
Vol. 5
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
263
BIODEGRADABLE POLYMERS,
MEDICAL APPLICATIONS
Introduction
Traditional applications of synthetic polymers are mostly based on their
relative inertness to biodegradation compared with natural macromolecules,
such as cellulose, and proteins. Biodegradation of the polymers can occur
in several ways including photodegradation, oxidation, and hydrolysis. Prac-
tically, biodegradation involves enzymatically promoted breakdown caused by
living organisms, usually microorganisms, but it is now well accepted that
the biodegradation can occur by hydrolysis, oxidation, or photooxidation in
a biological environment. These polymers have been used in many aspects
of life, eg, in environmentally friendly packaging materials (1,2), agricul-
ture (3,4), drug delivery (5,6), gene delivery (7,8), and tissue engineering
(9,10). (See C
ONTROLLED
R
ELEASE
T
ECHNOLOGY
; E
NVIRONMENTALLY
D
EGRADABLE
P
OLYMERS
; G
ENE
-D
ELIVERY
P
OLYMERS
; T
ISSUE
E
NGINEERING
). This article empha-
sizes the various biodegradable polymers obtained either synthetically or from
natural resources and their uses for biomedical applications.
Factors Affecting Biodegradation
Effect of Polymer Structures.
Synthetic biodegradable polymers contain
hydrolyzable linkages along the polymer chain; for example, amide enamine, ester,
phosphate, phosphazene, carbonate, anhydride, urea, and urethane linkages are
susceptible to biodegradation by microorganisms and hydrolytic enzymes. In ad-
dition, polymers containing substituents such as benzyl, hydroxy, carboxy, methyl,
and phenyl groups have been prepared in the hope that an introduction of these
Encyclopedia of Polymer Science and Technology. Copyright John Wiley & Sons, Inc. All rights reserved.

264
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
Vol. 5
substituents might increase biodegradability (11). Among benzylated polymers,
mixed results have been obtained for polyamides. Since most enzyme-catalyzed
reactions occur in aqueous media, the hydrophilic–hydrophobic character of syn-
thetic polymers greatly affects their biodegradability. A polymer containing both
hydrophobic and hydrophilic segments seems to have a higher biodegradability
than those polymers containing either hydrophobic or hydrophilic structures only.
A series of poly(alkylene tartrate)s was found to be readily assimilated by As-
pergillus niger. However, the polymers derived from C6 and C8 alkane diols were
more degradable than the more hydrophilic polymers derived from C2 and C4
alkane diols or the more hydrophobic polymers derived from the C10 and C12
alkane diols. Among the degradable poly(
α-aminoacid-co-ε-caproic acid)s, the hy-
drophilic copolyamide derived from serine was more susceptible than those con-
taining only hydrophobic segments. For a synthetic polymer to be degradable by
enzyme catalysis, the polymer chain must be flexible enough to fit into the active
site of the enzyme. This aspect most likely accounts for the fact that while the flex-
ible aliphatic polyesters are readily degraded by biological systems, the more rigid
aromatic poly(ethylene terephthalate) is generally considered to be inert (12).
Effect of Polymer Morphology.
Selective chemical degradation of
semicrystalline polymer samples shows certain characteristic changes (13–16).
During degradation, the crystallinity of the sample increases rapidly at first and
then levels off to a much slower rate as the crystallinity approaches 100%. This
effect is attributed to the eventual disappearance of the amorphous portions of
the sample. The effect of morphology on the microbial and enzymatic degradation
of poly(
ε-caprolactone) (PCL), a known biodegradable polymer with a number of
potential applications, has been studied (17,18). Scanning electron microscopy has
shown that the degradation of a partially crystalline polycaprolactone film by fila-
mentous fungi proceeds in a selective manner, with the amorphous regions being
degraded prior to the degradation of the crystalline region. The microorganisms
produce extracellular enzymes responsible for the selective degradation. This se-
lectivity can be attributed to the less-ordered packing of amorphous regions, which
permits easier access for the enzyme to the polymer chains. The size, shape, and
number of crystallites all have a pronounced effect on the chain mobility of the
amorphous regions and thus affect the rate of degradation. This effect has been
demonstrated by studying the effects of changing orientation via stretching on the
degradation (17,18).
Degradation in biological medium, cells, tissues, and body fluids proceeds
differently from chemical degradation as enzymes, biological reagents in the cell
organelles, and fluids are involved in the degradation process. Enzyme is able to
degrade the crystalline regions faster than hydrolysis. The enzyme system shows
no intermediate molecular weight material but shows a much smaller weight shift
with degradation as compared to chemical degradation. This shift indicates that
although degradation is selective, the crystalline portions are degraded shortly
after the chain ends are made available to the exoenzyme. The lateral size of the
crystallites has a strong effect on the rate of degradation because the edge of the
crystal is where degradation of the crystalline material takes place, because of
the crystal packing. A smaller lateral crystallite size yields a higher crystallite
edge surface in the bulk polymer. Prior to the saturation of the enzyme active

Vol. 5
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
265
sites, the rate is dependent on available substrate; therefore, a smaller lateral
crystallite size results in a higher rate of degradation. The degradation rate of a
PCL film is zero order with respect to the total polymer, but is not zero order with
respect to the concentrations of the crystallite edge material. The drawing of PCL
films causes an increase in the rate of degradation, whereas annealing of the PCL
causes a decrease in the rate of degradation (19). This phenomenon is probably
due to opposite changes in lateral crystallite sizes. in vitro chemical and enzymatic
degradations of polymers, especially polyesters, were analyzed with respect to
chemical composition and physical properties. It was found quite often that the
composition of a copolymer giving the lowest melting point is most susceptible
to degradation (19). The lowest packing order, as expected, corresponds with the
fastest degradation rate.
