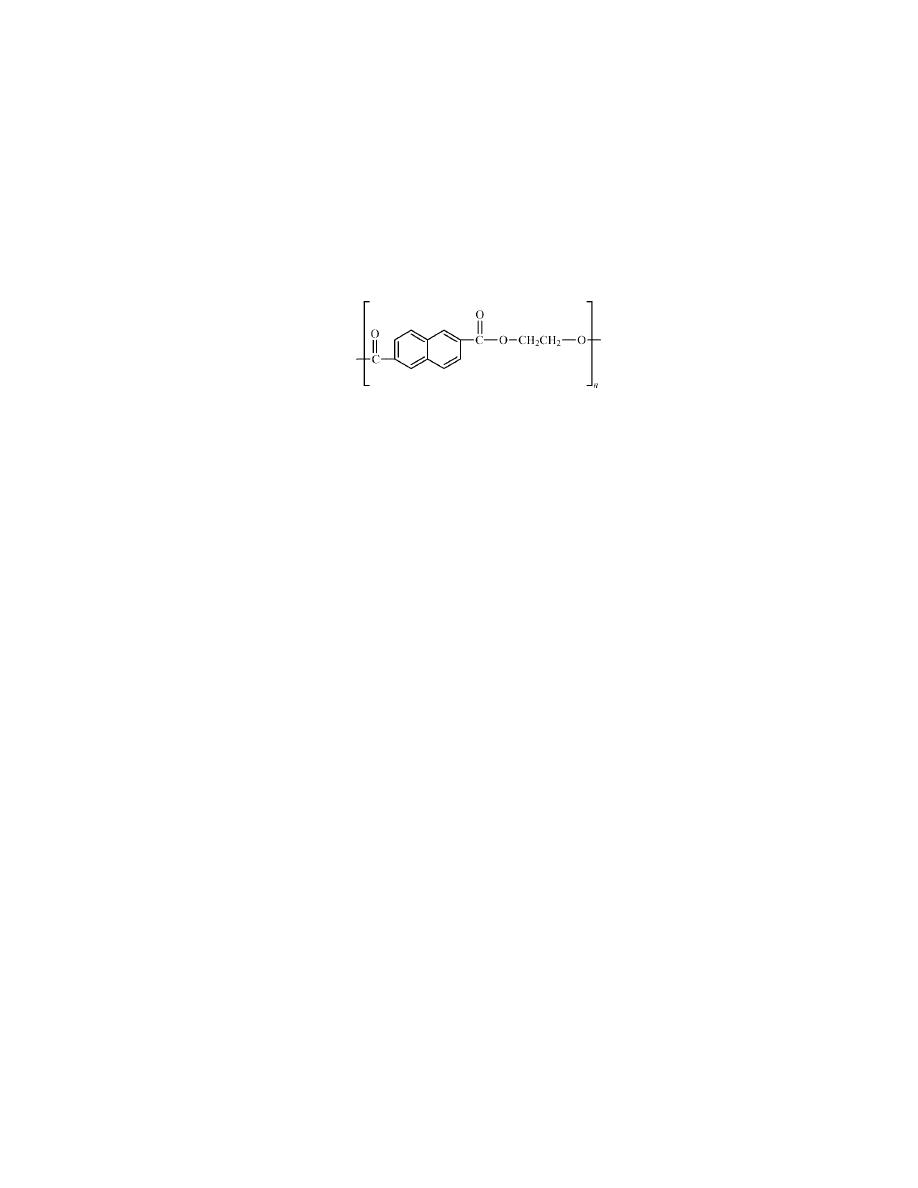
88
POLY(ETHYLENE NAPHTHALATE) (PEN)
Vol. 11
POLY(ETHYLENE NAPHTHALATE) (PEN)
Introduction
Poly(ethylene naphthalate) (PEN) is a thermoplastic polyester consisting of ethy-
lene naphthalate repeat units:
PEN
is
the
polycondensation
product
of
either
dimethyl-2,6-
naphthalenedicarboxylate (2,6-NDC) or naphthalate dicarboxylic acid (2,6-NDA)
with ethylene glycol. To date 2,6-NDC is the preferred feedstock in view of the
fact that it can be produced more economically in high purity form relative to
purified 2,6-NDA.
Other naphthalate-based polyesters include the butanediol-based analogue,
poly(butylene naphthalate) (PBN), as well as copolymers and blends of PET
[poly(ethylene terephthalate)] with PEN.
Naphthalate polyesters exhibit physical and mechanical properties gener-
ally superior to their terephthalate analogues made from terephthalic acid (TA)
or dimethyl terephthalate (DMT), such as PET or poly(butylene terephthalate)
(PBT). PEN polymerization was first reported by ICI in 1948 (1). Its superior
thermal, mechanical, and physical properties were recognized early on. However,
in the 40 years that followed, PEN did not find significant commercialization or
application development, due primarily to the lack of commerical availablity of
the key monomer, 2,6-NDC. Although studies on PEN film and fiber production
and applications were begun by Teijin in the 1960s, it was not until the 1970s
that 2,6-NDC became available in sufficient quantities for the first PEN films and
fibers to be produced on a semitechnical scale. High value speciality videotapes
were found to benefit from the use of PEN film in Japan during the 1980s. In the
early 1990s Amoco Chemical Co. announced their plan to invest in a world-scale
facility for production of 2,6-NDC in Decatur, Illinois. As a result of the promise
of larger scale and more economic raw material supply, plus greater interest from
the end market, PEN films were launched commercially in the early 1990s. Since
then, the start-up of commercial production at Amoco’s (now BP) 30,000 tons/year
2,6-NDC facility has significantly aided the economics of PEN production. This
has driven increasing use of PEN in a wide range of applications including films,
fibers, and rigid packaging.
An approximate percent breakdown of PEN usage worldwide per end-use
segment for the year 2002 is provided in Table 1.
At this time, commercial producers of PEN homopolymer and/or copolymer
resins are Teijin Ltd. (Japan), M&G (USA), 3M (USA), KoSa (Europe), Toyobo
(Japan), Kolon (Korea), MCC (Japan), Kimex (Mexico), and Shinkong (Taiwan).
Encyclopedia of Polymer Science and Technology. Copyright John Wiley & Sons, Inc. All rights reserved.

Vol. 11
POLY(ETHYLENE NAPHTHALATE) (PEN)
89
Table 1. Major PEN Market Segments and Approximate Percent Breakdown by End-Use
Segment
a
Approximate
End Use
consumption, %
Examples
Film
70
Imaging, recording tape, displays, memory
storage, insulators, capacitors
Rigid plastic
packaging
15
Beer returnable/refillable bottles, pharmaceutical
and cosmetic bottles
Fiber
15
Tirecord, hose reinforcement, sailcloth
a
Data from BP Amoco Chemical Co.
Polymer Properties
Naphthalate polyesters, such as PEN and PBN, derive their performance improve-
ment from the double ring structure of the naphthalene group. Incorporation of
this double ring structure in the polymer chain increases thermal, chemical, me-
chanical, and barrier performance versus polymers based on single aromatic rings.
Table 2 illustrates the wide variety of property improvements exhibited by PEN
versus PET.
Partial substitution of terephthalate-based polymers with the naphthalate
group, in a copolymer or blend form, can provide enhanced properties that are
Table 2. Comparative Properties of PEN vs PET
a
Property
PEN value
PET value
T
g
,
◦
C
122
80
Shrinkage, %
Dry at 150
◦
C
0.6
1.3
Wet at 100
◦
c
1
5
Mechanical continuous use temperature,
◦
C
160
105
UV absorbance at 360 nm, %
17
1
Radiation resistance, MGy
b
11
2
Tenacity retention, 45 min. at 150
◦
C, %
99
45
Young’s modulus, MPa
c
5200
3900
Tensile strength, MPa
c
60
45
Oligomer extraction, mg/m
2
0.8
20
Hydrolysis resistance, h
200
50
Permeability,
n mol
·m
m
2
·s·GPa
d
CO
2
12
61
O
2
3
12
Water vapor transmission,
n mol
·m
m
2
·s
e
0.129
0.452
a
Source: Teijin Ltd., Japan.
b
To convert MGy to Mrad, multiply by 100.
c
Convert MPa to Psi multiply by 145.
d
To convert
n mol
·m
m
2
·s·GPa
to
cm
3
·mm
m
2
·day·atm
, multiply by 0.2.
e
To convert
n mol
·m
m
2
·s
to
g
·mm
m
2
·day
, multiply by 1.55.
90
POLY(ETHYLENE NAPHTHALATE) (PEN)
Vol. 11
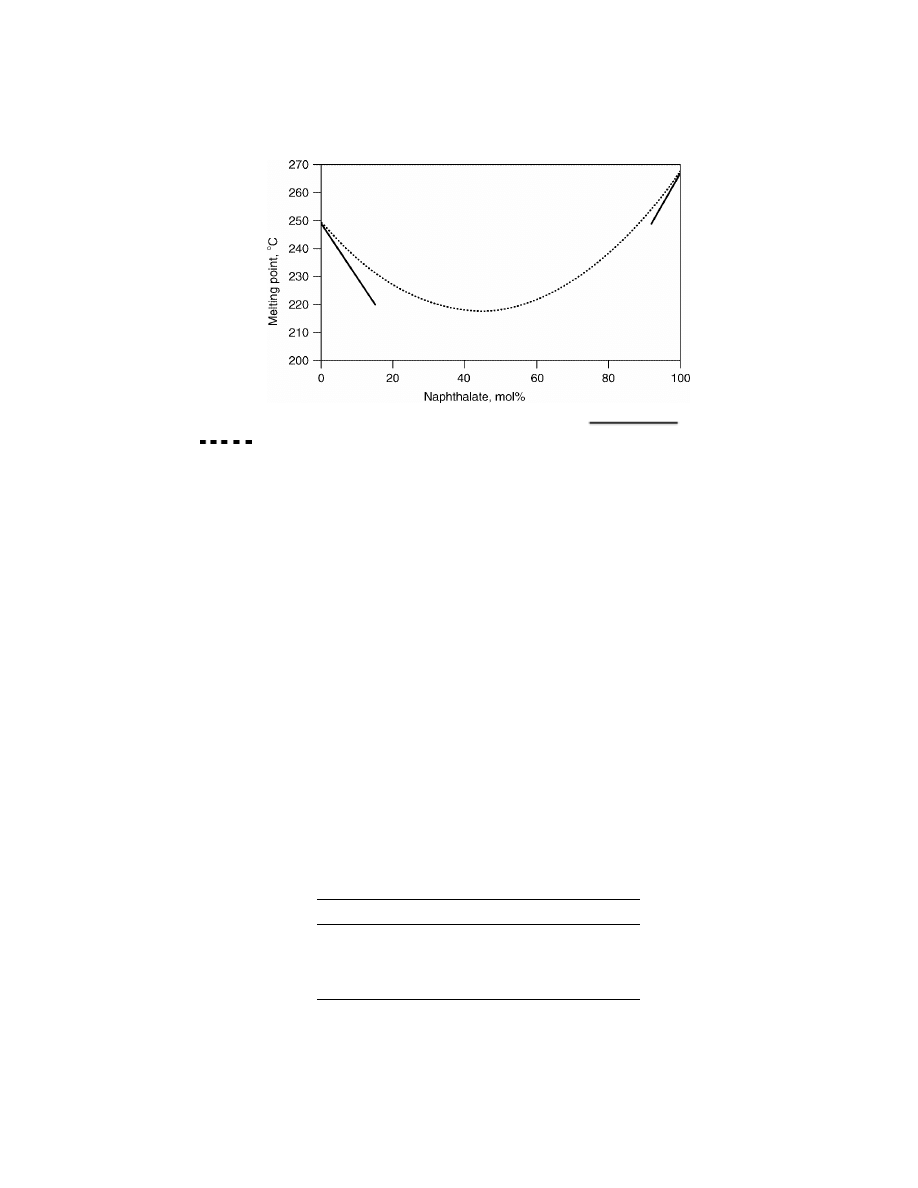
Fig. 1.
Melting point of PET/PEN copolymers and blends.
Copolymer,
Blend. Data from BP Amoco Chemcial Co.
typically intermediate between the respective homopolymers. A description of key
thermal, physical, and mechanical properties is provided below.
Thermal Properties of Naphthalate-Containing Polyesters.
Naph-
thalate homopolymers melt at higher temperatures vs terephthalate homopoly-
mers. Table 3 compares the T
m
of PEN and PBN vs PET and PBT respectively.
In random copolymers, where both TA and NDC are present, the melting
point decreases as NDC partially substitutes TA (eg, starting from PET) or when
TA partially substitutes NDC (eg, starting from PEN). This melting point depres-
sion effect is caused by a disruption in the regular crystalline pattern by the minor
component (Fig. 1).
Figure 1 shows that the melting point of a PEN copolymer modified with
8 mol% DMT (PENT-8) equals the melting point of a PET homopolymer. Use of
the lower melting PENT-8 copolymer in preparing PET/PEN blends is preferred
over use of a PEN homopolymer. Not only are lower melt temperatures required
in the blending process, which reduces the risk of PET degradation, but there
is a better viscosity match between the naphthalate and terephthalate polymers
which improves mixing and miscibility.
Within the range of approximately 10–90 mol% NDC, PET/PEN random
copolymers (PETN) remain essentially amorphous. On the other hand, PET/PEN
blends are capable of crystallizing and exhibiting a melting point at any T/N ra-
tio, which depends not only on this ratio but also on the degree of randomness or
Table 3. Melting Point of Polyester Homopolymers
a
Homopolymer
T
m
,
◦
C
PET
250
PEN
268
PBT
223
PBN
242
a
Data from BP Amoco Chemical Co.
