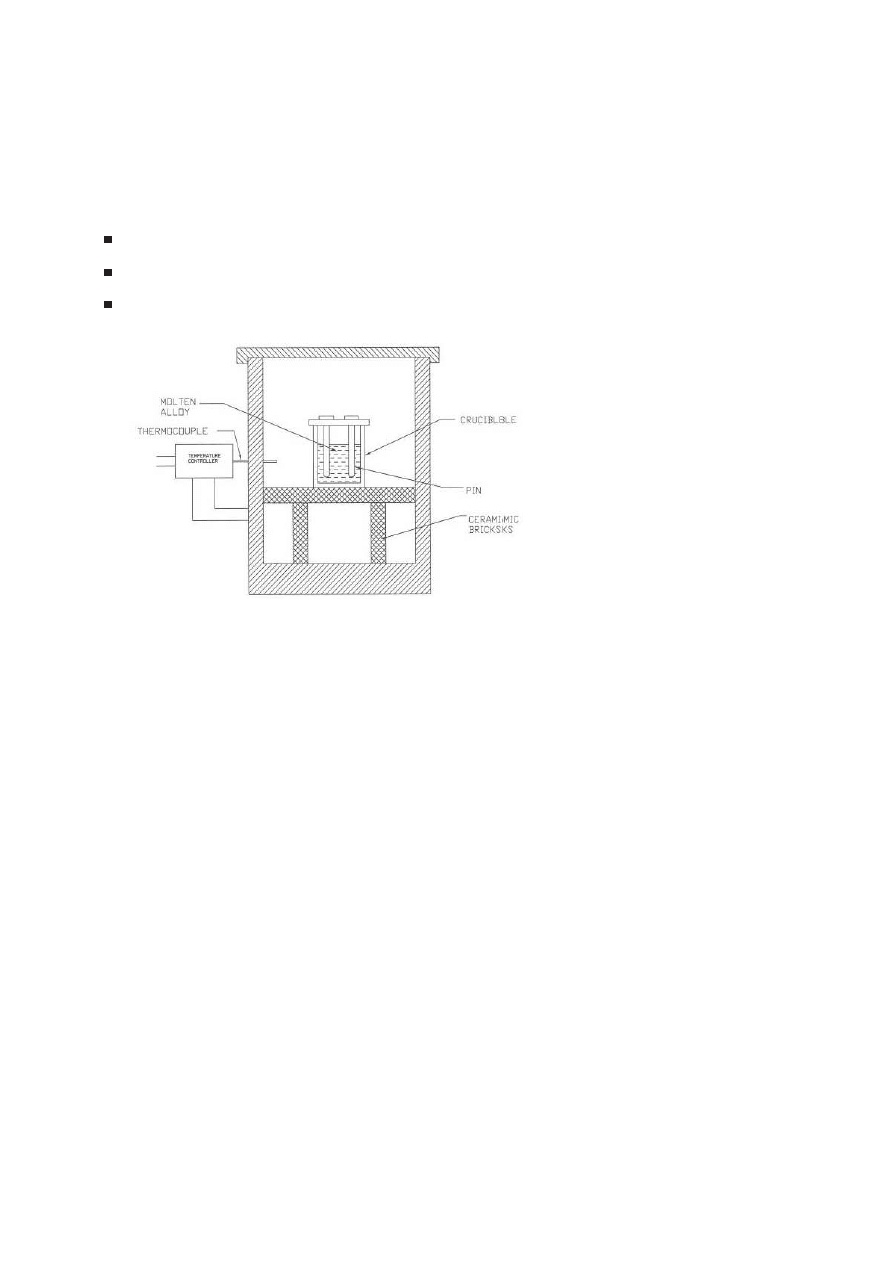
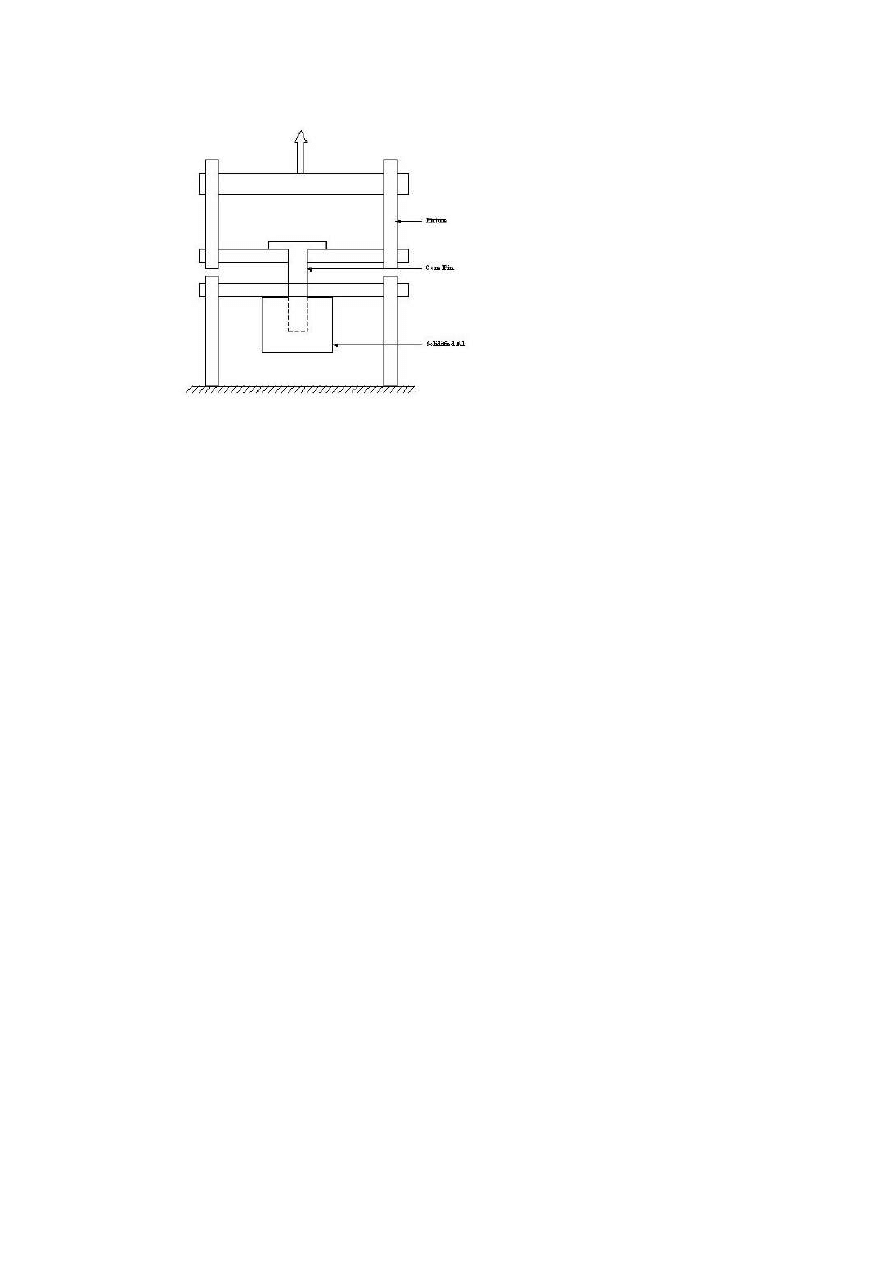
Soldering Dip-Test
These tests, set-up shown in Fig. 6, involved dipping
test coupons (core pins) in a crucible of molten A380 alloy kept at 680℃.
Fresh alloy was used for each round of tests. Nitrided coupons were loaded
Investigating Tribochemical Behavior of Nitrided Die Casting Die Surfaces
967
onto the fixture along with a hardened H13 pin to act as a control or reference
pin. After dipping for predetermined time, the coupons were extracted from
the melt and cooled. Five different dip times were used 5 seconds, 30
seconds, 5 minutes, 30 minutes and 2 hrs The cooled samples were then
subjected to the following analyses:
Macroscopic analysis.
Measuring the height of the soldered layer.
Microstructural analysis
Figure 6.
Schematic of Accelerated corrosion test set up at OSU.