Effect of Molecular Weight.
Microorganisms produce both exoenzymes,
which degrade polymers from terminal groups, and endoenzymes, which degrade
polymers randomly along the chain. One might expect a large molecular effect on
the rate of degradation in the ease of exoenzymes and a relatively small molecu-
lar weight effect in the case of endoenzymes. Low molecular weight hydrocarbons,
however, can be degraded by microbes. They are taken in by microbial cells, “acti-
vated” by attachment to coenzyme A, and converted to cellular metabolites within
the microbial cell. However, these processes do not function well (if at all) in an
extracellular environment, and the plastic molecules are too large to enter the
cell. This problem does not arise with natural molecules, such as starch and cel-
lulose, because conversions to low molecular weight components by enzyme reac-
tions occur outside the microbial cell. Photodegradation or chemical degradation
may decrease molecular weight to the point that microbial attack can proceed. Hy-
drolytic degradation of polyesters and polyanhydrides is affected by the molecular
weight as a result of differences in water accessibility to large molecular weight
polymeric materials.
Effect of Radiation and Chemical Treatment.
Photodegradation with
UV light and the
γ -irradiation of polymers generate radicals and/or ions that often
lead to cleavage and cross-linking. Oxidation also occurs, complicating the situ-
ation, since exposure to light is seldom in the absence of oxygen. Generally this
changes the material’s susceptibility to biodegradation. Initially, one expects the
observed rate of degradation to increase until most of the fragmented polymer is
consumed and a slower rate of degradation should follow for the cross-linked por-
tion of the polymer. Similarly, photooxidation of polyalkenes promotes (slightly in
most cases) the biodegradation (20). The formation of carbonyl and ester groups
is responsible for this change. Processes have been developed to prepare copoly-
mers of alkenes containing carbonyl groups so that they will be more susceptible
to photolytic cleavage prior to degradation. As expected,
γ -ray irradiation greatly
affects the rate of in vitro degradation of polyesters (21,22). For polyglycolide and
poly(glycolide-co-lactide), the pH of the degradation solution decreased as the pro-
cess proceeded. The change–time curves exhibit sigmoidal shapes and consist of
three stages: early, accelerated, and later; the lengths of these three regions were
a function of
γ -irradiation. Increasing radiation dosage shortens the time of the
early stage. The appearance of the drastic pH changes coincides with loss of tensile
breaking strength.

266
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
Vol. 5
Classification
On the basis of the formation, biodegradable polymers can be classified into two
groups including synthetic biodegradable polymers and natural biodegradable
polymers.
Synthetic Biodegradable Polymers.
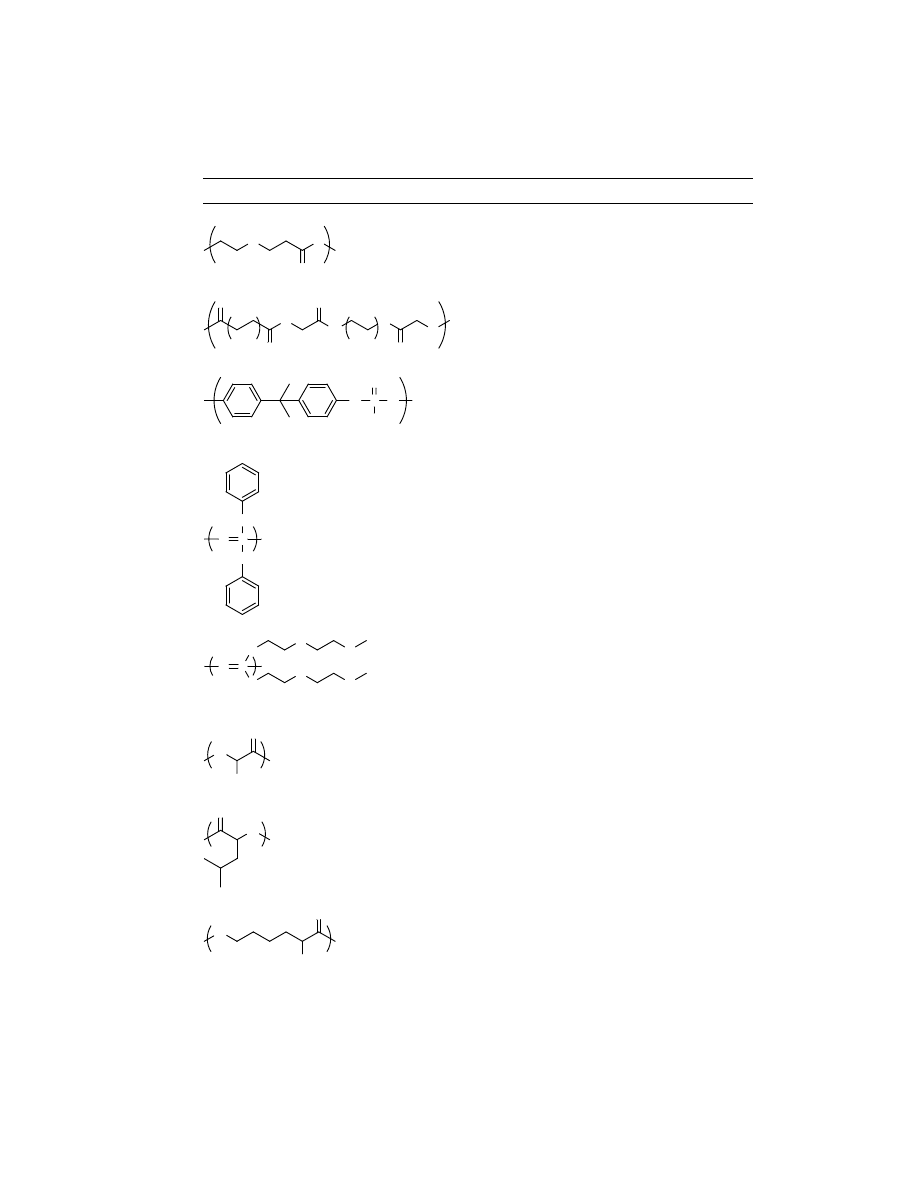
A representive list of synthetic