Vol. 11
POLY(ETHYLENE NAPHTHALATE) (PEN)
91
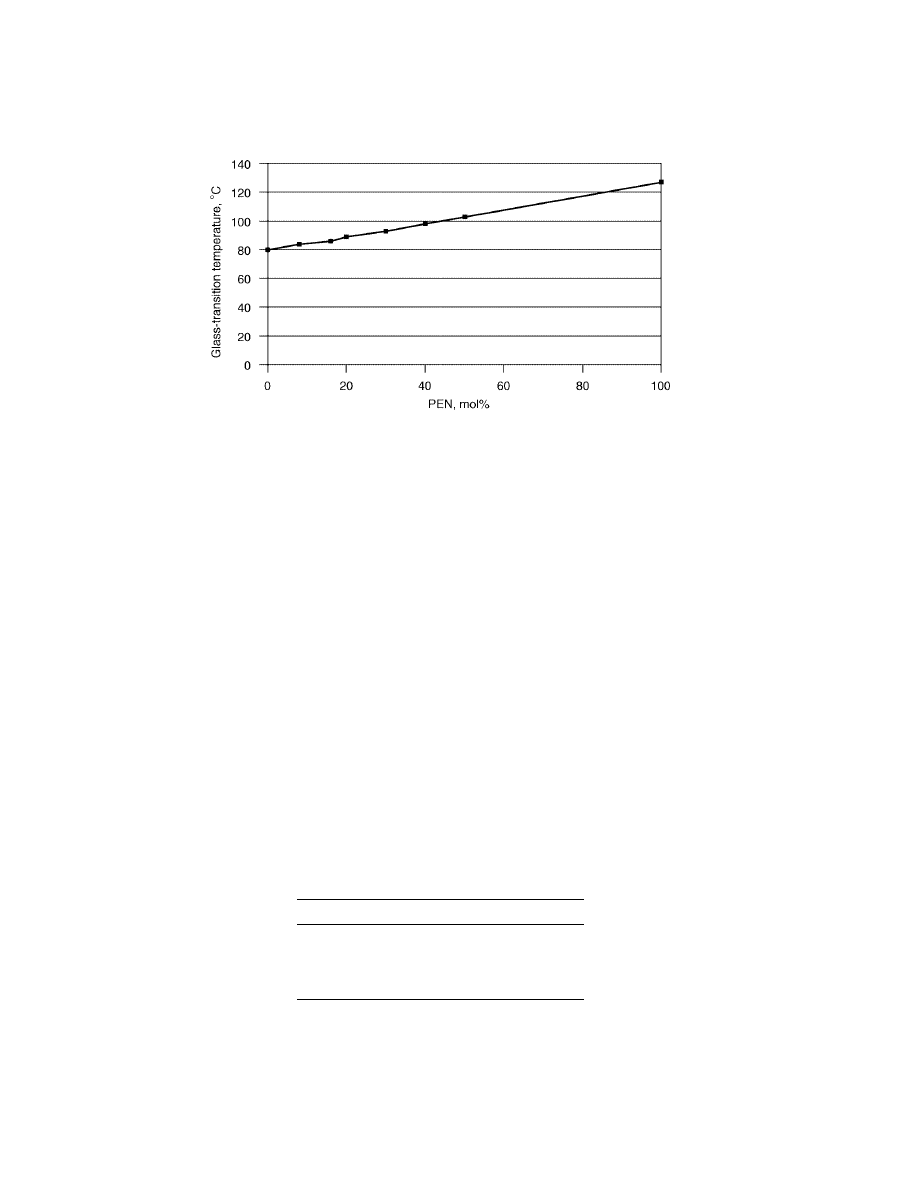
Fig. 2.
Glass transition of PET/PEN copolymers and blends. Data from BP Amoco Chem-
ical Co.
transesterification present. The dotted line in Figure 1 provides a cursory repre-
sentation of this behavior.
Naphthalate homopolymers have a glass-transition temperature signifi-
cantly higher than the corresponding terephthalate homopolymers (Table 4).
Unlike T
m
, the behavior of T
g
vs NDC content is linear and follows the mixing
rule (Fig. 2).
This linear relationship offers many opportunities for enhancing the thermal
resistance of PET by simply blending-in varying amounts of naphthalate. Practical
applications include hot-filled, pasteurized, or washable containers, and outdoor
glazing.
An increase in T
g
also affords improved long-term aging behavior or reduced
embrittlement of amorphous parts. It enables a reduction in cycle time during
injection molding (eg, of bottle preforms), since the parts can be released hotter.
A 20% faster cycle time has been achieved in the case of PEN preforms vs PET.
Finally, the higher T
g
of PEN contributes greatly to the reduction of thermal
shrinkage, which is of value in tire cord and other industrial fiber applications as
well as films (see Table 8).
Crystallization.
As in the case of PET, PEN can be quenched to amorphous
glass. Its amorphous density, 1.325 g/cm
3
[BP Amoco Chemical Co.], is slightly less
than that of PET (1.333) (2). Crystallization from the glass or from the melt at low
temperatures (
<200
◦
C) leads to a triclinic crystal form labeled
α (3), whose unit
Table 4. T
g
of Polyester Homopolymers
a
Homopolymer
T
g
,
◦
C
PET
80
PEN
125
PBT
46
PBN
82
a
Data from BP Amoco Chemical Co.
92
POLY(ETHYLENE NAPHTHALATE) (PEN)
Vol. 11
Table 5. PET and PEN Unit Cell Parameters
PET
a
α -PEN
b
β-PEN
c
Form
Triclinic
Triclinic
Triclinic
a, ˚A
4.56
6.51
13.14
b, ˚A
5.94
5.75
9.0.4
c, ˚A
10.75
13.2
12.19
α, (
◦
)
98.5
81.2
129.31
β, (
◦
)
118
144
88.62
γ , (
◦
)
112
100
90.77
Volume, ˚A
3
219.2
285.8
1120
Z (rpt units per cell)
1
1
4
Calculated density at 23
◦
C, g/cm
3
1.455
1.407
1.436
a
Ref. 5.
b
Ref. 4.
c
Ref. 3.
cell was determined by Mencik (4). Crystallization from the melt at temperatures
above 200
◦
C leads to a more dense crystalline form labeled
β (3).
PEN crystallizes in the same crystal pattern as PET, ie, triclinic space group
P¯I. The unit cell parameters are compared in Table 5.
Gas Permeability.
The oxygen, carbon dioxide, and water vapor perme-
ability of polyesters depend not only on composition but also on degree of crys-
tallinity, orientation, and temperature. Regardless of the variable, PEN always
exhibits significantly reduced gas permeability relative to PET. Some examples
are illustrated in Table 6.
Table 6. Gas Permeability
PET
PEN
Units and conditions
CO
2
transmission, amorphous sheet
69
a
15
a
cc-mil/100 in.
2
·day·atm @ 23
◦
C
and 0% RH
CO
2
transmission, 4
× 4 oriented film
(Ref. 8)
31
a
6.0
a
cc-mil/100 in.
2
·day·atm @ 30
◦
C
and 0% RH
O
2
transmission, amorphous
b
10.9
a
4.4
a
cc-mil/100 in.
2
·day·atm @ 23
◦
C
and 68% RH
O
2
transmission, oriented film (Ref. 6)
6.1
a
1.6
a
cc-mil/100 in
2
·day·atm @ 30
◦
C
and 68% RH
Water vapor transmission, amorphous
4.31
c
2.12
c
g-mil/100 in
2
·day·atm @ 38
◦
C
and 90% RH
Water vapor transmission, 4
× 4
oriented film (Ref. 6)
1.85
c
0.52
c
g-mil/100 in.
2
·day·atm @ 38
◦
C
and 90% RH
Water vapor transmission, commercial
biaxially oriented film
d
0.38
e
0.09
e
g
·mm/m2·day·atm @ 38
◦
C and
90% RH
a
For the value with units
n mol
·m
m
2
·s·GPa
, multiply by 2.
b
Data from BP Amoco Chemical Co.
c
For the value with units
n mol
·m
m
2
·s
, multiply by 0.253.
d
Source: DuPont Teijin Films (Melinex PET and Kaladex PEN respectively).
e
For the value with units
n mol
·m
m
2
·s
, multiply by 0.645.
Vol. 11
POLY(ETHYLENE NAPHTHALATE) (PEN)
93
Fig. 3.
Oxygen permeability of 4
× 4 biaxially oriented films from PET/PEN blends and
copolymers (7). For O
2
permeability values with the units
n mol
·m
m
2
·s·GPa
, multiply by 2.
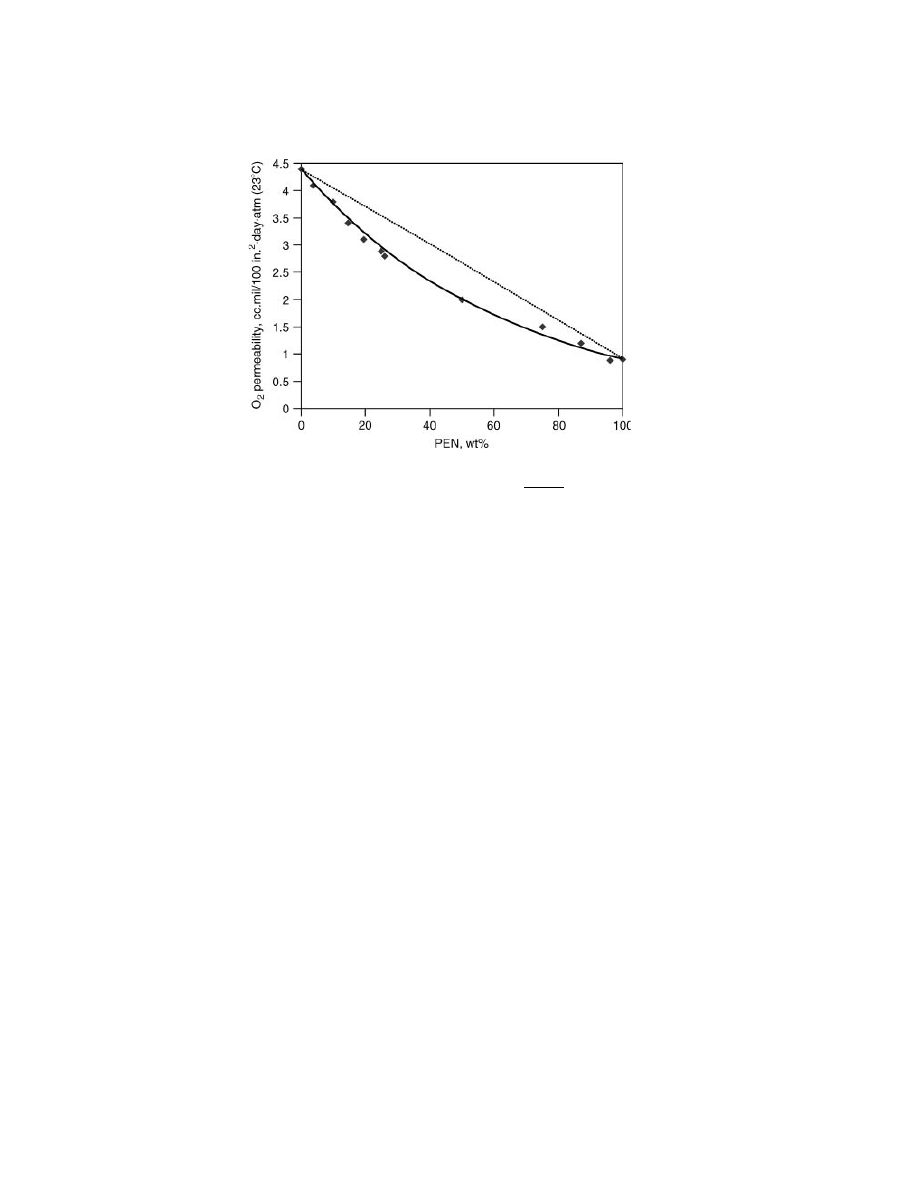
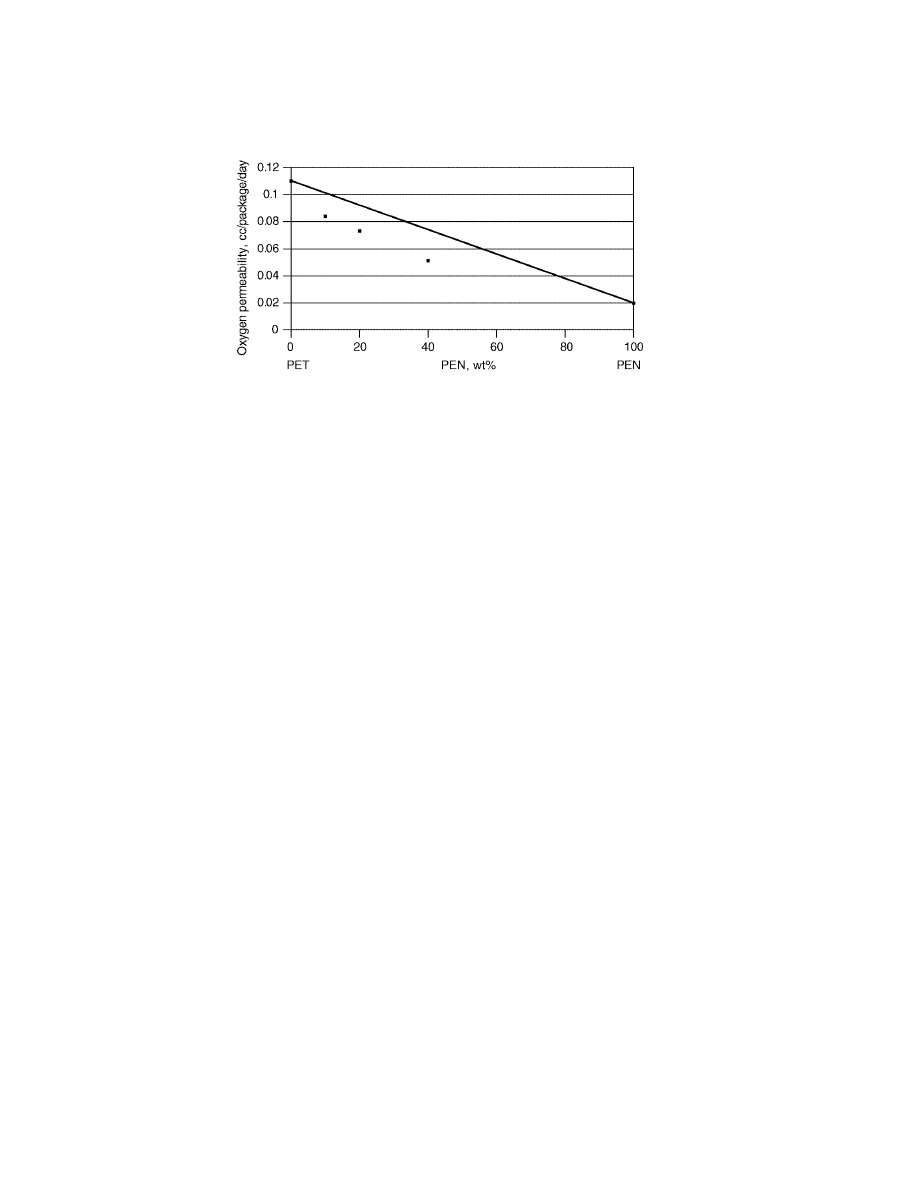
The permeability of PET/PEN copolymers and blends is as good or even lower
than that predicted by a linear fit between that of PET homopolymer and PEN
homopolymer. For example better-than-linear behavior was reported in the case
of O
2
permeability through 4
× 4 biaxially stretched films (Fig. 3).