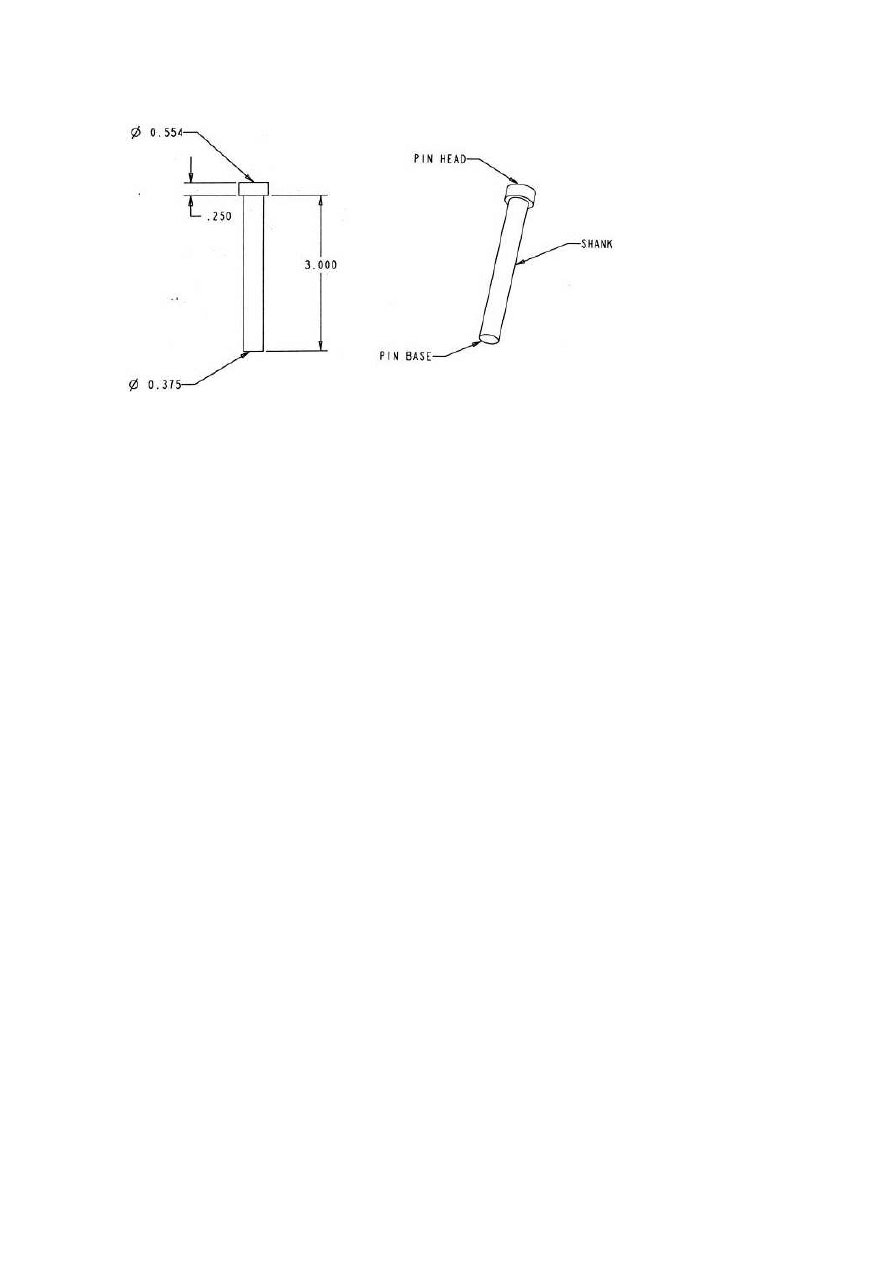
The schematic and dimensions of the test coupons are shown in Fig. 7.
They were standard H-13 core pins heat treated as follows: stress relieved for
0.5 hours at 537℃, vacuum hardened for 90 minutes at 1024℃, quenched,
tempered for 3 hours at 537℃, tempered again for 3 hrs at 550℃ and tem-
pered again for 2 hrs at 570℃. The final hardness was measured as 46–48
HRc. Three different nitrided case depths were used, 100, 250 and 390
microns.
Adhesion Ejection-Tests
Molten aluminum can have some affinity for
H13 dies, this affinity can result in adhesion of aluminum on the coating,
968
6TH INTERNATIONAL TOOLING CONFERENCE
Figure 7.
Schematic of the test coupons with dimensions.
which may solidify and increase the ejection force required to separate the
dies. The ejection tests consisted of solidifying a cylinder of cast metal
around the test coupon and then measuring the force required to extract this
coupon from the solidified cylinder.