biodegradable polymers is given in Table 1, which includes chemical backbone
structures of these polymers. These polymers can be catagorized into two types
on the basis of their backbone structures, namely (1) polymers with hydrolyzable
backbone and (2) polymers with carbon backbone.
Polyester.
Polyesters having hydrolyzable ester bond in their backbone are
considered the best biomaterial with regard to design and performance. Among
polyesters, poly(lactic acid) (PLA) can be made of the lactic acid monomers which
contain an asymmetric
α-carbon atom with three different isomers as
D
-,
L
-, and
DL
-lactic acid (see P
OLYLACTIDE
). The physiochemical properties of optically ac-
tive homopolymers poly(
D
-lactic acid) (PDLA) or poly(
L
-lactic acid) (PLLA) are the
same, whereas the racemic PLA has very different characteristics (25). Racemic
PLA and PLLA have glass-transition temperatures (T
g
’s) of 57 and 56
◦
C respec-
tively, but PLLA is highly crystalline with a melting temperature (T
m
) of 170
◦
C;
racemic PLA is purely amorphous. The polymer characteristics are affected by
the comonomer composition, the polymer architecture, and molecular weight (26).
The crystallinity of the polymer, an important factor in polymer biodegradation,
varies with the stereoregularity of the polymer. Sterilization using
γ -irradiation
decreases the polymer molecular weight by 30–40% (26). The irradiated polymers
continue to decrease in molecular weight during storage at room temperature.
This decline in molecular weight affects the mechanical properties and the re-
lease rate from the polymers. PLA and its copolymers with less than 50% gly-
colic acid content soluble in common solvents such as chlorinated hydrocarbons,
tetrahydrofuran, and ethyl acetate. Poly(glycolic acid) (PGA) is insoluble in com-
mon solvents but in hexafluoroisopropanol and hexafluoroacetone sesquihydrate
(HFASH). In its highly crystalline form, PGA has a very high tensile strength
of 69–138 MPa (10,000–20,000 psi) and modulus of elasticity of about 6900 MPa
(
∼1,000,000 psi). The solubility parameters were in the range of 16.2 and 16.8,
which are comparable to those of polystyrene and polyisoprene (27).
A comparison study on the physicomechanical properties of several
biodegradable polyesters was reported (28). The thermal properties, tensile prop-
erties, and the flexural storage modulus as a function of temperature were deter-
mined. The following polymers were compared: poly(
L
-lactic acid), poly(
DL
-lactic
acid), poly(glycolic acid), poly(
ε-caprolactone), poly(hydroxybutyrate) and copoly-
mers with hydroxyvaleric acid, and poly(trimethylene carbonate). The thermal
and mechanical properties of several of the polymers tested are summarized in
Table 2 (28). A comprehensive review on the mechanical properties of several
biodegradable materials used in orthopedic devices has been published (29). The
tensile and flexural strength and modulus, as well as the biodegradation of vari-
ous lactide/glycolide polymers, poly(orthoester), and polycaprolactone have been
summarized in a tabular or diagram format.