Chemical Resistance.
PEN offers significant improvement in chemical,
oxidative, and hydrolytic stability vs PET. This has led to increased use of PEN in
demanding engineering resin, fiber, film, and plastics applications and in return-
able/refillable, pharmaceutical, and chemical packaging (Table 7).
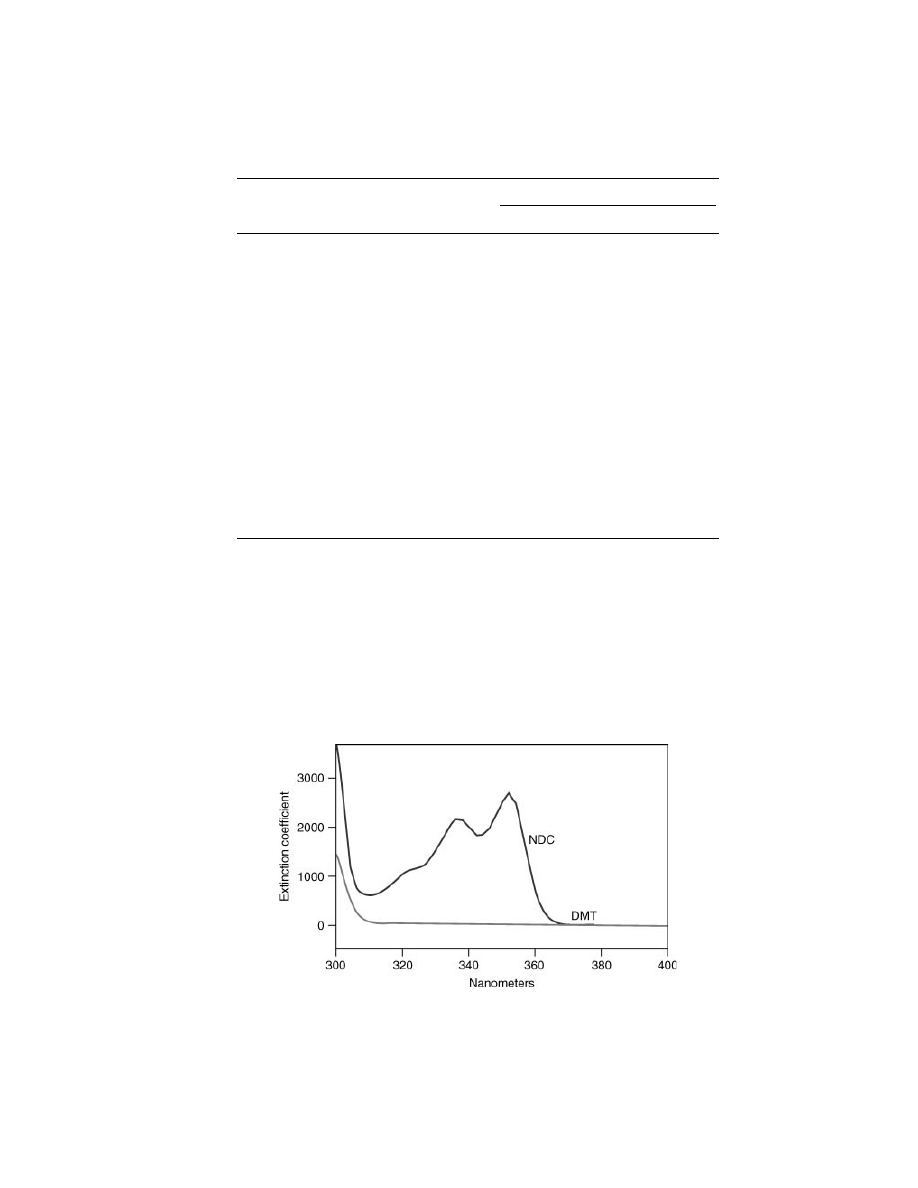
UV Absorption Characteristics.
Naphthalate-containing polyesters
demonstrate a dramatically improved barrier to UV radiation. They also exhibit a
greater retention of mechanical strength under external exposure or weathering
conditions. This behavior is due to the fused aromatic structure of the naphtha-
late ring of NDC, which absorbs UV light at wavelengths as high as 370 nm. The
benzene ring structure of DMT or TA is essentially transparent at wavelengths
above 310 nm. This UV absorption behavior is also exhibited in the corresponding
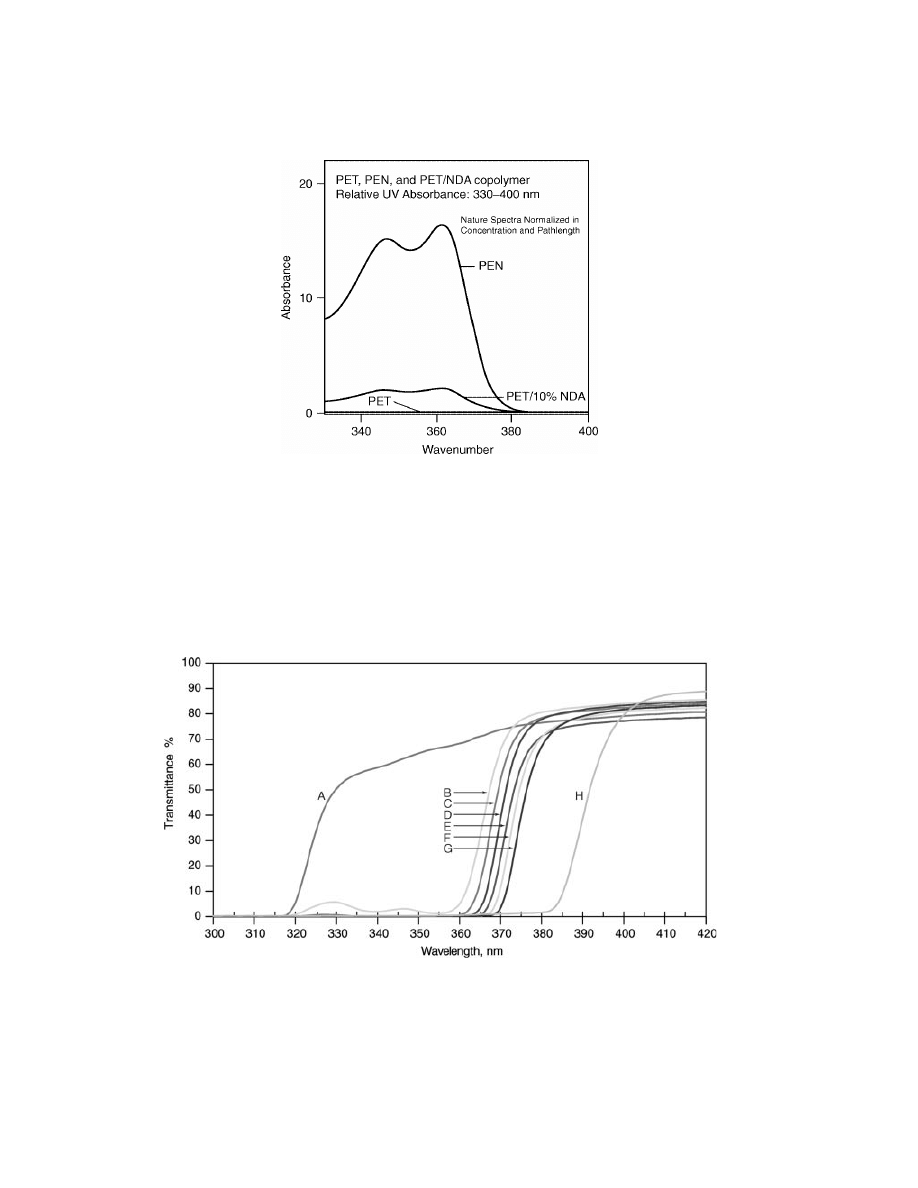
polymers (Figs. 4 and 5).
The combination of even very low levels of PEN with PET (by copolymer-
ization or blending) can be a very effective UV barrier. This effectiveness, in the
form of transmission versus wavelength through constant thickness (0.33 mm) is
illustrated in Figure 6.
Light transmittance T is defined as T
= I/I
0
, where I
0
and I are intensities
of incident and transmitted light respectively. The effect of PEN incorporation on
light transmission properties at a given wavelength follows the Beer–Lambert
Law, A
= εCD, where A = −log T (“absorbance”), C is PEN concentration, D
is light path length, and
ε is the naphthalate extinction coefficient at the given
wavelength.
94
POLY(ETHYLENE NAPHTHALATE) (PEN)
Vol. 11
Table 7. Comparison of PET and PEN Chemical Resistance
a
% Retention of elongation at break
Chemical Agent
PET
PEN
1% hydrochloric acid
2 weeks
72
85
5 weeks
74
106
10% hydrochloric acid
2 weeks
4
69
5 weeks
0
60
1% sodium hydroxide
2 weeks
76
97
10 weeks
97
126
10% sodium hydroxide
1 week
0
70
2 weeks
0
50
Ammonia gas
2 weeks
15
93
10 weeks
0
96
Thermal aging
2 weeks at 180
◦
C, 0% RH
0
80
2 weeks at 130
◦
C, 100% RH
20
50
a
From Ref. 8.
Mechanical Properties.
Naphthalate presence improves mechanical
properties such as modulus and tensile strengths. Like PET, PEN can be oriented
uniaxially (fiber) or biaxially (film and bottle) and heat-treated in a variety of
ways. Consequently, mechanical properties vary over a wide range depending upon
the exact fabrication parameters used. Furthermore, in films, mechanical prop-
erties may differ between the machine (MD) and transverse (TD) directions; bal-
anced films have very similar properties between the MD and the TD, whereas in
Fig. 4.
UV absorption spectrum of NDC vs DMT. Data from BP Amoco Chemical Co.
Vol. 11
POLY(ETHYLENE NAPHTHALATE) (PEN)
95
Fig. 5.
UV absorption of PEN and PET. Data from BP Amoco Chemical Co.
tensilized films, the properties in MD and TD differ substantially. In general how-
ever, PEN bottle sidewalls, fibers, and films are capable of attaining tensile or flex
modulus 1.5–3 times higher versus PET. Correspondingly, the elongation at break
of PEN is generally lower versus PET.
Table 8 compares mechanical properties of PEN vs PET in typical films and
high strength fibers.
Fig. 6.
Light transmission through 0.33 mm for PET, PEN, and PEN/PET combinations.
A PET, B 0.3% PEN, C 0.6% PEN, D 1.3% PEN, E 2.5% PEN, F 3.1% PEN G 6.2% PEN, H
PEN. Data from BP amoco Chemical Co.

96
POLY(ETHYLENE NAPHTHALATE) (PEN)
Vol. 11
Table 8. Mechanical Properties of PEN and PET Films
a
and Fibers
b
PEN
PET
50-
µm films
Young’s modulus, Mpa
c
MD
5000
3800
TD
5350
4200
Tensile Strength, Mpa
c
MD
265
190
TD
150
110
Stress at 5% elongation, MPa
c
MD
80
140
TD
70
100
Elongation at break, %
MD
80
140
TD
70
100
Thermal shrinkage at 150
◦
C, %
MD
0.6
1.3
TD
0.4
1.3
High strength industrial fibers
Tenacity, N/tex
d
0.9
0.8
Modulus, N/tex
d
32
9.9
Elongation at break, %
8
14
Boiling water shrinkage, %
1
5
Dry heat shrinkage at 177
◦
C, %
4
8
a
Taken from 1995 DuPont Films PEN Film Brochure.
b
Refs. 9–11.
c
To convert MPa to psi, multiply by 145.
d
To convert N/tex to g/den multiply by 11.33.
PEN Polymerization
Melt-Phase Polymerization.
Commercially PEN is produced from 2,6-
NDC (abbreviated to “NDC” in the rest of this article) in a process analogous to
producing PET from DMT. The reactivity of NDC in a PEN polymerization process
is similar to that of DMT in a PET polymerization process and similar catalyst
and conditions can be used. Because of this, PEN preparation can be typically
accomplished in existing DMT-based polymerization facilities with only minor
modification. This modification is generally limited to the NDC feed system and
changes required to handle the physical property differences of NDC vs DMT and
of PEN vs PET.
Comparative physical properties of DMT and NDC are summarized in
Table 9.
The primary differences between PEN and PET polymers from a preparation
point of view are the glass-transition temperature and the melt viscosity.