A small crucible was used as a mold. Measured amount of A380 was
melted at 680℃ in this crucible and coated coupon was dipped in to the
crucible up to a constant depth. After a predetermined time, the whole
assembly was cooled to room temperature.
The casting formed was then ejected from the pin on the tensile testing
machine using a specially designed fixture and the force of ejection was
measured. The schematic of the test set up on a SpecTester tensile testing
machine is as shown in the Fig. 8. The crosshead distance in this machine
is 30 inches and the stroke length is 5 inches. The testing machine was
connected to a Accutek data acquisition system, which transfers the required
data for the peak loads during the tests. A constant test speed of 75 mm per
minute was used in all experiments.
Investigating Tribochemical Behavior of Nitrided Die Casting Die Surfaces
969
Figure 8.
Schematic of the adhesion test set up.
TEST RESULTS AND DISCUSSIONS
Dip Tests: Results
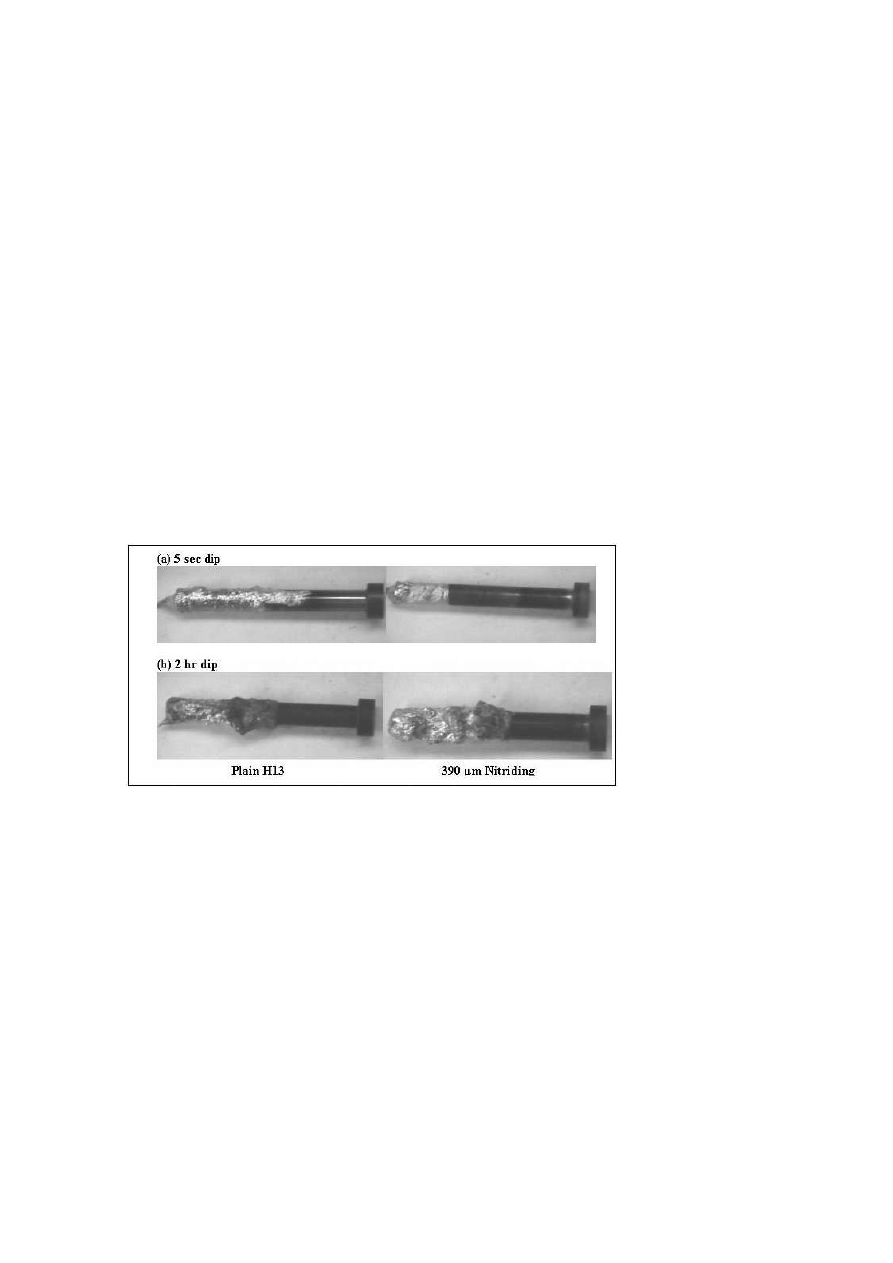
Figures 9(a) and (b) shows the appearance of pins after
dipping in molten aluminum for 5 seconds and 2 hrs respectively. Figure 10
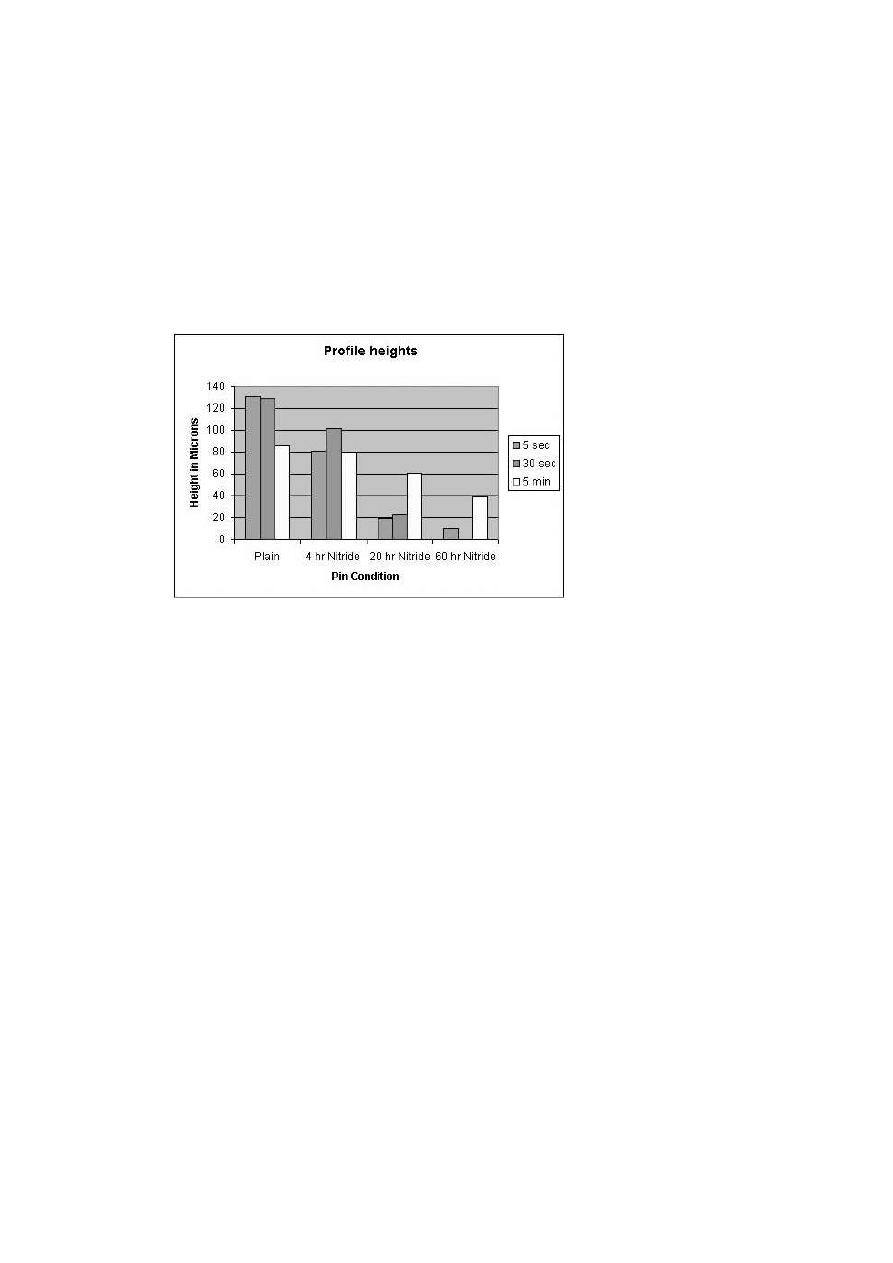
shows the results of the height of the soldering profile for 5 seconds, 30
seconds and 300 seconds dip time. Figure 11 shows the microstructural
analysis of the soldered layer for 5 seconds and 5 minutes dipping time
for both plain H13 and nitrided coupons. The Fig. 12 shows the SEM
photographs of the intermetallic layers after various dip times.