Polycaprolactone. Poly(
ε-caprolactones) (PCL) are synthesized by ring-
opening polymerization of
ε-caprolactones and are soluble in chlorinated and

Vol. 5
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
267
Table 1. Examples of Synthetic Biodegradable Polymers
a
Structure
Name
Polyesters
O
O
n
Poly(glycolic acid) (PGA)
O
O
n
Poly(lactic acid) (PLA)
O
n
O
Poly(
ε-caprolactone) (PCL)
O
O
n
Poly(
β-hydroxybutyrate)
n
HO
O
O
OH
O
O
Poly(propylene-fumarate)
O
O
O
O
x
y
Poly(lactide-glycolide)
O
O
O
O
x
O
y
Poly(p-dioxane-co-glycolide)
O
O
O
x
y
O
O
Poly(p-dioxane-co-
caprolactone)
Poly(orthoesters)
O
O
OR
m
Poly(orthoester) I
O
OCH
2
OCH
2
CH
2
O
CH
2
O
O
m
Poly(orthoester) II
O
C
O
O
R
R
m
Poly(orthoester) III
268
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
Vol. 5
Table 1. (continued)
Structure
Name
Other polyesters
O
O
O
n
Poly(1,5-dioxipan-2-one)
O
N
H
O
O
O
H
N
O
O
x
y
n
Poly(ester amide)
O
P
R
O
O
m
Polyphosphate ester
Polyphosphazenes
O
P
O
N
m
Poly(aryloxyphosphazene)
N
P
O
O
O
O
O
O
m
Poly(PEG-phosphazene)
Amino acid derived polymers
H
N
O
R
m
Poly(amino acid)
H
N
O
m
Polyleucine
H
N
NH
2
O
m
Polylysine

Vol. 5
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
269
Table 1. (continued)
Structure
Name
H
N
O
HO
O
m
Poly(glutamic acid)
Polyanhydrides
O
O
O
m
Poly(fumaric acid)
O
O
O
O
O
O
x
m
y
Poly(terephthalic-co-
isophthalic acid)
O
O
O
O
CH
3
O
x
m
y
Block-poly(sebacic
anhydride)-co-poly(lactic
acid)
Polysaccharides
O
CH
2
O
OH
O
O
OH
OH
OH
OH
OH
O
O
OH
OH
OH
m
Pullulan
O
O
OH
O
O
OH
OH
OH
OH
O
O
OH
OH
OH
m
OH
Elsinan
O
O
OH
NH
2
NH
2
OH
m
OH
OH
O
O
Chitosan
O
CH
2
OH
O
O
OH OH
O
OH
OH
OH
m
Levan

270
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
Vol. 5
Table 1. (continued)
Structure
Name
Polyurethanes
R
N
H
O
O
n
Poly(urethanes)
Poly(amide-amines)
R
N
H
O
H
N
n
Poly(amide-enamines)
Natural polypeptides
H
N
R
O
m
A plasma glycoprotein belonging structurally to the
keratin–myosin group. Synthesized and secreted by
hepatic parenchymal cells. Essential to the clotting
of blood. The molecular weight is 340,000. Soluble,
but forms an insoluble gel on conversion to fibrin.
Fibrin monomer is fibrinogen from which one or two
peptides have been removed by means of thrombin.
Fibrinogen
Polypeptide substance comprising one third of the total
protein in mammalian organisms; main constituent
of skin, connective tissue, and the organic substance
of bones and teeth. Different types of collagens exist.
Collagen (qv)
A heterogeneous mixture of water-soluble proteins of
high average molecular weight. Derived from
collagen. Swells up in water to form a gel/insoluble
in organic solvents.
Gelatin (qv)
a
The structures of polymers and description of their properties and applications can be found in either
Ref. 23 or Ref. 24.
aromatic hydrocarbons, cyclohexanone, and 2-nitropropane but insoluble in
aliphatic hydrocarbons, diethyl ether, and alcohols (30). The homopolymer of PCL
melts at 59–64
◦
C with a T
g
of
−60
◦
C. Copolymerization with lactide increases the
T
g
with the increase in the lactide content in the polymer (31). The crystallinity
of the polymer decreases with the increase in polymer molecular weight; polymer
of weight 5000 is 80% crystalline whereas the polymer of weight 60,000 is 45%
crystalline (32).
Tokiwa and Suzuki (33) have discussed the hydrolysis and biodegradation of
PCL by fungi, and have shown that PCL can be degraded enzymatically. Blends of
PCL and polyesters prepared from alkanediols and alkane dicarboxylic acids with
natural substances such as tree bark have been molded into shaped containers
for horticultural seeding plantouts (34). After 3 months of soil burial, the PCL

Vol. 5
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
271
Table 2. Thermal and Mechanical Properties of Biodegradable Polyesters
a
Elongation
Tensile
Tensile
T
g
,
T
m
, strength, modulus, Yield, Break,
Polymer
M
w
◦
C
◦
C
MPa
b
MPa
b
%
%
Poly(lactic acid)
L
-PLA
50,000
54
170
28
1200
3.7
6.0
L
-PLA
100,000
58
159
50
2700
2.6
3.3
L
-PLA
300,000
59
178
48
3000
1.8
2.0
DL
-PLA
107,000
51
—
29
1900
4.0
5.0
Poly(glycolic acid) (PGA)
50,000
35
210
NA
NA
NA
NA
Poly(
β-hydroxybutyrate)
PHB
370,000
1
171
36
2500
2.2
2.5
P(HB-11%HV)
529,000
2
145
20
1100
5.5
17
Poly(
ε-caprolactone) (PCL)
44,000
−62
57
16
400
7.0
80
Poly(trimethylene
carbonate) PTC
48,000
−15
—
0.5
3
20
160
Poly(orthoesters)
t-CDM:1,6-HD
c
(35:65)
99,000
55
—
20
820
4.1
220
t-CDM:1,6-HD
c
(70:30)
101,000
84
—
19
800
4.1
180
a
Taken from Ref. 28.
b
To convert MPa to psi, multiply by 145.
c
t-CDM: 1,6-HD
=trans-cyclohexane dimethanol: 1,6-hexanediol.
containers were found to be embrittled, disintegrated, and biodegraded, which
suggests that the extracellular enzymes in the soil may cleave the polymer chain
prior to the assimilation of the polymer by microorganisms.