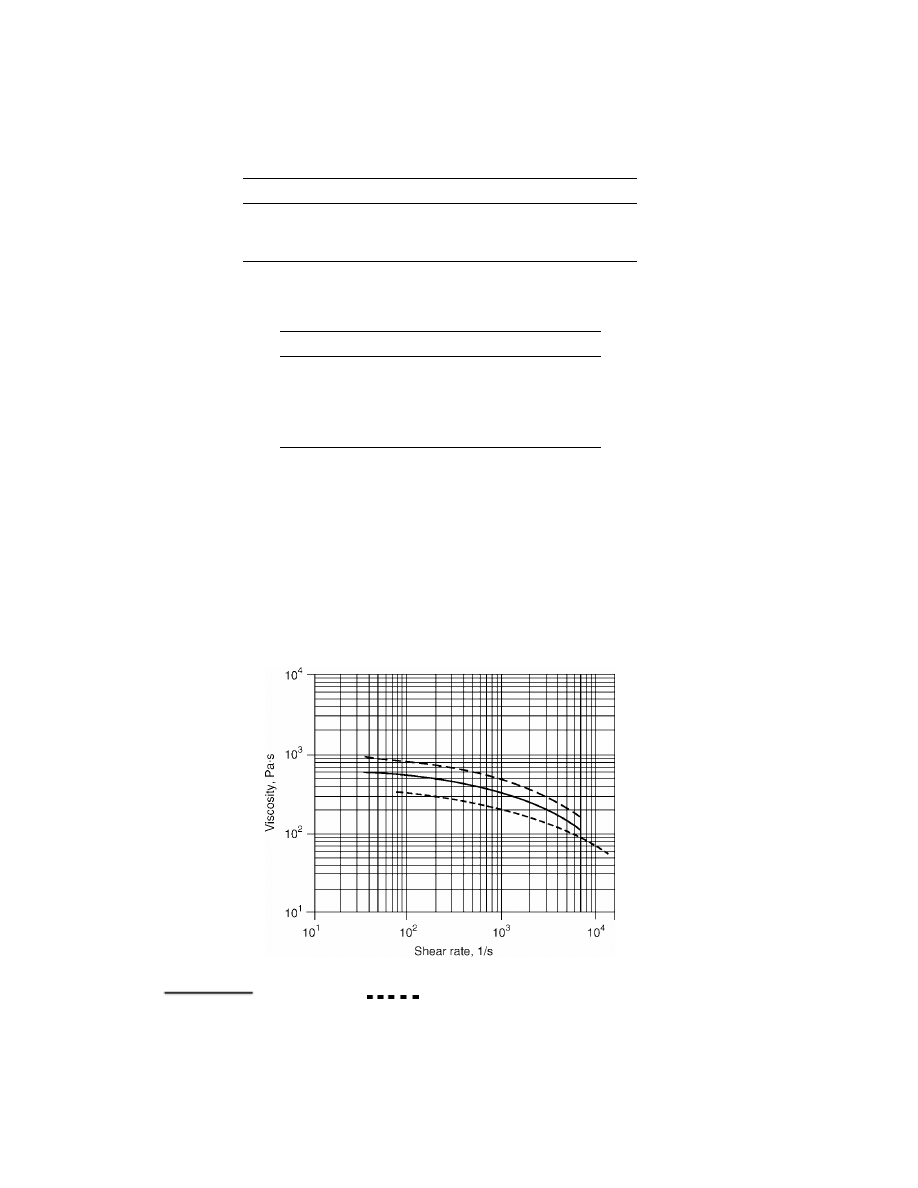
The melt rheology of PEN resin is significantly higher than that of PET even
at lower inherent viscosities (IVs) and/or higher temperatures. The rheology of
PEN at an IV typically produced in a melt-phase polymerization (0.49–0.53) was
Vol. 11
POLY(ETHYLENE NAPHTHALATE) (PEN)
97
Table 9. Properties of NDC and DMT
a
NDC
DMT
Molecular weight
244.26
194.19
Melting point,
◦
C
190
140
Specific gravity
1.35
1.28
a
Data from BP Amoco Chemical Co.
Table 10. Solubility of NDC in Diols Used in
Polyester Polymerization
a
Ethylene glycol at:
Solubility in g/100 g diol
135
◦
C
1.0
160
◦
C
9.1
167
◦
C
23.1
170
◦
C
31.1
178
◦
C
79.9
a
Data from PB Amoco Chemical Co.
measured at 295
◦
C and compared to typical PET melt-phase resin. As Figure 7
indicates both PEN IVs had higher viscosities than that of PET.
PEN and copolyesters are readily prepared from NDC and ethylene glycol
using either batch or continuous polymerization (12). Solubility data for NDC in
ethylene glycol is summarized in Table 10. An example of a typical continuous
polymerization process is shown in Figure 8.
NDC, ethylene glycol, and a suitable catalyst are metered into the first of a se-
ries of ester-exchange reactors. Normally, several ester exchange reactors provide
Fig. 7.
Viscosity vs shear rate for PEN and PET at 295
◦
C. –—PEN IV
= 0.53,
PEN IV
= 0.49,
PET IV
= 0.64. To convert Pa·s to poise, mul-
tiply by 10.
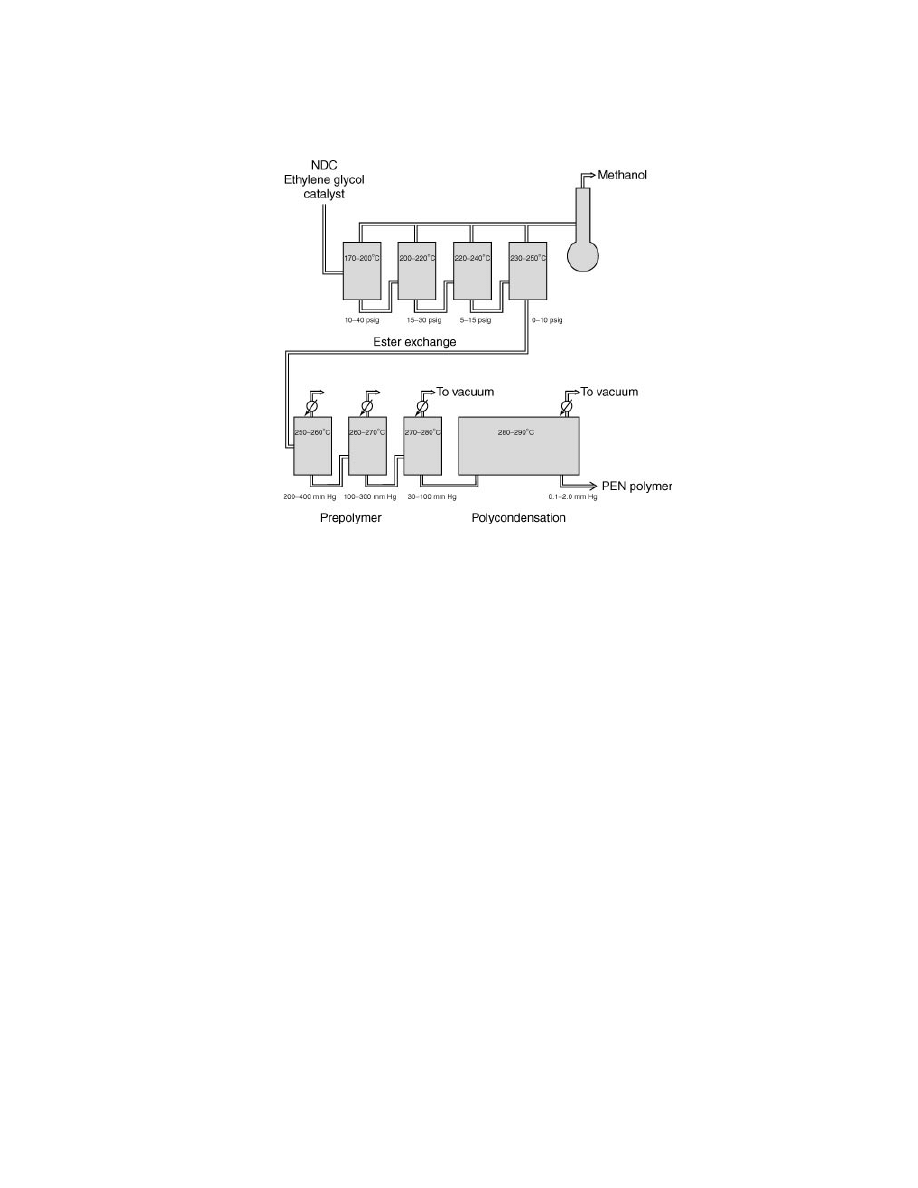
98
POLY(ETHYLENE NAPHTHALATE) (PEN)
Vol. 11
Fig. 8.
Continuous PEN polymerization.
control of the temperature profile and conversion to 2-hydroxyethyl terminated
oligomers, while the by-product methanol is removed and recovered. Glycol-to-
ester feed mole ratios typically range from 1.5 to 3.0, while reactor temperatures
range from 170
◦
C in the initial reactor to 250
◦
C in the final reactor. The initial reac-
tor pressure can range from 10 to 40 psig, dropping to 0–10 psig in the final reactor.
The ester-exchange reaction product, a mixture of oligomers, is subsequently
metered into the first of a series of prepolymer reactors. Generally, several agitated
reactors are used. Operating in a temperature range of 250–280
◦
C, excess ethy-
lene glycol is removed and the resulting prepolymer is metered into a final re-
actor to complete the polymerization. According to this process, PEN polymer is
produced having an IV of 0.4–0.6 dL/g. Examples of suitable final polymeriza-
tion reactors include the horizontal types supplied by Zimmer AG (Germany) and
Hitachi (Japan).
Catalysts, such as manganese, zinc, calcium, cobalt, and titanium, have been
widely used as effective ester-exchange catalysts. Polycondensation catalysts, such
as antimony, are commonly employed. Phosphorus compounds can be added to
increase the thermal stability of the finished resin.
Crystallization/Solid-State Polymerization.
As in the case of PET, pro-
duction of PEN polymer for container or packaging applications will typically
include crystallization/solid-state polymerization step. Because PEN has a higher
melt viscosity and gas barrier, the volatile byproducts of a solid stating process,
such as water, ethylene glycol, or acetaldehyde, are more easily trapped inside the
amorphous pellet. This may result in a tendency of the pellets to undergo sudden
expansion during crystallization or solid stating.

Vol. 11
POLY(ETHYLENE NAPHTHALATE) (PEN)
99
Several procedures have been described for crystallizing/solid stating PEN.
These procedures include adding a devolatilization step (13), or conducting the
crystallization under elevated pressure (14) or in the presence of a liquid (15).
Because of its higher T
g
vs PET, the optimum crystallization temperature range
for PEN (180–220
◦
C) is higher than that of PET (150–190
◦
C). Similarly, because
of its higher T
m
, the typical SSP temperature of PEN (240–260
◦
C) is higher than
that of PET (210–240
◦
C). On the other hand, PEN copolymers with 8–10 mol% TA,
which match the PET melting point, should be solid-stated at the same tempera-
ture conditions as PET. A typical solid-stated PEN resin has an IV in the range
0.55–0.70 dL/g.
Fiber Applications
The principal applications of PEN and PEN/PET blends and copolymers are as
films (see P
OLYESTER
F
ILMS
).
PEN multifilament fibers are commercially available from several polyester
fiber producers. These include Teijin in Japan, Honeywell (formally AlliedSignal)
in the United States and Europe, Kosa in Europe, and Hyosung in Korea. PEN
fibers are produced in a melt spinning/post-drawing process similar to that used
in PET fiber manufacture. All production to date is in the form of continuous
multifilament yarn, although staple and monofilament fabrication have also been
reported.
PEN-based fibers extend the performance of polyesters. PEN fibers have
excellent heat resistance, modulus, and dimensional stability relative to PET
and demonstrate better retention of mechanical properties in a hot/wet environ-
ment and offer improved chemical resistance versus PET fibers (see P
OLYESTERS
,
F
IBERS
). With naphthalate modification, polyester fibers can meet the application
requirements, which are currently served by other high performance industrial
fibers such as rayon, nylon-6,6, aramids, poly(phenylene sulfide) (PPS), and even
steel.
The primary commercial application of PEN fiber today is in rubber rein-
forcement. Fibers with high molecular weight, crystallinity, and high molecular
orientation are required. To produce high orientation PEN fibers requires opti-
mization of both resin design and spinning technology. Typically, high IV (
>0.9)
solid-stated PEN resin is used. High IV PEN resin is available from suppliers
such as M&G and Teijin. In addition to rubber reinforcement (for tires, belts, and
hoses) applications for PEN fibers include ropes and cordage, and sailcloth.
Tire Reinforcement.
PEN’s superior thermal and mechanical properties
make it a natural candidate for the reinforcement of radial passenger and light
truck tire carcasses. These superior properties are summarized in Table 11.
PEN yarn has higher tenacity and improved modulus vs PET, nylon, and
rayon. Its thermal properties, eg, dry heat shrinkage, are also superior. This lower
thermal shrinkage is a significant benefit in tire cord manufacture because of the
heat-setting treatment and preshrinkage relaxation required to convert yarn to
twisted cord. Compared to PET, the lower thermal shrinkage of PEN enables less
preshrinkage treatment, which results in a final cord that has improved tenacity
and modulus.
100
POLY(ETHYLENE NAPHTHALATE) (PEN)
Vol. 11
Table 11. Physical Properties of High Strength Industrial Yarns Used in Reinforcement
PEN
PET
a
Nylon
b
Rayon
c
Aramid
d
Density
1.36
1.39
1.14
1.52
1.44
Tenacity, N/tex
0.9
f
0.8
0.8
0.44
2
Tensile modulus, N/tex
e
32
g
9.7
4.4
11
49
Tensile elongation, %
8
h
14
15
12
4
Boiling water shrinkage,%
1
g
5
8
Decomposes
<0.1
Dry heat shrinkage (1 min at 177
◦
C)
4
g
8
10
6
<0.1
a
Ref. 11.
b
Ref. 16.
c
Ref. 17.
d
Ref. 18
e
To convert N/tex to g/den, multiply by 11.33.
f
Refs. 9 and 10.
g
Ref. 9.
h
Ref. 10.
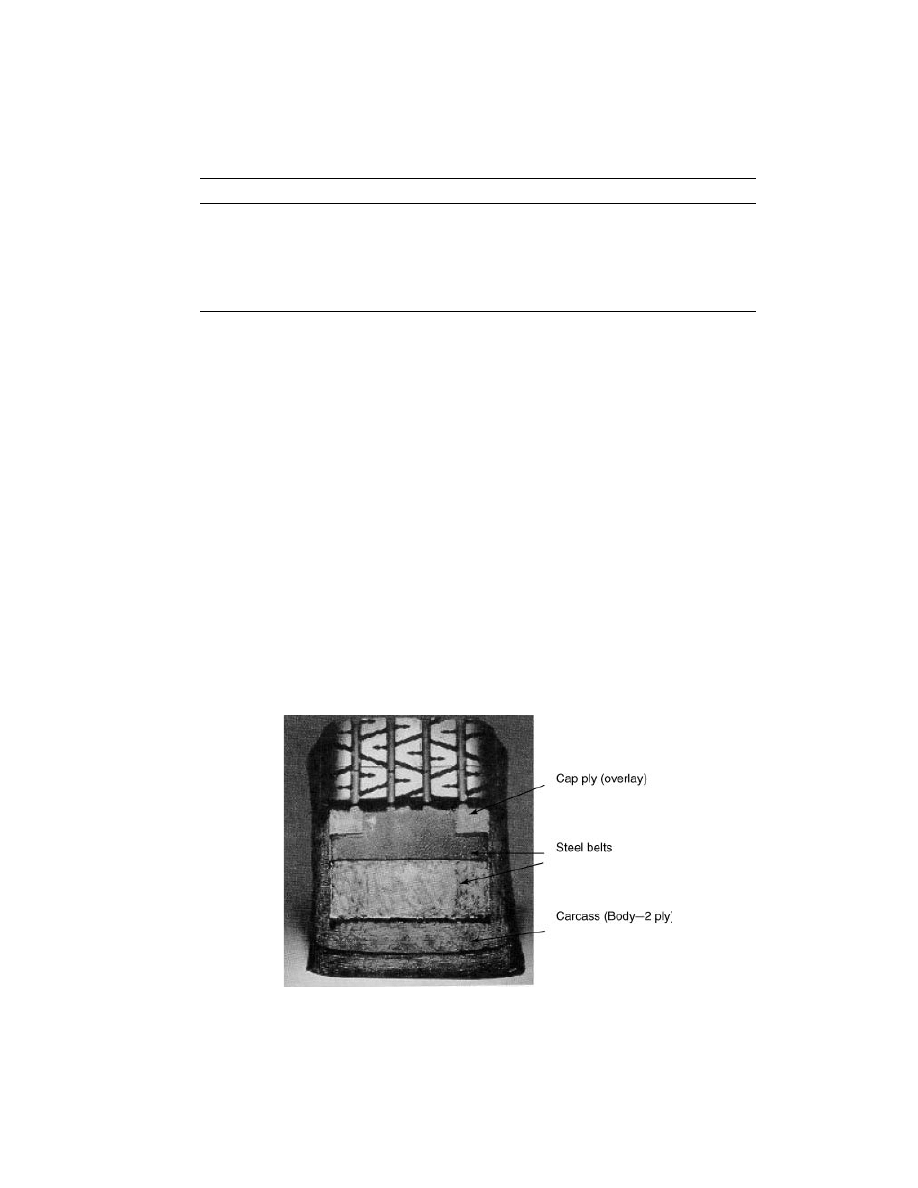
There are multiple opportunities for PEN fiber use in tire construction.