For smaller dip times (5 sec), nitriding compared to plain H-13 signifi-
cantly decreases the amount of soldered material on the pin (Fig. 9(a) with
the reduction in soldering directly proportional to the depth of the nitrided
case (Fig. 10). At these times, the corrosive interaction between the pin
surface and the soldered material (Fig. 11(a) is negligible. This interaction
is even less with heavier nitrided case (Fig. 11(b). This lack of wetting is
important for tribology of the interface.
At higher dip times, intermetallic layer forms on the plain H-13 surface.
The nitrided case successfully protects the substrate from this chemical in-
teraction (Fig. 11(b). There are two possible ways an intermetallic layer
can be formed at the interface, either by deposition or by chemical reac-
970
6TH INTERNATIONAL TOOLING CONFERENCE
tion. Deposition of the aluminum on the die surface will occur when the
aluminum melt is supersaturated with iron. The deposition of the aluminum
on the die surface will in turn lead to solid-state diffusion between iron and
the aluminum leading to intermetallic formation. Intermetallic formation,
by the deposition process is time consuming. During a single die casting
cycle, the die surface is in contact with the molten metal for under a minute,
which implies that the formation of an intermetallic layer at the interface
takes place in a short time.
From the diffusion theory, it is known that mass diffusion is a function of
the concentration gradient as well as the exposed surface area or contact area.
The contact area is defined by the wettability of the liquid metal to the die
surface. In the wetting phenomena, surface energy plays a very important
part. It is known that the surface tension of liquid determines if the solid will
be wetted and also that the tension of the interface between the solid and the
liquid metal exerts a dominant influence. It has been found that the mutual
solubility of the metals bears a relationship with the surface tension between
interfaces. The greater the mutual solubility, the more effective wetting will
be.
This logic is supported by evidence in Figs. 11 (a), (b). After a short
dipping time (5 sec), there is no reaction between steel and molten aluminum.
Figure 9.
Appearance of Soldering after: (a) 5 sec dip (b) 2 hr dip.

Investigating Tribochemical Behavior of Nitrided Die Casting Die Surfaces
971
However as shown in Fig. 12(a), after 5 minute of dip, in plain H13 steel,
the aluminum starts diffusing towards steel and an intermetallic layer build
up starts (around 25 microns). But for the nitrided steel, there is no reaction
between the nitriding layer and molten aluminum and hence no intermetallic
formation. The same trend continued for the dipping time of 30 minutes.
For plain H13 steel, the intermetallic region grew substantially (82 microns).
The nitrided layer is still protecting the steel surface from attack from the
molten aluminum as seen in Fig. 12(b).
The trend changes at very long dip times. At 2 hrs of dipping, the effect of
nitriding as a protective barrier virtually ends and an intermetallic layer starts
building up as shown in Fig. 12(c). From this picture, one can also judge the
difficulty of polishing required for theses samples, as the aluminum tries to
flake out during polishing. This is especially true for the nitrided samples,
where the bonding between nitrided steel and aluminum alloy is not very
strong.
Ejection Tests: Results
Molten aluminum has affinity for the steel sur-
face, bare or coated H13 die steel. This affinity results in chemisorption and
adhesion (welding) of aluminum on the pin surface. This adhered surface
substantially increases the ejection force required to separate the casting
from the die surface. A tribologically sound surface (well lubricated with
no adhesion) will permit clean/low force ejection of the casting. Therefore,
the adhesive strength between a die casting alloy and die steel is related
to the soldering tendency of the two materials. This adhesive strength was
measured by a specially developed ejection test.