Poly(
β-hydroxybutyrate). Poly(β-hydroxybutyrate) (PHB) is made by a con-
trolled bacterial fermentation (see P
OLY
(3-
HYDROXYALKANOATES
). The producing
organism occurs naturally. An optically active copolymer of 3-hydroxybutyrate
(3HB) and 3-hydroxyvalerate (3HV) has been produced from propionic acid or
pentanoic acid by Alcaligenes eutrophus. PHB is characterized as having a high
molecular weight (
>100,000, [η] > 3 dL/g) with a narrow polydispersity and a
crystallinity of around 50%. The melting point depends on the polymer composi-
tion; P(3HB) homopolymer melts at 177
◦
C with a T
g
at 9
◦
C, the 91:9 copolymer
with 4HB melts at 159
◦
C, and the 1:1 copolymer with 3HV melts at 91
◦
C. The
PHB properties in the living cells of A. eutrophus were determined using X-ray
and variable-temperature
13
C NMR relaxation studies (35). PHB is an amorphous
elastomer with a T
g
around
−40
◦
C in its “native” state within the granules. The
biodegradation of these polymers in soil and activated sludge show the rate of
degradation to be in the following order (36):
P(3HB
−co−9%4HB) > P(3HB) = P(3HB−co−50%3HV)
Microspheres degraded slowly in phosphate buffer at 85
◦
C and after
5 months, 20–40% of the polymer eroded under these conditions. Copolymers
having a higher fraction of 3HV and low molecular weight polymers were more
susceptible to hydrolysis (36).

272
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
Vol. 5
Poly(phosphoesters).
Poly(phosphoesters) are synthesized from the reac-
tion of ethyl or phenyl phosphorodichloridates and various dialcohols including
bisphenol A and poly(ethylene glycol) of various molecular weights (37). Leong
and co-workers (38) have incorporated phosphoester groups into poly(urethanes).
Poly(urethanes) have been used as blood-contacting biomaterials because of their
having a broad range of physical properties: from hard and brittle to soft and tacky
(38). Leong and co-workers has designed inert biomaterial for controlled release
application by introducing phosphoester linkage in poly(urethane) (38). Introduc-
tion of phosphoester linkage does not change the mechanical properties inherent
in the poly(urethanes) and provides excellent biodegradable materials.
Polycarbonates.
Polycarbonates are synthesized from the reaction of dihy-
droxy compounds with phosgene or with bischloroformates of aliphatic dihydroxy
compounds by transesterification, and by polymerization of cyclic carbonates (39).
These polymers have been synthesized from carbon dioxide and the corresponding
epoxides in the presence of organometallic compounds as initiators. Poly(ethylene
carbonate) and poly(propylene carbonate) are linear thermoplastic polyesters of
carbonic acid with aliphatic dihydroxy compounds (39). Poly(dihydropyrans) were
developed for contraceptive delivery. The in vivo and in vitro release of contra-
ceptive steroids and antimalarial agents from polymer matrices has been studied.
Poly(p-dioxanone) is clinically used as an alternative to poly(lactide) in absorbable
sutures with similar properties to poly(lactide) with the advantage of better irradi-
ation stability during sterilization (40). This polymer has not yet been developed
as a carrier for controlled drug delivery. Biodegradable polymers derived from
naturally occurring, multifunctional hydroxy acids and amino acid have been in-
vestigated by Lenz and Guerin (41).
Poly(amides).
The utilization of amide-based polymers, especially natural
proteins, in the preparation of biodegradable matrices have been extensively in-
vestigated in recent years (42). Microcapsules and microspheres of cross-linked
collagen, gelatin, and albumin have been used for dug delivery (43). The synthetic
ability to manipulate amino acid sequences has seen its maturity over the last
two decades with new techniques and strategies continually being introduced.
Poly(amides) such as poly(glutamic acid) and poly(lysine) and their copoly-
mers with various amino acids have also been studied as drug carriers (41,44,45).
Pseudopoly(amino acids), prepared from N-protected trans-4-hydroxy-
L
-proline,
and poly(iminocarbonate) from tyrosine dipeptide as monomeric starting mate-
rial have been reported (46–48). The properties, biodegradability, drug release,
and biocompatibility of this class of polymers have been reviewed (42,46).
Polyphosphazenes.
The polymers are most commonly synthesized by a
substitution reaction of the reactive poly(dichlorophosphazene) with a wide range
of reactive nucleophiles such as amines, alkoxides, and organometallic molecules.