Figure 9 is a typical tire construction schematic, showing the three different tire
cord textile layers, carcass, belt reinforcement, and cap ply, where PEN fiber can
be used.
Cap Ply Reinforcement with PEN.
The cap ply is wound over the shoul-
ders or the entire body of the belt to provide a compressive force, which resists the
centrifugal forces created in the belt at high rpm. Nylon-6,6 has been typically
used for cap ply applications because of its high retractive force at elevated tem-
peratures. PEN cord has been demonstrated to offer similar properties to nylon at
177
◦
C and is an excellent replacement material. Honeywell (formally AlliedSig-
nal) has commercialized high tenacity/high modulus PEN fiber under the trade
name PENTEX. In January 1999, Italian tire company Pirelli launched a new
Fig. 9.
Radial tire anatomy (19).

Vol. 11
POLY(ETHYLENE NAPHTHALATE) (PEN)
101
tire reinforced with caps made from PENTEX (20). Pirelli uses tire cord made
from PENTEX in its Dragon EVO Corsa line of radial motorcycle tires. PEN’s
significantly higher modulus vs nylon or rayon, 22, 4.4, and 11 N/tex (250, 50, and
125 g/den) respectively, gives it the dimensional stability required for these high
performance uses.
Bridgestone, the largest tire company in Japan, is also using PEN cord in
nylon cap replacement applications in its Regno GR7000 tire (21). In addition to
the dimensional stability improvements, the rigidity that PEN brings to the tire
prevents noise generated at the road surface from being transmitted to inside the
car. Tests show that these PEN-containing tires reduce road noise by as much as
30%, making the tires ideally suited for high end luxury cars, such as the Lexus.
Carcass Reinforcement.
There are several fibers currently used in carcass
reinforcement. High modulus/low shrinkage PET is typically used in most radial
passenger tires and offers a good mix of properties. Nylon is used extensively in
bias ply tires where strength is paramount; this has more value in developing
countries with poorly maintained roads and in off-the-road equipment tires. In
most of the developed world nylon carcass tires are not longer preferred in pas-
senger cars because of the problem of flat-spotting associated with nylon’s high
moisture regain. Rayon is the reinforcement cord of choice in Europe for high
performance tires, due to its ability to maintain mechanical properties at
high temperature.
The performance properties of PEN present opportunities for rayon replace-
ment in carcass construction (22). The use of PEN cord in these applications is
currently being evaluated in both Asia and Europe. PEN has a demonstrated
acceptable flexural fatigue equivalent to PET and rayon. It has equivalent tough-
ness to rayon, which is important for sidewall impact resistance and curb scuffing
resistance. PEN’s superior mechanical properties also afford opportunities to use
less fiber in carcass construction, enabling production of lighter weight, more fuel-
efficient tires.
Steel Belt Replacement with PEN.
In radial tires, the carcass provides
reinforcement along the radial direction. To provide reinforcement in the rolling
direction, belts are necessary. Steel is the traditional material of choice but there
has been a drive to replace it with lighter weight synthetic cords. Aramids offer
a synthetic alternative but their cost has been prohibitive except for high per-
formance racing tires and luxury cars. PEN has good fatigue at low twist and
superior compressive modulus to aramid. This translates into a significant reduc-
tion in tire weight and improvement in fuel usage. In addition, use of a polyester
fiber-like PEN offers greater opportunity to recycle used tires.
Belts and Hoses.
As automotive design trends toward higher engine op-
erating temperatures, the requirements for under the hood reinforcement fibers
have changed. Today, fibers not only require good mechanical and thermal proper-
ties, but also improved chemical/hydrolytic resistance in a hot-wet environment.
PEN fiber meets these more demanding requirements. Performance advantages
of PEN include the following:
(1) cost (half compared to aramid)
(2) very low tension decay
102
POLY(ETHYLENE NAPHTHALATE) (PEN)
Vol. 11
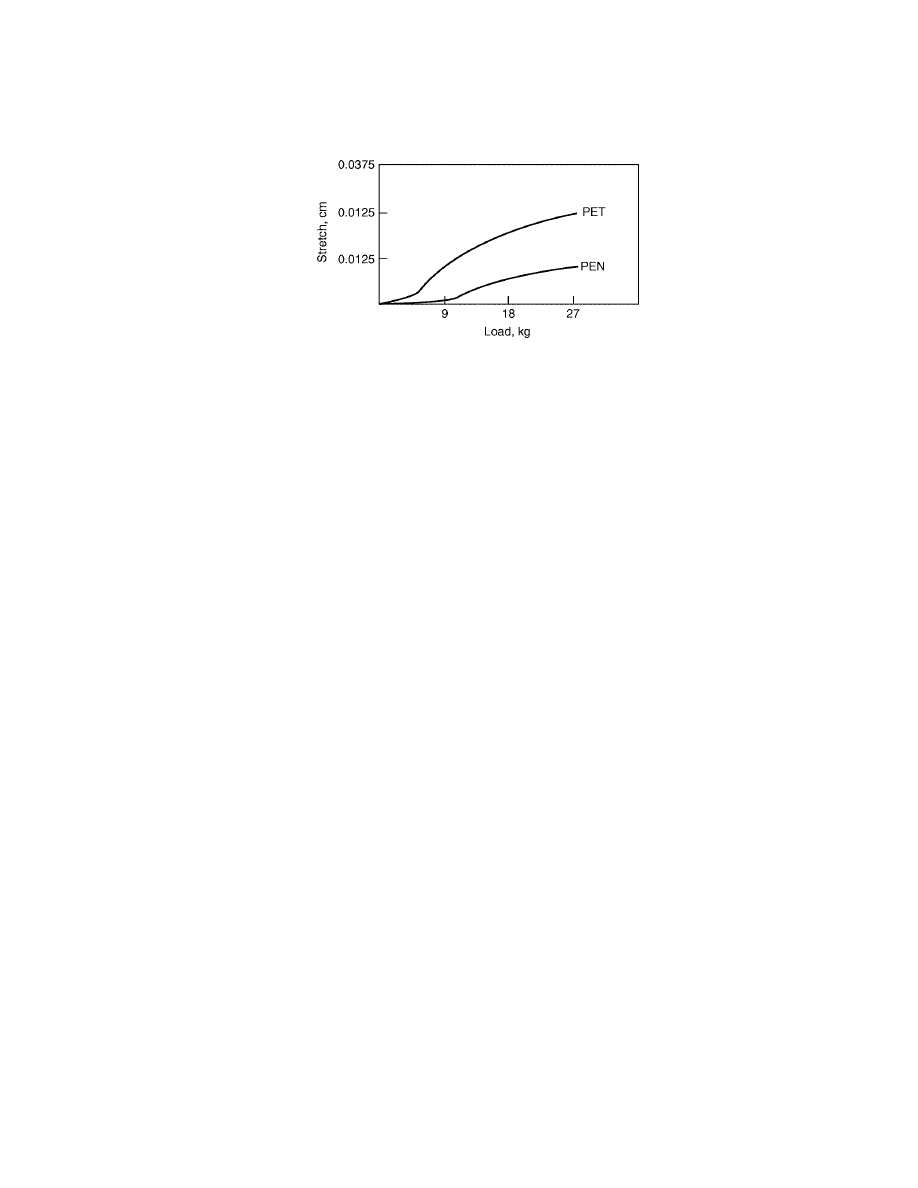
Fig. 10.
Stretch at equivalent load of PEN yarn compared to standard polyester (23).
(3) high modulus (two times of PET)
(4) low shrinkage after cure
(5) high adhesion and adhesion retention
(6) improved fatigue resistance
Several types of PEN- or PBN-reinforced belts and hoses, ranging from trans-
mission belts to oil brake hoses, have been produced. Additional applications
include timing belts, conveyer belts, high pressure hydraulic, steam, fuel, and
coolant hoses, as well as polychain belt cord.
Ropes and Cordage.
PEN’s high strength, hydrolytic stability, and re-
sistance to UV light degradation create numerous opportunities for its use in rope
and cordage applications. The combination of strength and low stretch make PEN
cordage the material of choice for rescue rope applications (Fig. 10). For example,
Sterling Rope utilized Honeywell’s high tenacity PEN yarn, Pentex, in their high
performance rescue rope products (24). The dimensional stability of the PEN res-
cue rope, with less than 1% stretch per 300-lb load, ensures steady support for
rescue operations.
Sailcloth.
PEN filament yarn has found applications in high performance
sailcloth construction. Challenge Sailcloth Inc. has introduced its SuperModulus
sailcloth made from Honeywell’s high tenacity PEN fiber, Pentex (23). The im-
proved UV resistance of Pentex fibers more than doubles the shelf life of sails in
the Caribbean, Mediterranean, and other high UV areas. The low stretch charac-
teristics of PEN also provide a significant advantage in performance and enables
production of a much lighter weight sail.
Rigid Packaging Applications
The use of naphthalate-modified polyester resins in packaging applications of-
fers improvement in gas barrier, UV barrier, thermal, and mechanical proper-
ties versus PET alone. Naphthalate resins suitable for packaging applications
are commercially available in either homopolymer (PEN), PET/PEN copolymers
(PETN-x), or PET/PEN blends.
Naphthalate-containing polyester resins at all compositions, from 0 to 100%,
are fully regulated for use in food contact applications. Naphthalate polymers are
Vol. 11
POLY(ETHYLENE NAPHTHALATE) (PEN)
103
Table 12. Naphthalate Resin Benefits in Rigid Packaging Applications
Application
Naphthalate Benefit
Typical resin type
Returnable/refillable
(Beer, mineral water,
water fountain jugs,
juice milk)
Improved thermal resistance
(washing)
Improved chemical resistance
(washing)
Reduced flavor carryover
Reduced adsorption
Improve gas barrier
(CO
2
loss and O
2
ingress)
Increased UV barrier
PEN, copolymers, blends
Beer—one way
Pasteurization capability
Increased UV barrier
Copolymers
Small-size carbonated
soft drinks (
<0.5 L)
Improved barrier against CO
2
loss
Increased UV barrier
Copolymers, blends
Hot-fill applications
(Foods, sport drinks,
fruit juice)
Improved thermal resistance
Improved barrier
Increased UV barrier
PEN, copolymers, blends
Sterilizable applications
(baby bottle)
Improved thermal resistance
PEN, high PEN
copolymers
Retort applications
(baby food)
Improved thermal resistance
PEN, high PEN
copolymers
Pharmaceutical/cosmetic
Improved barrier
Improved chemical resistance
UV barrier
PEN, copolymers, blends
Industrial and speciality
Improved barrier
Improved chemical resistance
PEN, copolymers, blends
permitted for use with all food types and under all conditions of use as defined
by the FDA. These include packaging for foods that undergo high temperature
sterilization in the container, to frozen foods reheated in the container.
A summary of naphthalate rigid packaging commercial and potential appli-
cations is provided in Table 12. Applications of naphthalate-modified polyesters
can be segmented by resin type and naphthalate content. This includes PEN
homopolymer, medium level PET/PEN blends and PETN-x, or low naphthalate
level copolymers. The use of a particular resin type is often dictated by opti-
mization of cost versus the thermal and barrier requirements of the application.
Figure 11 summarizes current uses and potential opportunities for naphthalate
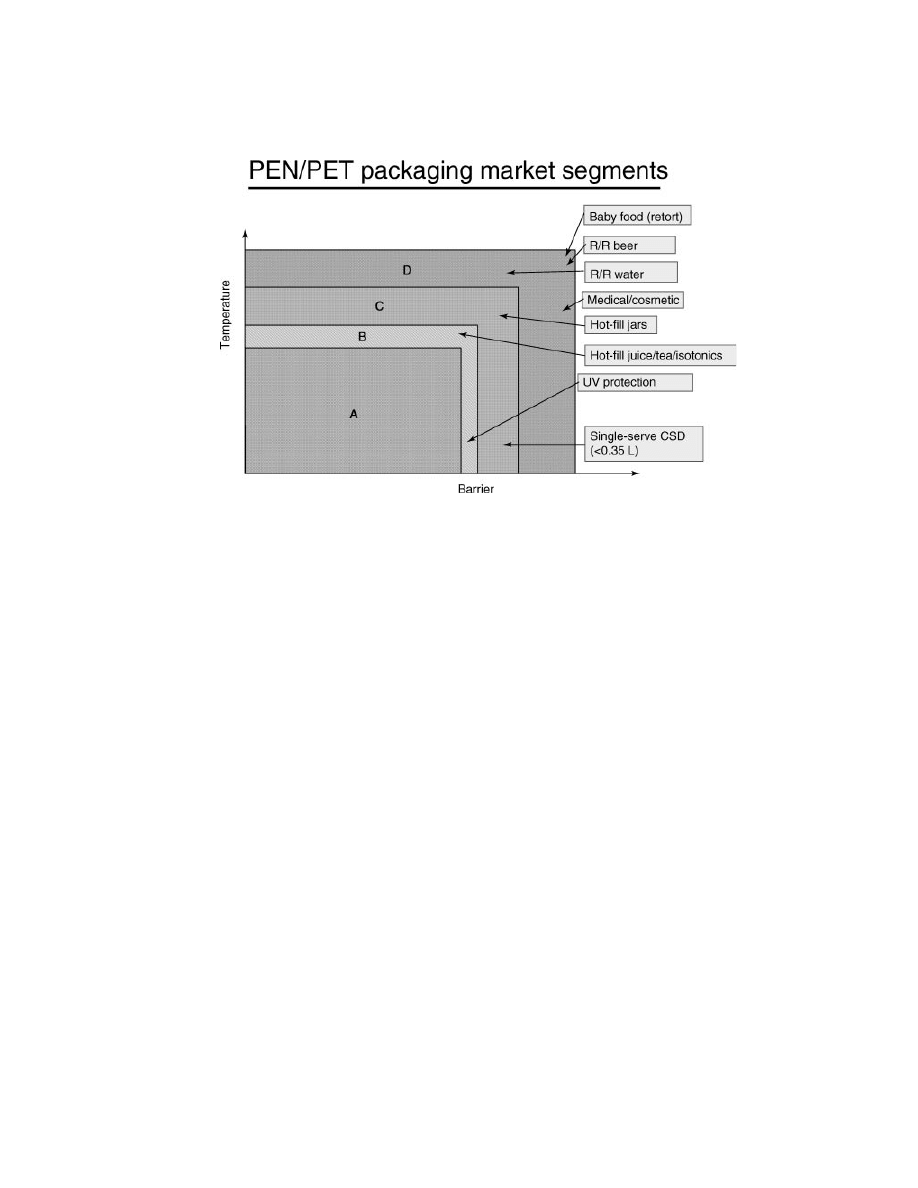
polyester resins in rigid packaging.