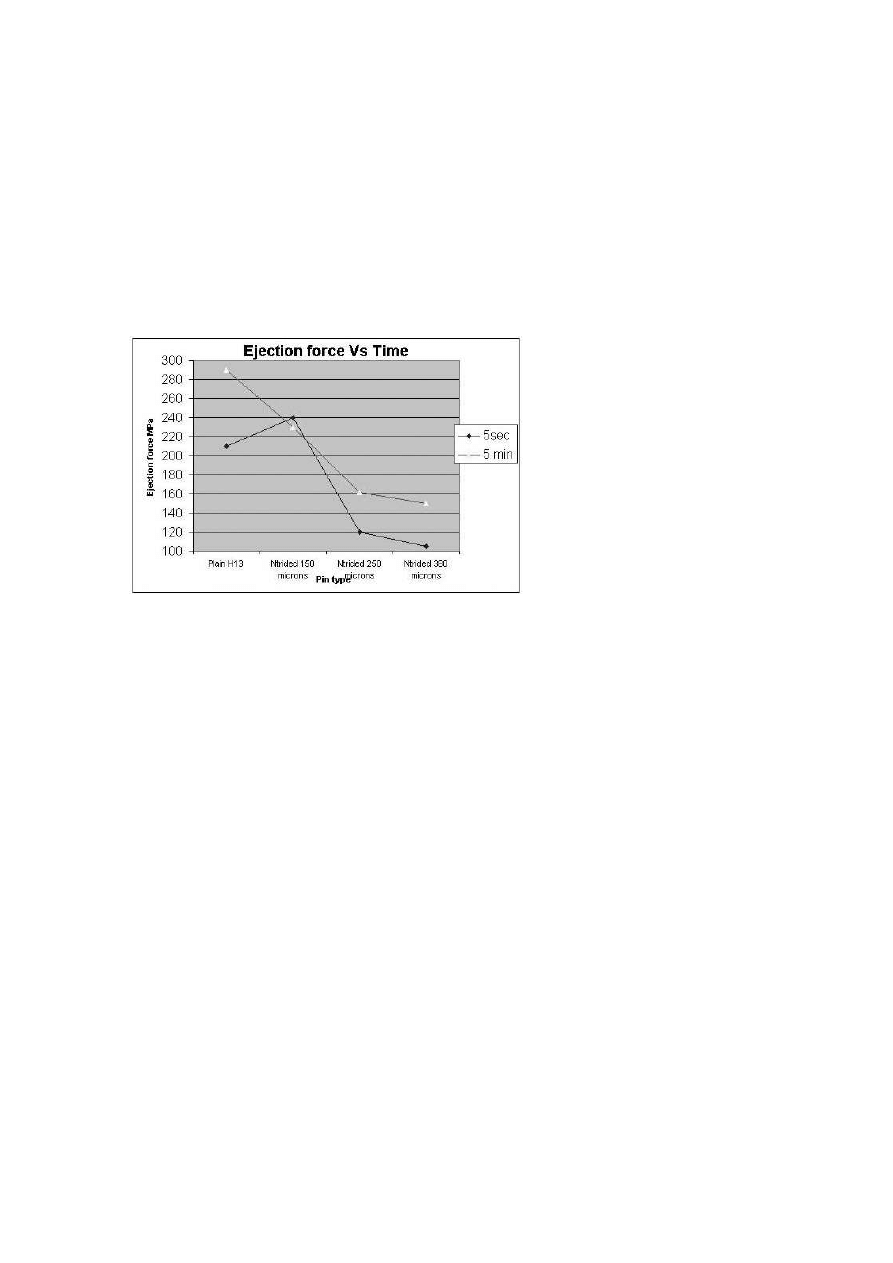
Figure 13 shows the plots for the ejection tests for nitrided pins and H-13
pins. The ejection force is plotted for dipping times of 5 seconds and 5 min-
utes. For the very small dip time of 5 seconds, there is no reaction between
steel and aluminum alloy. Hence the soldering is simply the solidified alu-
minum alloy on steel surface (typically called as cold soldering). Hence, the
ejection forces depend on the surface finish of the pins. Plain H13 pin was
very nicely polished and hence with very low coefficient of friction whereas,
the nitrided pins with low nitriding depths had rough surface finish (coeffi-
cient of friction around 0.6). Forces are lower for plain surface compared to
the nitrided surface.
The trend changes for higher dip times. Even after 5 minutes of dipping,
the small pits start forming on plain H13 steel. This leads to poorer surface

972
6TH INTERNATIONAL TOOLING CONFERENCE
finish. Further, as shown in Fig 11(b), an intermetallic layer is formed
between steel and aluminum alloy at this temperature, which is harder and
difficult to separate. This leads to higher ejection forces for plain H13 steel.
