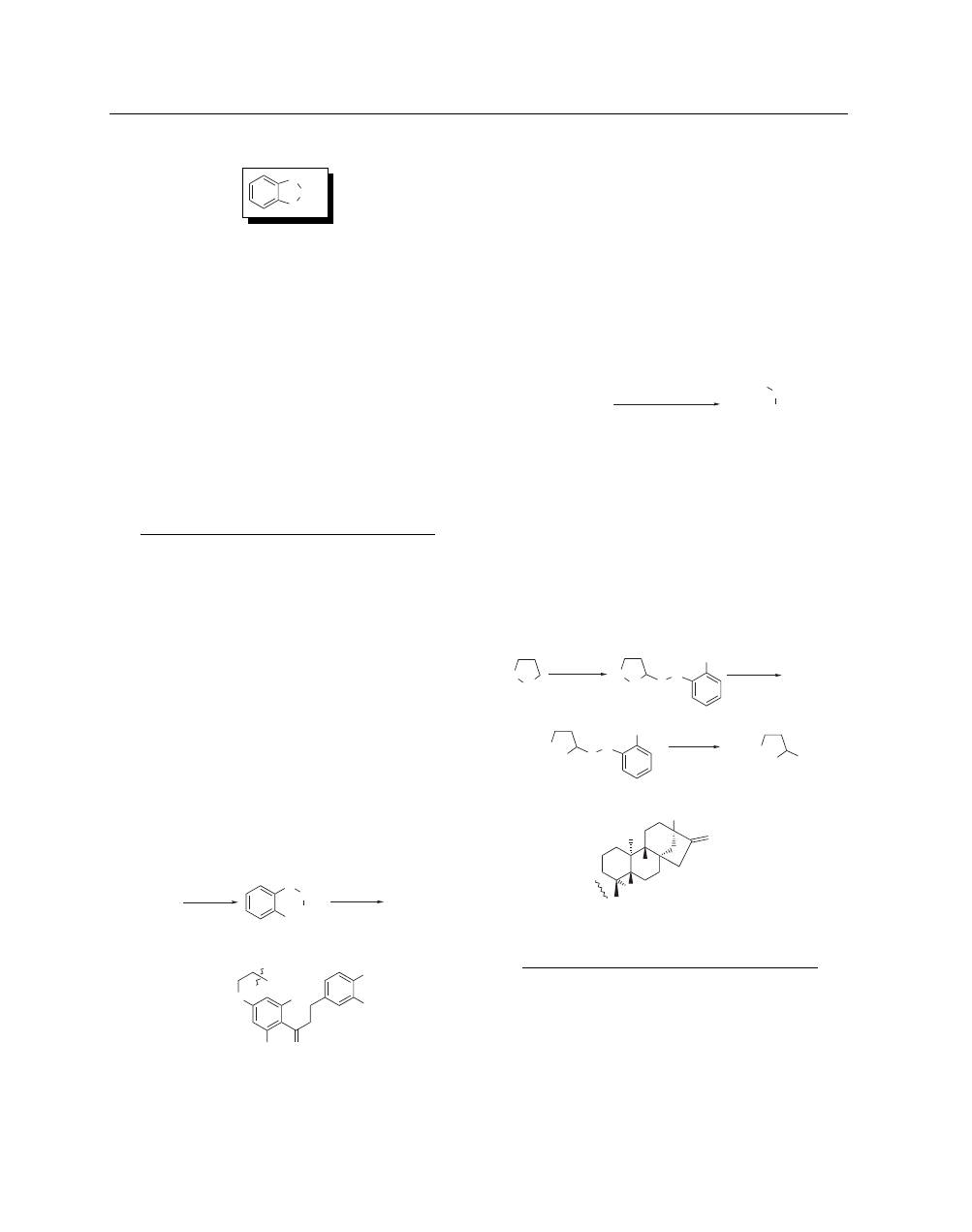
CATECHOL SULFATE
1
Catechol Sulfate
O
SO
2
O
[4074-55-9]
C
6
H
4
O
4
S
(MW 172.16)
InChI = 1/C6H4O4S/c7-11(8)9-5-3-1-2-4-6(5)10-11/h1-4H
InChIKey = ORZMSMCZBZARKY-UHFFFAOYAJ
(selective reagent for the conversion of amines to sulfamate
salts
4
and sulfamides;
9
mild reagent for sulfonation of carbon
acids
16
)
Alternate Names:
pyrocatechol cyclic sulfate; 1,3,2-benzo-
dioxathiole 2,2-dioxide.
Physical Data:
mp 35.5–36
◦
C; bp 76–78
◦
C/1.25 mmHg.
4
Solubility:
Sparingly sol water and hexane; sol most organic
solvents.
Form Supplied in:
white solid or colorless needles.
Preparative Method:
reaction of catechol with SO
2
Cl
2
.
4
Purification:
distillation and recrystallization (hexane).
Handling, Storage, and Precautions:
stable indefinitely at
ambient temp; no information available on toxicity.
The reactivity of catechol sulfate (CS) with hydroxide ion is
anomalous relative to other sulfuric acid diesters. Surprising is the
very high rate of cleavage
1
and that only S–O fission is observed.
2
X-ray crystal data suggest this unusual reactivity to be due to ring-
strain effects and a distorted nonplanar conformation.
3
Conversion of Amines to Sulfamate Salts.
Amines may
be cleanly converted to catecholyl esters of sulfamic acids on
reaction with CS.
4
These readily isolable esters may be effi-
ciently hydrolyzed on mild alkaline treatment. This two-step pro-
cess affords greater selectivity than possible with other reagents
such as ClSO
3
H and SO
3
complexes,
5
fuming H
2
SO
4
,
6
,
7
and
SO
2
Cl
2
/SbCl
5
.