Rigid Packaging Applications for PEN Homopolymer and High PEN
Copolymers.
PEN homopolymers and high level PEN copolymers are best
suited to rigid packaging application requiring the maximum in organoleptics,
temperature, and gas barrier improvement. Specific published examples, includ-
ing market trials and commercial launches, are listed below.
Returnable/Refillable Beer Bottle.
In August 1999, Carlsberg A/S
launched PEN returnable/refillable bottles for their Carlsberg Lager and Tuborg
Green Label brands sold in Denmark (20,25). Danish law requires that all carbon-
ated bottles filled in Denmark are returnable and refillable. Although for one-way
104
POLY(ETHYLENE NAPHTHALATE) (PEN)
Vol. 11
Fig. 11.
Opportunities for PEN homopolymers, copolymers, and blends in rigid packaging.
A PET, B Low PEN copolymer, C PEN blends, D PEN.
beer coated plastic bottles multilayer PET solutions are available, PEN is the only
plastic material that combined the necessary barrier, pasteurization capability
and the ability to withstand repeated washings at temperatures used to sterilize
glass bottles—up to 85
◦
C. The PEN bottles keep carbon dioxide in and oxygen out,
enabling a shelf life of greater than 4 months. The ability to reuse the bottle, an
80% reduction in weight versus glass, makes the PEN return refill package the
preferred environmental and total system cost solution. PEN returnable/refillable
beer bottles were also launched in Norway and in Brazil (24,25).
PEN Returnable/Refillable Water Bottle.
The washing temperature used
in returnable/refillable water bottles (up to 90
◦
C in caustic solutions) is higher
than the washing temperatures typically used for returnable/refillable carbon-
ated soft drink (CSD) bottles (45–60
◦
C). This is dictated by higher requirements
for removal of trace foreign contaminants that might contribute to bad taste.
In addition, antiscalping and carbonation retention performance are equally
critical. In view of all these factors, PEN, with its high T
g
(125
◦
C vs 80
◦
C
for PET), high carbonation retention (four times relative to PET) and excellent
anti-sorption/desorption characteristics have been an ideal material for packag-
ing returnable/refillable water. In Uruguay, a PEN returnable/refillable bottle was
introduced for mineralized water sold under the Bonaqua brand by MONRESA
(Montevideos Refrescos, SA), a bottler of The Coca-Cola Co. (26). With PEN bot-
tles, carbonation retention was demonstrated to be four times greater than with
PET bottles. The returnable/refillable PEN bottles easily withstand washing with
a 2% sodium hydroxide solution at 90
◦
C. No stress cracking and less than 1%
dimensional change was shown after 100 washing and refilling cycles.

Vol. 11
POLY(ETHYLENE NAPHTHALATE) (PEN)
105
PEN Cosmetic Bottle.
Replacing glass with clear plastic in small bottle
applications, like ampules for cosmetics or medicines, requires a polymer that has
acceptable resistance against heat distortion, environmental stress cracking, va-
por barrier, and creep in the amorphous state. This is because the injection blow
process used to fabricate clear small containers typically affords an amorphous or
low crystalline bottle. PEN homopolymer with its high T
g
(125
◦
C) and low gas per-
meability and chemical resistance is ideal for these applications. One commercial
example is a skin care product from Japan’s leading mail-order cosmetics com-
pany, FANCL (20). Substitution from glass to plastic in this case was driven by
two factors: waste disposal in incinerators (glass does not burn) and reduction in
total packaging size and cost. With the PEN bottle, FANCL reduced the amount
of required protective packing, decreased the size of the box, and substantially
reduced the total costs of packaging.
PEN for Medical Applications.
Another application for PEN is medical
packages, where material selection is driven by chemical resistance, safety, and
strength. One example is a package from Abbott Laboratories (24), used for the
drug Sevoflurane, a volatile anesthetic used in hospital operating rooms. PEN
was chosen to replace glass because of its chemical resistance, low extractables,
and strength. The container needs to be able to contain pressure buildup if the
contents increase in temperature.
PENT-8 Reusable Baby Milk Bottle.
Although polycarbonate (PC) has tra-
ditionally been the preferred alternative to glass in reusable baby milk bottles,
health concerns with PC usage have been raised in some countries about possible
presence of residual bisphenol A. The use of a high PEN-containing copolymer,
PENT-8, has been demonstrated to provide a bisphenol A free substitute for PC.
In Korea, Kolon has found that PENT-8 has adequate T
g
(123
◦
C) to withstand
required sterilization in boiling water (20). At the same time, the lower melting
point of PENT-8 (250
◦
C) relative to PEN (268
◦
C) makes it processable at PET
melt conditions.
PEN School Lunch Tableware.
Another example where PEN replaced PC
owing to concerns about bisphenol A was in tableware for school cafeterias in
Japan (24). The producer, Sanshin, selected PEN homopolymer for its ability to
withstand multiple washings and its antiscalping properties.
PEN Bottle Fabrication by Injection Stretch Blow Molding.
PEN
is semicrystalline polyester-like conventional PET, and those familiar with and
skilled in the fabrication of conventional PET should be able to produce good qual-
ity bottles from PEN. However, there are some differences between PEN and PET
that must be considered when fabricating PEN. The most important of these are
the higher glass-transition temperature and melting point of PEN (Tables 2 and 3).
Because of its higher melting point, PEN must be injection-molded at higher
temperatures than PET, and because of the higher glass-transition tempera-
ture, PEN preforms must be preheated to higher temperature when stretch blow
molded.
With respect to preform design, optimization of bottle properties will result
from using a slightly higher stretch ratio for PEN than for conventional PET. This
is illustrated in Figure 12, which shows stretch ratios of PET and PEN of various
IVs. The data were obtained by free blow experiments that were conducted at the
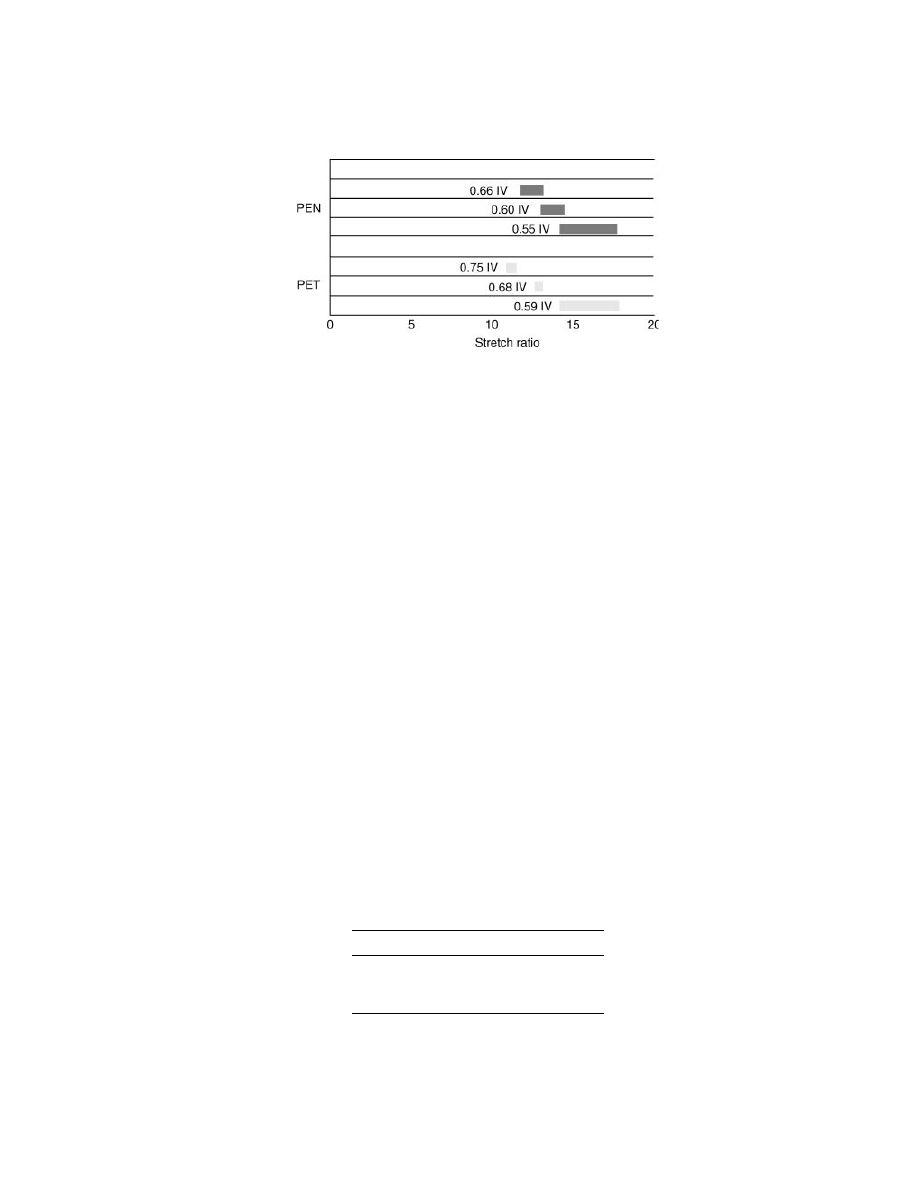
106
POLY(ETHYLENE NAPHTHALATE) (PEN)
Vol. 11
Fig. 12.
Stretch ratios of PET and PEN.
optimum stretch temperature range for each resin, which was 105–110
◦
C in the
case of PET and 165–170
◦
C in the case of PEN.
The results show that stretch ratio decreases with increasing IV for both
PET and PEN but that for a given IV, the stretch ratio of PEN is greater than that
of PET. This means that for typical commercial PEN with an IV of about 0.65, one
would want to design the preform tooling with a slightly higher stretch ratio than
used for typical 0.80 IV commercial PET.
Resin Drying.
Like conventional PET, PEN is sensitive to hydrolysis and
must be dried prior to melt processing. Recommended drying conditions for PEN
are shown in Table 13.
The recommendations are based on a polyester pellet size of 40–50 pellets/g
and may need to be adjusted if the pellets are smaller (shorter time) or larger
(longer time). The polyester should be dried to a moisture level of 50 ppm or less
prior to melt fabrication. Drying temperature greater than 175
◦
C (350
◦
F) should
be avoided to prevent discoloration of the polyester.
Preform Injection Molding.
PEN homopolymer must be molded at signifi-
cantly higher temperatures than PET to accommodate the higher melting point
and melt viscosity of PEN. In general, for a given injection molding machine,
the initial temperature settings should be about 20
◦
C higher for PEN than those
typically used for PET. From that starting point, conditions can be adjusted to
provide optimum quality preforms. Representative processing conditions for PET
and PEN are shown in Table 14 and Figure 13. The data are intended to provide
a relative comparison of molding conditions; optimum conditions will depend on
the specific resin and injection molding machine used.
Bottle Stretch Blowing.