There is no pitting and no intermetallic layer for the nitrided pins and hence
the ejection forces are lower. It is also seen that as the depth of nitriding
increased, lower ejection forces are required. More in-depth understanding
of this phenomenon will require a detailed analysis of the nitrided steels such
as types of nitrides with different depths and degradation of nitrided layers
during increased dipping.
CONCLUSIONS
In this study, H-13 hot working die steel coupons were ion nitrided with
varying case depths. The nitrided pins were then dipped in liquid aluminum
alloy for predetermined time, removed, and examined metallographically.
Soldering tendency of the surface was measured from the amount of soldered
metal and the dissolution tendency by the dissolution in pin surface. It is
seen that nitriding significantly reduced the both the amount of soldering and
the dissolution interaction (thickness of intermetallic layer) of the surface
with higher reductions seen at higher case depths. This improvement in
tribo-chemical interaction, results in lower wettability and adhesion of the
cast metal to the nitrided surface. This conclusion is confirmed by measuring
the forces of ejection between the nitrided surface and the pre-solidified cast
metal.
ACKNOWLEDGMENTS
This research was made possible by a grant (DE-FG02-98ER82702) from
the Office of Industrial Technologies, Department of Energy to UES, Inc
under the SBIR program. The authors thank Drs. Rabi Bhattacharya and
Satish Dixit of UES, Inc. for their support.
REFERENCES
[1] THE FOSECO FOUNDRYMAN’S HANDBOOK, 9th edition, Pergamon Press, 1986
[2] E. K. HOLZ, Light Metal Age, Oct. 1972, 30, (9-10), pp14–16
[3] Y. NAERHEIM and E. HENNIE, Foundry Trade Journal, January 3, pp 11–15, 1980
[4] Y. L.CHU, P. S.CHENG and R. SHIVPURI, Nadca Transactions, Paper T93–124,
1993, pp 361-371

Investigating Tribochemical Behavior of Nitrided Die Casting Die Surfaces
973
[5] T. ARAI and T. IWAMA, Paper# G-T81-092, Nadca Transactions, 1981
[6] M. Yu, PhD Dissertation, The Ohio State University, 1994
[7] S. GOPAL, Masters Thesis, The Ohio State University, 1994
[8] A. LAKARE, S. GOPAL and R. SHIVPURI, T99-111, Transactions of 20th Interna-
tional Die-Casting Congress, NADCA, 1999.
[9] ASM Handbook Heat treating, Volume 4, August 1991, pp 420 – 424
[10] V. JOSHI, R. SHIVPURI, et. al Surface Coating and technology, Volume: 146-147,
September - October, 2001. pp. 338–343.
974
6TH INTERNATIONAL TOOLING CONFERENCE
Figure 10.
Soldering Profile heights.

Investigating Tribochemical Behavior of Nitrided Die Casting Die Surfaces
975
Figure 11.
Interface microstructures after 5 seconds and 30 seconds dipping.

976
6TH INTERNATIONAL TOOLING CONFERENCE
Figure 12.
SEM images of interface microstructures.
Investigating Tribochemical Behavior of Nitrided Die Casting Die Surfaces
977
Figure 13.
Adhesion test results.
Wyszukiwarka
Podobne podstrony:
43 597 609 Comparison of Thermal Fatique Behaviour of Plasma Nitriding
Investor Psychology A Behavioral Explanation Of Six Finance Puzzles
Corrosion behavior of titanium nitride
16 197 208 Material Behaviour of Powder Metall Tool Steels in Tensile
36 495 507 Unit Cell Models for Thermomechanical Behaviour of Tool Steels
50 707 719 Thermal Fatique and Softening Behaviour of Hot Work Steels
76 1075 1088 The Effect of a Nitride Layer on the Texturability of Steels for Plastic Moulds
44 611 624 Behaviour of Two New Steels Regarding Dimensional Changes
Electrochemical behavior of exfoliated NiCl2–graphite intercalation compound
DYNAMIC BEHAVIOUR OF THE SOUTH Nieznany
49 687 706 Tempering Effect on Cyclic Behaviour of a Martensitic Tool Steel
Ecology and behaviour of the tarantulas
Fatigue Behavior of Polymers
Behaviour of precast concrete floor slabs exposed to standar
[Elsevier] Corrosion Behaviuor Of Stressed Magnesium Alloys
więcej podobnych podstron