The reaction is carried out in general at room temperature in tetrahydrofuran or
aromatic hydrocarbon solutions (49). Two different types of polyphosphazenes are
of interest as bioinert materials: those with strongly hydrophobic surface charac-
teristics and those with hydrophilic surfaces. Polycarbonates (qv) bearing fluo-
roalkoxy side groups are some of the most hydrophobic synthetic polymers known
(50,51). Such polymers are as hydrophobic as poly(tetrafluoroethylene) (Teflon),
but unlike Teflon, polyphosphazenes of this type are flexible or elastomeric, easy
to prepare, and they can be used as coatings for other materials.

Vol. 5
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
273
The uniqueness of the polyphosphazenes stems from its inorganic backbone
(N P) which with certain organic side groups can be hydrolyzed to phosphate and
ammonia. Several polymer structures have been used as matrix carriers for drugs
(51) or as a hydrolyzable polymeric drug, where the drug is covalently bound to
the polymer backbone and released from the polymer by hydrolysis (52). A com-
prehensive review on the synthesis, characterization, and medical applications of
polyphosphazenes was published (53).
Poly(orthoesters).
Poly(orthoesters) were first designated as Chronomer
and later as Alzamer (54). They were prepared by a transesterification reaction.
The molecular weight of poly(orthoesters) were significantly dependent on the type
of diol and catalyst used for synthesis. A linear, flexible diol like 1,6-hexanediol
gave molecular weights greater than 200 kDa, whereas bisphenol A in the pres-
ence of catalyst gave molecular weights around only 10,000 kDa (55). Mechanical
properties of the linear poly(orthesters) can be varied over a large range by select-
ing various compositions of diols. It was shown that the T
g
of the polymer prepared
from 3,9-diethylidene-2,4,8,10-tetraoxaspiro[5,5]undecane can be varied from 25
to 110
◦
C by simply changing the amount of 1,6-hexanediol in trans-1,4 cyclohex-
ane dimethanol from 100% to 0% (29). A linearly decreasing relationship between
the T
g
and percentage of 1,6 hexanediol is observed. One could take advantage of
the above relationship in selecting the polymer for in vivo applications because in
vivo the T
g
of the polymer would drop because of the inhibition of water, resulting
in the loss of stiffness and rigidity of the polymer.
Polyanhydrides.
The majority of the polyanhydrides (qv) are prepared by
melt polycondensation. The sequence of reaction involves first the conversion
of a dicarboxylic acid monomer into a prepolymer consisting of a mixed anhy-
dride of the diacid with acetic anhydride. This is achieved by simply refluxing
the diacid monomer with acetic anhydride for a specified length of time. The
polymer is obtained subsequently by heating the prepolymer in vacuo to elimi-
nate the acetic anhydride (56). Almost all polyanhydrides show some degree of
crystallinity as manifested by their crystalline melting points. An in-depth X-ray
diffraction analysis was conducted with the homopolymers of sebacic acid (SA),
bis(carboxyphenoxy)propane (CPP), bis(carboxyphenoxy)hexane (CPH), and fu-
maric acid, and the copolymers of SA with CPP, CPH, and fumaric acid (57). The
results indicated that the homopolymers were highly crystalline and the crys-
tallinity of the copolymers was determined, in most cases, by the monomer of
highest concentration. Copolymers with a composition close to 1:1 were essen-
tially amorphous (57). The melting point of the aliphatic–aromatic copolyanhy-
drides is proportional to the aromatic content. For this type of copolymers there
is characteristically a minimum T
m
between 5 and 20 mol% of the lower melting
component (57).
The majority of polyanhydrides dissolve in solvents such as dichloromethane
and chloroform. However, the aromatic polyanhydrides display much lower solu-
bility than the aliphatic polyanhydrides. In an attempt to improve the solubility
and decrease the T
m
, copolymers of two different aromatic monomers were pre-
pared. These copolymers displayed a substantial decrease in T
m
and an increase
in solubility than did the corresponding homopolymers of aromatic diacids (57).
Natural Biodegradable Polymers.
Biopolymers formed in nature dur-
ing the growth cycles of all organisms are referred to as natural polymers. Their

274
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
Vol. 5
synthesis generally involves enzyme-catalyzed, chain-growth polymerization re-
actions of activated monomers, which are typically formed within cells by complex
metabolic processes.
Polysaccharides.
For materials applications, the principal polysaccharides
of interest are cellulose and starch because of their easy and cheap resources, but
increasing attention is being given to the more complex carbohydrate polymers
produced by bacteria and fungi, especially to polysaccharides such as xanthan,
curdlan, pullulan, and hyaluronic acid (see C
ARBOHYDRATE
P
OLYMERS
).
Starch. Starch thermoplastic (qv) is a polymer that occurs widely in plants.
The principal crops used for its production include potatoes, corn, and rice. In
all of these plants, starch is produced in the form of granules, which vary in size
and somewhat in composition from plant to plant. In general, the linear polymer,
amylose, makes up about 20 wt% of the granule, and the branched polymer, amy-
lopectin, the remainder. Research on starch includes investigation of its water-
adsorptive capacity, the chemical modification of the molecule, its behavior under
agitation and high temperature, and its resistance to thermomechanical shear.