The key difference in stretch blow molding of PEN
vs PET is related to the higher glass-transition temperature of PEN. Since the
Table 13. PEN Drying Conditions
Temperature
Drying time, h
150
◦
C (300
◦
F)
6.0
162
◦
C (325
◦
F)
5.0
175
◦
C (350
◦
F)
4.0
Vol. 11
POLY(ETHYLENE NAPHTHALATE) (PEN)
107
Table 14. Indicative Processing Conditions for Making
One-Way Amber Beer Bottle Preforms from PEN in
Comparison to PET
a
PEN
PET
Temp. settings,
◦
C
Feed zone
291
271
Zone 2
290
270
Zone 3
290
270
Zone 4
290
275
Nozzle
295
275
Back pressure, bar
50
35
Screw speed, m/min
10.0
10.0
Cooling time, s
18.0
18.0
Total cycle time, s
48.8
50.1
Shot size, g
36.9
36.9
Hot runner,
◦
C
Body
300
275
Tip
305
280
a
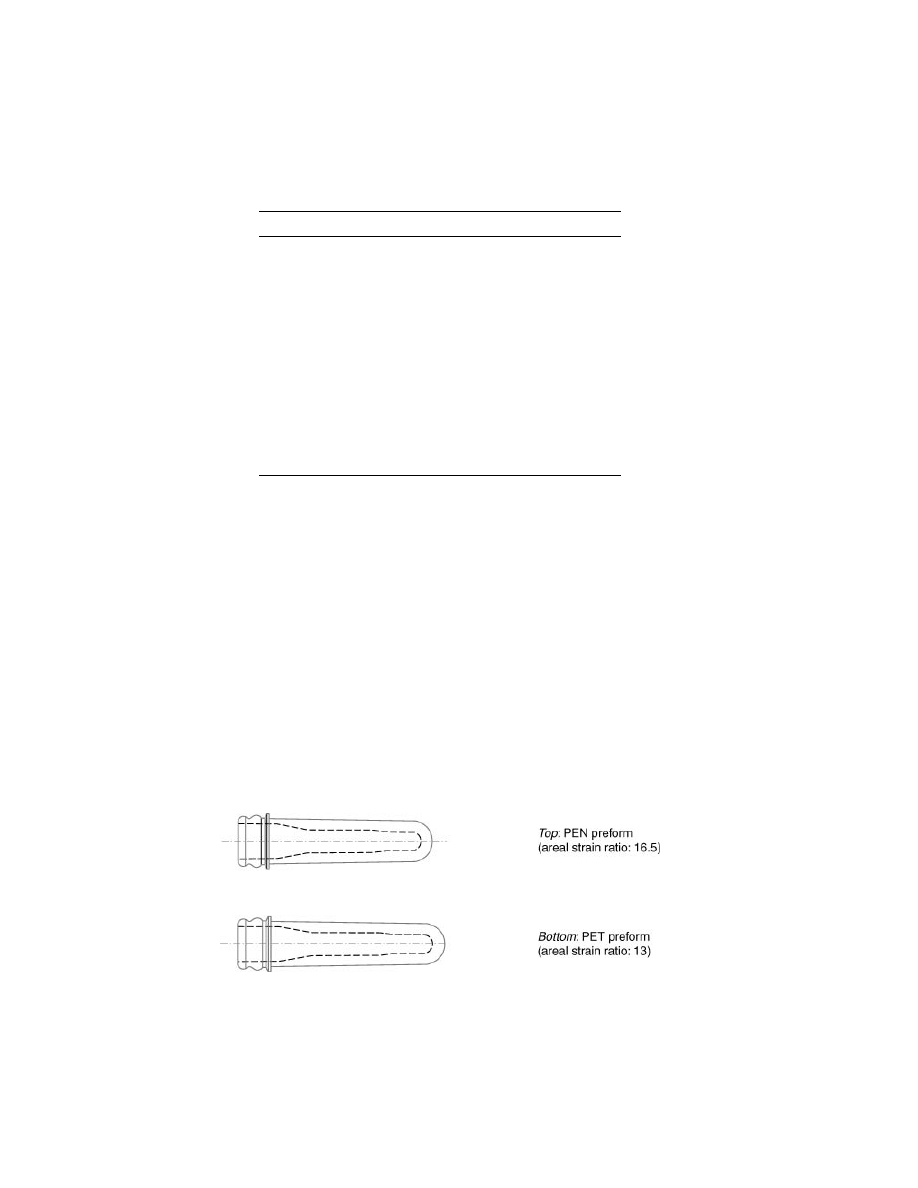
Bottle type: 500 mL, 37 gram weight, long neck with crown finish;
preform areal strain: 16.5 for PEN, 13 for PET (see Fig. 13). Amber
Colorant: Colormatrix #89-767-1 Tr Amber.
glass-transition temperature of PEN is approximately 40
◦
C higher than that of
standard PET, the PEN preforms must be heated approximately 40
◦
C higher than
PET preforms prior to stretch blow molding. This can be accomplished by increas-
ing the infrared lamp power settings. If not enough power is available at a given
line speed, the next step is to reduce line speed in order to increase residence time
in the infrared oven. Since the preform reheat temperature is significantly above
the water boiling temperature, it is recommended that amber-colored preforms
are kept dry or dried prior to stretch blowing, since the amber colorant particles
might nucleate boiling of moisture present, resulting in blistering. A representa-
tive set of stretch blow molding conditions (used in blowing the preforms shown
in Fig. 13, made according to Table 14) is shown in Table 15. The resulting bottle
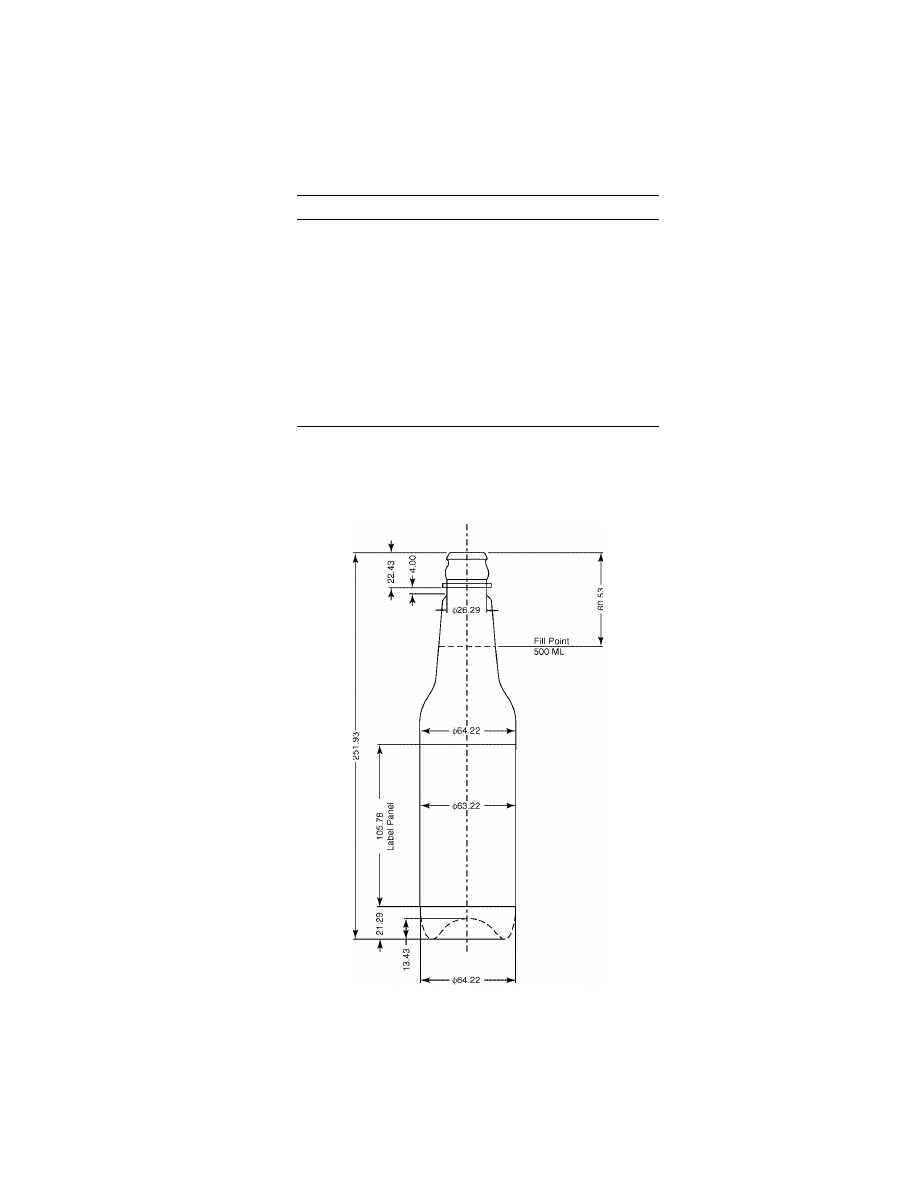
is shown in Figure 14.
Fig. 13.
Difference in preform design for making the same bottle from PEN vs PET (as
per Table 14).
108
POLY(ETHYLENE NAPHTHALATE) (PEN)
Vol. 11
Table 15. Representative PEN Stretch Blow Molding
Conditions vs PET
PEN
PET
Speed, bph
500
a
800
Mold temp.,
◦
C
4
4
Mold base temp.
4
4
Overall Oven Power, %
80
60
Oven lamp settings
Zone 6
50
70
Zone 5
0
70
Zone 4
65
0
Zone 3
30
60
Zone 2
50
32.5
Zone 1
72.5
85
Preform temp.,
◦
C
141
99
a
Slowed down vs. PET to provide additional residence time in the
oven for heating up preform to the required temperature (141
◦
C).
Fig. 14.
Schematic of bottle referred to in Table 15
Vol. 11
POLY(ETHYLENE NAPHTHALATE) (PEN)
109
Fig. 15.
Oxygen permeation of 0.35-L jars from PET/PEN blends (31).
Rigid Packaging Applications for PET/PEN Blends.
Physical blends
of PET with PEN are best suited for rigid packaging applications when perfor-
mance requirements demand a naphthalate content in a range of 15–90%. Random
copolymers within this range lack the ability to develop the strain–induced crystal-
lization needed in most bottle applications. Although PET/PEN blends are initially
immiscible, melt-blending PET and PEN under conditions where a limited amount
of transesterification takes place enhances miscibility, which results in a single T
g
and clear blend. The processing window required to produce an acceptable degree
of transesterification is wide enough to produce blends that are clear but still capa-
ble of developing strain-induced crystallinity. Several studies have been published
describing the necessary blending residence time and temperature conditions
(27–29). Aoki has reported barrier properties of containers prepared from blends
of PET with PEN (30). The data show that the barrier performance observed
for 10, 20, and 40 wt% blends exceed that predicted by extrapolation of simple
mixtures (Fig. 15), in agreement with the data shown earlier on films (Fig. 3).
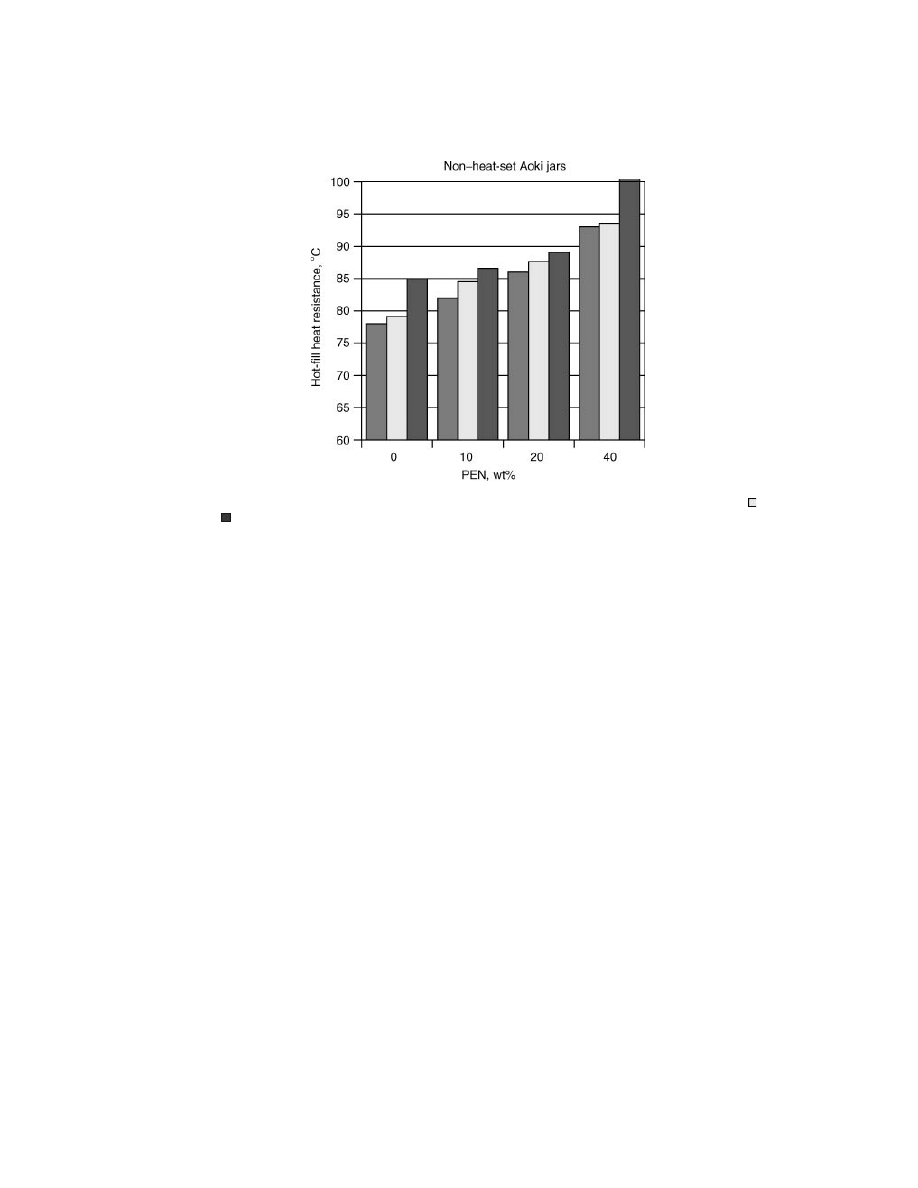
Small Hot-Fillable Wide Mouth Jars.
PEN/PET blends have been suc-
cessfully used in wide mouth, nonheat set hot-fill container applications. Aoki
and Guelph Food Technology Center established that the hot-fill performance for
wide mouth containers made from PET/PEN blends matched closely the glass-
transition temperature of the material (31). As shown in Figure 16, containers
from each material were tested under a special protocol for hot-filling performance.
The jars were filled at various temperatures. The maximum temperature tried at
which a jar gave satisfactory performance is marked as “pass” and the next higher
temperature tried is marked as “fail.” As seen in all cases the material’s T
g
lies
between the “pass” and “fail” temperature.
The biggest benefit of this performance attribute is in the case of small
wide-mouth containers, where development of crystallinity during stretching is
minimal and application of heat-setting is not practical. A commercial example
was a PET/PEN blend container with 40 wt% PEN introduced under the Aran
brand in Scotland (32). The size of the container, made on a one-step Aoki ma-
chine, was 28 g and the filling was done at 85
◦
C.
CSD Bottles from PET/PEN Blends.
As the container size decreases the
need for gas barrier (carbonation retention in the case of CSD) increases. This
110
POLY(ETHYLENE NAPHTHALATE) (PEN)
Vol. 11
Fig. 16.
Hot-fill performance of amorphous jars made from PET/PEN blends.
ⵧ
Pass
T
g
,
Fail.
is a direct effect of the increase in specific area or area per unit volume. In gen-
eral, when considering normal transportation logistics and desired shelf life, the
practical CSD bottle size limit attainable with PET is 500 mL. At the same time,
market research has shown that smaller single-serve sizes (eg, 350 mL) are desir-
able in many regions worldwide. The Coca-Cola Co. had demonstrated, through
an extended test market introduction in Australia, that a PET/PEN blend is suit-
able for fabricating a 350-mL bottle that meets the shelf life requirements for
Coca-Cola and Diet Coke carbonated beverages (32).
Packaging Applications Using Low PEN Level PET/PEN Copoly-
mers.
PETN-x low level copolymers, where x
≤ 1% to typically 10% are used
in rigid packaging applications where increased UV barrier resistance or small
improvements in thermal performance versus PET are required.