8
As example of the selectivity observed with CS,
amine 1 is quantitatively converted to ester 2 which is then cleanly
hydrolyzed to sulfamate salt 3 (eq 1). In contrast, while reaction
of 1 with Me
3
N/SO
3
gives 3 directly, it is formed as a minor com-
ponent (15–20%) of a complex mixture. Ester 2 is then cleanly
converted to 3 by reaction with alkali.
R-NH
2
OH
NHR
SO
2
O
OH
OH
O
OH
OMe
O
RNHSO
3
K
(1)
(2)
CS
Et
3
N, DMF
(3)
R =
(1)
0 °C, 1 h
100%
100 °C, 1 h
89%
KOH
H
2
O
The CS sulfamation procedure is limited to primary aliphatic
amines. Aromatic amines react only slowly with CS and secondary
amine/CS condensation products are not cleaved by alkali.
Conversion of Amines to Sulfamides. Catecholyl sulfamate
esters, which are available as described above, may be combined
with a second amine to provide sulfamides.
9
Other known pro-
cedures for sulfamide preparation employ strongly electrophilic
agents such as SO
2
Cl
2
.
10
–
13
The two-step CS procedure is ad-
vantaged in its selectivity and in that either symmetrical or un-
symmetrical sulfamides may be obtained. It is limited in that the
amine which reacts with CS must be primary and thus tetrasubsti-
tuted products cannot be obtained directly. Sulfamides of the type
R
1
R
2
NSO
2
NH
2
have been prepared by using a primary amine in
which the alkyl substituent is acid labile (e.g. 4-MeOPhCH
2
).
14
Exemplary of the two-step CS sulfamide methodology is the
preparation of 4 (eq 2).
1. CS, Et
3
N, CH
2
Cl
2
0 °C, 1 h
89%
(2)
(4)
NHBn
SO
2
Et
2
N
BnNH
2
2. HNEt
2
, dioxane
100 °C, 2 h
96%
Conversion of Carbon Acids to Sulfonate Salts. Reaction
of a carbanion with CS yields the catecholyl ester of a sulfonic
acid. The ester is easily hydrolyzed with alkali to give the desired
sulfonic acid salt.
15
This procedure is selective and the interme-
diate esters may easily be isolated in pure form. Carbon acids
may also be sulfonated by SO
3
and various of its complexes.
16
However, the yields are generally low and the desired products
difficult to obtain in pure form. Exemplary of CS sulfonation is
the preparation of substituted methionic acid 5 (eq 3).
15
O
SO
2
RCOO
KO
3
S
S
O
2
O
HO
O
SO
2
S
O
2
O
HO
O-
β-
D
-Glu-2-O-
β-
D
-Glu
H
H
RCOO
KO
3
S
SO
3
K
R =
(3)
(5)
n
-BuLi , CS
25 °C, 24 h
49%
THF, –78 °C
40%
100 °C, 8 h
60%
KOH
H
2
O
RCOOK
DMF
Related Reagents. Chlorosulfonic Acid; Sulfur Trioxide;
Sulfuryl Chloride.
1.
Kaiser, E. T.; Katz, I. R.; Wulfers, T. F., J. Am. Chem. Soc. 1965, 87,
3781.
2.
Kaiser, E. T.; Zaborsky, O. R., J. Am. Chem. Soc. 1968, 90, 4626.
3.
Boer, F. P.; Flynn, J. J., J. Am. Chem. Soc. 1969, 91, 6604.
4.
DuBois, G. E.; Stephenson, R. A., J. Org. Chem. 1980, 45, 5371.
5.
Gilbert, E. E. Sulfonation and Related Reactions; Interscience: New
York, 1965; Chapter 7.
6.
Bieber, T., J. Am. Chem. Soc. 1953, 75, 1405.
Avoid Skin Contact with All Reagents

2
CATECHOL SULFATE
7.
Bieber, T., J. Am. Chem. Soc. 1953, 75, 1409.
8.
Weiss, G.; Schulze, G., Justus Liebigs Ann. Chem. LA 1969, 729, 40.
9.
DuBois, G. E., J. Org. Chem. 1980, 45, 5373.
10.
Dorlars, A., Methoden Org. Chem. (Honben-Weyl) 1958, XI/2, p 711.
11.
Andersen, K. K. In Comprehensive Organic Chemistry, Barton, D. H.
R., Ollis, W. D., Eds.; Pergamon: New York, 1979; Vol. 3, p 363.
12.
Spillane, W. J., Int. J. Sulfur Chem., Part B 1973, 8, 469.
13.
Gilbert, E. E. Sulfonation and Related Reactions, Interscience: New
York, 1965; Chapter 7.
14.
Lee, C.-H.; Lee, M. S.; Lee, Y.-H.; Chung, B. Y., Bull. Korean Chem.
Soc. 1992
, 13, 357.
15.
DuBois, G. E.; Stephenson, R. A., J. Med. Chem. 1985, 28, 93.
16.
Gilbert, E. E. Sulfonation and Related Reactions, Interscience: New
York, 1965; p 33.
Grant E. DuBois
The Coca-Cola Company, Atlanta, GA, USA
A list of General Abbreviations appears on the front Endpapers
Wyszukiwarka
Podobne podstrony:
mercury II sulfate eros rm044
potassium permanganate copper II sulfate eros rp245
palladium on barium sulfate eros rp003
catechol eros rc031
benzyl chloride eros rb050
hydrobromic acid eros rh031
chloroform eros rc105
magnesium eros rm001
oxalyl chloride eros ro015
potassium permanganate eros rp244
peracetic acid eros rp034
p toluenesulfonic acid eros rt134
hexamethylenetetramine eros rh019
copper II chloride eros rc214
glyoxylic acid eros rg009
więcej podobnych podstron