The starch molecule has two important functional groups, the
OH group that is
susceptible to substitution reactions and the C O C bond that is susceptible to
chain breakage. By reaction of its
OH group, modification of various properties
can be obtained. One example is the reaction with silane to improve its dispersion
in polyethylene (58). Cross-linking or bridging of the
OH groups changes the
structure into a network while increasing the viscosity, reducing water retention
and increasing its resistance to thermomechanical shear. Acetylated starch does
have several advantages as a structural fiber or film-forming polymer as compared
to native starch. Starch acetate has an improved solubility compared to starch and
is easily cast into films from simple solvents. The degree of acetylation is easily
controlled by transesterification, allowing polymers to be produced with a range
of hydrophobicities.
Since isocyanates are highly reactive with hydroxyl groups, they can be used
to prepare a number of reactive resins that cross-link with starch. The addition
of starch to isocyanate resins considerably reduced costs and improved solvent
resistance and strength properties (59). Starch can be modified with nonpolar
groups, such as fatty esters, before the isocyanate reaction to improve the degree
of reactivity (60).
Cellulose. Cellulose (qv) is a very highly crystalline, high molecular weight
polymer, which is insoluble in water and organic solvents. It is soluble in ag-
gressive, hydrogen bond-breaking solvents such as N-methylmorpholine-N-oxide.
Because of its insolubility, cellulose is usually converted into derivatives to make
it more processable. The important derivatives of cellulose are reaction products
of one or more of the three hydroxyl groups, which are present in each glucopy-
ranoside repeating unit, including (1) ethers (61,62), eg, methyl cellulose and hy-
droxylethyl cellulose; (2) esters (63), eg, cellulose acetate and cellulose xanthate,
which are used as soluble intermediates for processing cellulose into either fibre
or film forms, during which the cellulose is regenerated by controlled hydrolysis;
and (3) acetals (64), especially the cyclic acetal formed between the C2 and C3
hydroxyl groups and butyraldehyde.
Chitin and Chitosan.
Chitin is a macromolecule found in the shells of
crabs, lobsters, shrimps, and insects (see C
HITIN AND
C
HITOSAN
). It consists of

Vol. 5
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
275
2-acetamide-2-deoxy-b-d-glucose through the b-(1-4)-glycoside linkage. Chitin can
be degraded by chitinase. Chitin fibers have been utilized for making artifi-
cial skin and absorbable sutures (65). Chitin is insoluble in its native form but
chitosan, the partly deacetylated form, is water-soluble. The materials are bio-
compatible and have antimicrobial activities as well as the ability to chelate
heavy metal ions. They also find applications in the cosmetic industry because
of their water-retaining and moisturizing properties. Using chitin and chitosan
as carriers, a water-soluble prodrug has been synthesized (66). Modified chi-
tosans have been prepared with various chemical and biological properties (67).
N-Carboxymethylchitosan and N-carboxybutylchitosan have been prepared for
use in cosmetics and in wound treatment (68). Chitin derivatives can also be used
as drug carriers (69), and a report of the use of chitin in absorbable sutures shows
that chitins have the lowest elongation among suture materials including chitin
(70), PGA, plain catgut, and chromic catgut. The tissue reaction of chitin is similar
to that of PGA (71).
Alginic Acid. Alginate is a binary linear heteropolymer containing 1,4-
linked
α-l-guluronic acid and β-d-mannuronic acid. Alginates have been stud-
ied extensively for their ability to form gels in the presence of divalent cations
(72,73). Alginic acid forms water-soluble salts with monovalent cations, low molec-
ular weight amines, and quaternary ammonium compounds. It becomes water-
insoluble in the presence of polyvalent cations such as Ca
2
+
·, Be
2
+
·, Cu
2
+
·, Al
3
+
·,
and Fe
3
+
·. Alginate gels have been used widely in controlled release drug delivery
systems (73). Alginates have also been used to encapsulate various herbicides,
microorganisms, and cells.
Naturally Occurring Polypeptides.
The proteins that have found appli-
cations as materials are, for the most part, neither soluble nor fusible without
degradation and so they are used in the form in which they are found in nature.
This description is especially true for the fibrous proteins wool (qv), silk (qv),
and collagen (qv). All proteins are specific polymers with regular arrangements
of different types of a-amino acids; so the biosynthesis of proteins is an extremely
complex process involving many different types of enzymes. In contrast, the en-
zymatic degradation of proteins, with general-purpose proteases, is a relatively
straightforward, amide hydrolysis reaction.
Gelatin. Gelatin (qv), an animal protein, consists of 19 amino acids joined
by peptide linkages and can be hydrolyzed by a variety of proteolytic enzymes
to yield its constituent amino acids or peptide components (74). This nonspeci-
ficity is a desirable factor in intentional biodegradation. Gelatin is a water-soluble,
biodegradable polymer with extensive industrial, pharmaceutical, and biomedi-
cal uses, which has been employed for coatings and microencapsulating various
drugs (75,76) and for preparing biodegradable hydrogels (77).
A method was developed to prepare a simple, flexible gelatin film-based ar-
tificial skin that could adhere to an open wound and protect it against fluid loss
and infection. The approach was to mix polyglycerols, either as is or after reac-
tion with epichlorohydrin, with commercially available gelatin and then cast films
on Teflon-covered trays (78). The films were tough and adhered to open wounds
spontaneously. They could be loaded with bioactive molecules, such as growth fac-
tors and antibiotics that would be released over several days. The films could be
sterilized with
γ -rays or prepared under sterile conditions.