Naphthalate UV Property Benefits in Packaging Applications.
As dis-
cussed in the Performance Property section, NDC incorporation—even at a
low level—into polyesters affords substantial UV-absorber absorption in the
310–370 nm wavelength range. The ability of NDC to function as a UV radia-
tion blocker can be used to protect container contents from the harmful effects of
UV light in applications ranging from dairy products to fruit juices and pharma-
ceuticals. A fundamental advantage offered by NDC-based UV barrier protection
in polyester containers is its stability to migration. Unlike many standard UV
stabilizers that can leach or migrate out of the polymer matrix, NDC is reacted
directly into the polyester backbone and cannot migrate.
Studies completed on model color-sensitive beverages further illustrate the
effectiveness of low level naphthalate modification in protecting against UV
radiation exposure (33). The model beverage consisted of 20 ppm Yellow #5,
1000 ppm citric acid, 200 ppm sodium benzoate, and 100 ppb iron. This model
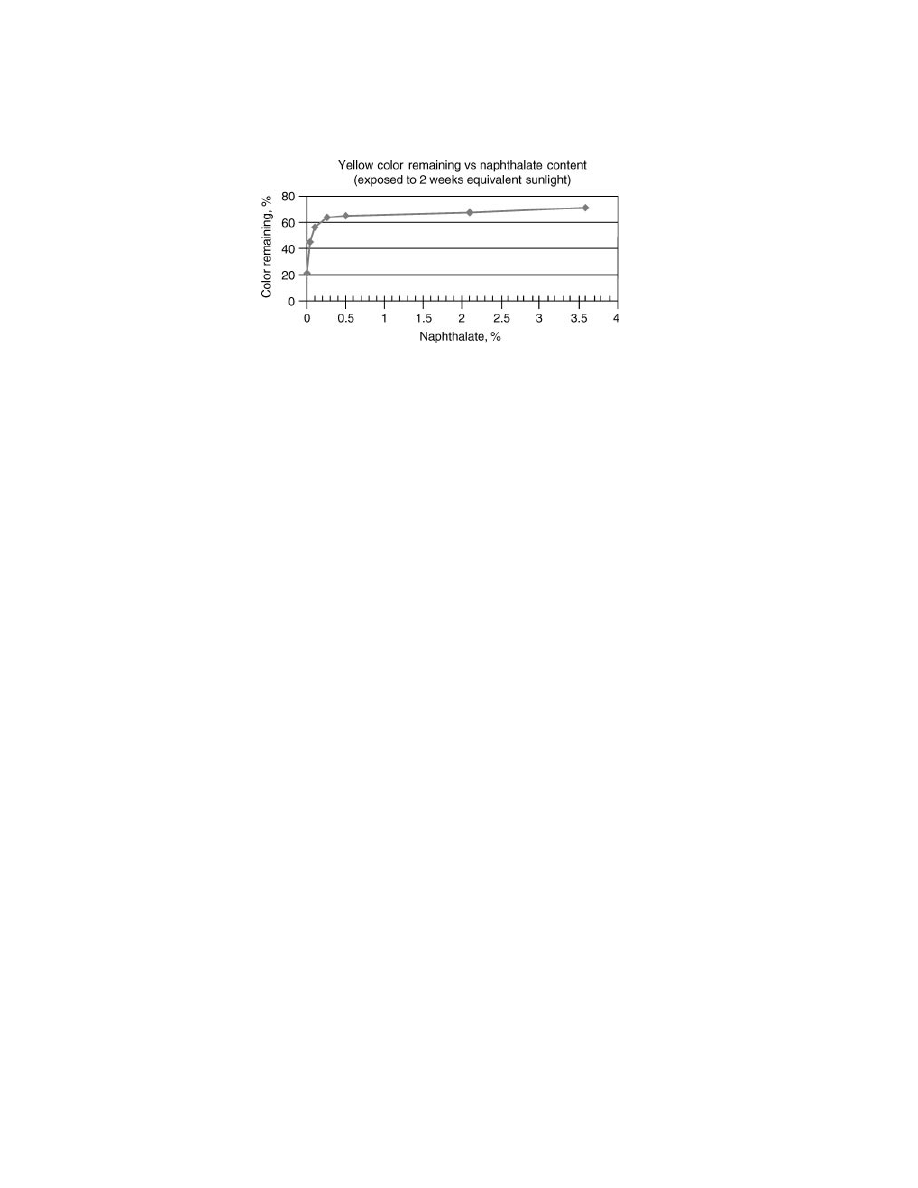
Vol. 11
POLY(ETHYLENE NAPHTHALATE) (PEN)
111
Fig. 17.
Color retention of a model beverage in PET and PETN bottles (33).
solution is extremely sensitive to yellow color bleaching upon UV exposure. As
Figure 17 illustrates, after accelerated testing in a Xenon weatherometer (corre-
sponding to 2 weeks of equivalent sunlight), substantial protection against color
loss occurred even at very low levels of naphthalate content in the PET package.
This plot indicates that an NDC level as low as 0.25 mol% should be sufficient for
many UV barrier applications.
The same study demonstrated the UV protective capability of PETN copoly-
mers on the color stability of a commercial juice drink containing artificial colorant
Blue No. 1. As shown in Figure 18, PET modified with 0.25 mol% NDC offers sub-
stantial protection against a dramatic loss in color occurring after only 5 h of
sunlight exposure when unmodified PET is used instead.
Low level NDC modification of PET has been used in Japan for Listerine
Cool Mint mouthwash, a product of Warner-Lambert Japan. The PETN copoly-
mer in these 80-mL to 700-mL bottles is used to extend shelf life by protection
from UV exposure, as well as to prevent absorption of the active ingredients of
the mouthwash into the bottle (30). Another commercial packaging application,
relying on the UV barrier property of naphthalates, was Fujiya’s Lemon Squash
(34), packaged in a 1.5-L PETN copolymer bottle. A small amount of naphthalate
was incorporated into the PET resin to protect the drink’s natural fruit juices and
added Vitamin C.
PETN Copolymer Hot-Fill Applications.
PETN copolymers at naphtha-
late modification levels up to 10 mol% are capable of developing strain-induced
crystallinity. These copolymers have been used in several bottle geometries and
offer both improved thermal resistance and UV barrier versus PET. These ad-
vantages derive in part from the increase in T
g
(as shown in Fig. 1), and partly
from optimizing the bottle stretch blow process and preform design. For example,
bottles from PETN that were subjected to a one-stage heat-setting treatment (hot
mold) without neck crystallization have been capable of hot-fill temperatures up to
93–95
◦
C. Table 16 shows an example of a hot-fill test comparing a PETN-10 and
a PET 500-mL tea-drink bottle.
This increased temperature stability has stimulated several commercial
PETN commercial introductions. Examples are illustrated below.
Hero Group, U.K.-based Carters Packaging, had produced a one-way and
multitrip container for hot-fill applications (30). Produced on a Sidel standard
heat-set, one-wheel blow-molding machine, the package could be hot-filled to 95
◦
C
112
POLY(ETHYLENE NAPHTHALATE) (PEN)
Vol. 11
Table 16. Hot-Water Hot-Fill Test
a
,b
Volume change at:
PET
PETN-10
85
◦
C
−1.0%
−0.2%
90
◦
C
−2.5%
−0.4%
93
◦
C
−2.0%
−0.4%
a
Data from BP Amoco Chemical Co.
b
One-step heat-set 500 mL tea-drink bottles, amorphous neck.
after standard aging practices, without having to crystallize the neck to prevent
deformation. The one-trip container had a weight of 59 g and a 28-mm neck, while
the returnable and rewashable bottle had a weight of 76 g and a 43-mm neck finish.
The latter could be washed at 75
◦
C with a 2% caustic solution. For comparison,
heat-set PET allows only a washing temperature as high as 59
◦
C.
S ¨antis Kunstoffe, a Swiss bottle producer, had introduced a 0.5-L PETN-8
container. This bottle contained “Sports
+ Fit” athletic beverage, which comes in
Orange or Citrus Flavor (23). The bottle, which was made on a MAG machine,
weighed 40 g and had a filling temperature of 85
◦
C. A similar bottle has been
used in Switzerland by the canner Bischofszell AG for its 100% orange juice brand
“Sunqueen.” which is distributed by the Swiss Migros supermarket chain.
Log Plastics Products Co. of Israel had developed a 1.0-L bottle from PETN
copolymer for hot-fill applications (28). The bottle was produced on an Aoki one-
step machine. It was used by Gan Schmuel Foods Ltd. for its 100% juices.
Water bottler Powwow has introduced in Europe 5-gallon return-
able/refillable containers from PETN copolymers, for use in drinking water foun-
tains, an application typically served by PC (35). Unlike PC containers, which
are made by extrusion blow molding, the PETN 5-gallon containers are fabri-
cated by one-step injection/stretch blow molding. Incorporation of PEN repeat
units enhances the container’s thermal stability against washing with hot caustic
solutions, for increased number of trips.
Fig. 18.
Blue-colored fruit drink exposed to sunlight for 5 h (33).

Vol. 11
POLY(ETHYLENE NAPHTHALATE) (PEN)
113
BIBLIOGRAPHY
1. BP604,073 (1948), J. G. Cook and co-workers (to ICI).
2. E. Fischer and S. Fakirov, J. Mater. Sci. 11, 104 (1976).
3. J. Liu, J. Myers, P. H. Geil, J. C. Kim, and M. Cakmak, in ANTEC’97, Technical Papers
Volume XLIII, Society of Plastics Engineers, 1997, p. 740.
4. Z. Mencik, Chem. Prim. 17(2), 78 (1978).
5. C. Bunn and W. Daubney, Proc. R. Soc. 226, 531 (1954).
6. Research Disclosures 28,340 (1987).
7. D. C. Hoffman and J. K. Caldwell, in Specialty Polyesters’95, pp. 223–242
8. I. Ouchi, H. Aoki, S. Shimotsuma, T. Asai, and H. Hosoi, in Proc. 17th Japan Congr.
Materials Res., 1974, p. 217
9. GB Pat. 1,445,464 (1973) (to Teijin).
10. World Pat. WO 93/14252 (1993) (to AlliedSignal)
11. Allied Technical Bulletin P-1, 1300–192.
12. Research Disclosure 29,487 (1988).
13. U.S. Pat. 4,963,644 (Oct. 16, 1990) (to The Goodyear Tire & Rubber Co.).
14. U.S. Pat. 5,750,644 (Dec. 5, 1998) (to Shell Oil Co.).
15. U.S. Pat. 5,744,578 (Apr. 28, 1998) (to Shell Oil Co.).
16. DuPont Technical Bulletin X-272, Type 717.
17. Avtex Fibers Inc. Brochure, Type 120 Yarn.
18. DuPont Technical Bulletin X-272, Kevlar 29, Type 964.
19. F. Fourn´e, Synthetic Fibers, Carl Hansern Verlag, Munich, Germany, 1999,
p. 588.
20. BP Amoco “Elements” Naphthalate News Update, Feb. 2000.
21. BP “Elements” Naphthalate News Update, Summer 2001.
22. J.-F. Fritsch (Honeywell Performance Fibers), Technical and Cost Optimization of Tex-
tile Constructions for Advanced Reinforcement of Passenger and Van Tires, in IRC
2000 Rubber Conference, Helsinki, Finland, June 13, 2000.
23. Amoco NDC “Elements” News Update, Spring 1997.
24. BP “Elements” Naphthalate News Update, Winter 2002.
25. Mod. Plast. 51 (Sept. 2002).
26. Amoco NDC “Elements” News Update, Spring 1995.
27. M. E. Stewart, A. J. Cox, and D. M. Naylor, Polymer, 34, 4060 (1994).
28. Continuing Development of Process Technology for PET/PEN Blend Preforms, by Laura
Martin, Husky Injection Molding Systems. Presented at Bev-Pak America ’95.
29. Further Blow Molding Developments of PET/PEN Blends Bottles, by Luc Desoutter,
Group Sidel. Presented at Bev-Pak America’95.
30. Fabrication of Naphthalate-Containing Wide Mouth Jars, by I. Nakajima, Aoki. Pre-
sented at Bev-Pak America ’95.
31. A Food Processor’s Perspective on Performance Opportunities for Heat Resistant Plas-
tic Packaging, by Nina Goodrich, Ian Britt, and Marvin Tung, Guelph Food Technology
Centre. Presented at 1995 Institute of Packaging Professionals (IDPP).
32. Shell Chemicals Bulletin SC:2306–98, 1998.
33. Barnaby Wallace, End-User Executive Perspective—PET @ Coca-Cola, in PET Strate-
gies 2001 Conference Proceedings, Atlanta, Ga.
34. Amoco NDC “Elements” News Update, Autumn 1994.
35. BP “Elements” Naphthalate News Update, Fall 2002.
S. L. S
AKELLARIDES
BP Amoco Chemical Company

114
POLY(ETHYLENE NAPHTHALATE) (PEN)
Vol. 11
POLY(ETHYLENE-NORBORNENE).
See E
THYLENE
-N
ORBORNENE
C
OPOLYMERS
.
POLY(3-HYDROXYALKANOATES).
See Volume 3.
POLYIMIDES.
See Volume 7.
POLYISOBUTYLENE.
See B
UTYL
R
UBBER
.
POLYKETONES.
See Volume 3.
POLYMER BLENDS.
See A
RTICLE
O
NLINE
.
Wyszukiwarka
Podobne podstrony:
poly(ethylene oxide) amphiphilic block copolymers
of poly(ethylene glycol) and poly(N isopropylacrylamide)
Cytoszkielet - histologia, I rok, I rok, gieldy, pen, medycyna, 2 semestr, HISTOLOGIA
Possibilities of polyamide 12 with poly(vinyl chloride) blends recycling
Ethylene—Acrylic Elastomers
Gielda 2008 - 2, I rok, I rok, gieldy, pen, medycyna, Anatomia, pierdoly, GIEŁDA, Egzamin 2 2008
Ethylene Polymers, HDPE
Ethylene—Norbornene Copolymers
Ethylene Polymers, LLDPE
choroby genetyczne tabelka, I rok, I rok, gieldy, pen, medycyna, 1 semestr, Biologia medyczna, Genet
lab2 pen !!!!!!!!!!
Czwórniki, Politechnika Lubelska, Studia, sem III, pen
Knowledge?out Gases Ethylene or Ethene
23 Modelowanie za pomocą?itable Poly
PEN w06
06 Antro pen
Doman MM pen
Poly(trimethylene terephthalate)
więcej podobnych podstron