276
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
Vol. 5
Toxicity and Biocompatibility
In all the potential uses of polymeric materials, a direct contact between the poly-
mer and biological tissues is evident; therefore, for the eventual human application
of these biomedical implants and devices, an adequate testing for safety and bio-
compatibility of the specific polymer matrix is essential. Biocompatibility deals
with how the tissue reacts to foreign materials and the ability of a material to
perform with an appropriate host response in a specific application.
Poly(lactic-co-glycolide).
Whenever a synthetic polymer material is to
be utilized in vivo, the possible tissue–implant interactions must be taken into
consideration. In the case of biodegradable matrices, not only the possible toxicity
of the polymer has to be evaluated, but also the potential toxicity of its degra-
dation products. Moreover, biocompatibility is considered as the foundation for
biocompatibility of degradable polymer systems. Thus, PLLA is defined as a safe
biomaterial for in vivo use because its degradation product
L
-lactic acid is a natural
metabolite of the body. Even though PLGA is extensively used and represents the
gold standard of degradable polymers, increased local acidity due to its degrada-
tion can lead to irritation at the site of polymer implant. Agrawal and Athanasiou
(79) have introduced a technique in which basic salts are used to control the pH
in local environment of PLGA implant. The feasibility of lactide/glycolide poly-
mers for the controlled release of bioactive agents is well proven, and they are
the most widely investigated biodegradable polymers for drug delivery. The lac-
tide/glycolide copolymers have been subjected to extensive animal and human
trials without any significant harmful side effects (80). However, some limited
incompatibility of certain macromolecules with lactide/glycolide polymers was ob-
served. Bezwada and co-workers (81) studied in vitro and in vivo biocompatibility
and efficacy of block copolymer of poly(glycolide) and PCL in the form of Monocryl
(Ethicon) sutures.
Poly(caprolactone).
The
biocompatibility
and
toxicity
of
poly-
(caprolactone) have mostly been tested in conjuction with evaluations of
Capronor (Schering), which is an implantable 1-year contraceptive delivery sys-
tem composed of a levonorgestrel-ethyl oleate slurry within a poly(caprolactone)
capsule. In a preliminary 90-day toxicology study of Capronor in female rats
and guinea pigs, except a bland response at the implant site and a minimal
tissue encapsulating reaction, no toxic effects were observed (82). The Capronor-
polycaprolactone contraceptive delivery system was also tested implanted in rats
and monkeys (83). Based on animal clinical and physical data such as blood
and urine analysis, ophthalmoscopic tests, and histopathology after necropsy, no
significant differences between the test and control groups was observed. Phase I
and II clinical trials with Capronor were recently carried out in different medical
centers (83).
Polyphosphazenes.
Biocompatibility and safety testing of polyphosp-
hazenes by subcutaneous implantation in animals have shown minimal tissue
response (84). The connection between hydrophobicity and tissue compatibility
has been noted for classical organic polymers (85). Thus, these hydrophobic
polyphosphazenes have been mentioned as good candidates for use in heart
valves, heart pumps, blood vessel prostheses, or as coating materials for

Vol. 5
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
277
pacemakers or other implantable devices; however, more in vivo testing and
clinical trials are needed (53).
In their bioerosion reactions polyphosphazenes display a uniqueness that
stems from the inorganic backbone, and the appropriate side groups can undergo
facile hydrolysis to phosphate and ammonia. The phosphate can be metabolized
and the ammonia excreted. Theoretically, if side groups attached to the poly-
mer are released by the same process being excretable or metabolizable, then
the polymer can be eroded under hydrolytic conditions without the danger of a
toxic response. Polyphosphazenes of this type are potential candidates as erodible
biostructural materials for sutures, or as matrices for controlled delivery of drugs
(53).
Poly(orthoesters).
As
mentioned
previously,
the
Chronomer
poly(orthoester) material from Alza Corp. or Alzamer has been investigated
as bioerodible inserts for the delivery of the narcotic antagonist naltrexone,
and for the delivery of the contraceptive steroid norethisterone (86,87). In
vitro studies have shown that good control over release of tetracycline could be
achieved, and very good in vitro adhesion to bovine teeth demonstrated (88).
However, studies in beagle dogs with naturally occurring periodontitis were
not successful because ointment-like polymers with a relatively low viscosity
are squeezed out of the pocket within about 1 day, despite good adhesiveness
(54).
Naturally Occurring Polymers.
The use of natural biodegradable poly-
mers to deliver drugs continues to be an area of active research despite the advent
of synthetic biodegradable polymers (43). Natural polymers remain attractive pri-
marily because they are natural products of living organisms, readily available,
relatively inexpensive, and capable of a multitude of chemical modifications (89).
Most investigations of natural polymers as matrices in drug delivery systems have
focused on the use of proteins (polypeptides or polyamides) such as gelatin, colla-
gen, and albumin. Collagen is a major structural protein found in animal tissues
where it is normally present in the form of aligned fibers. Because of its unique
structural properties, collagen has been used in many biomedical applications as
absorbable sutures, sponge wound dressings, composite tissue tendon allografts,
injectables for facial reconstructive surgery, and as drug delivery systems espe-
cially in the form of microspheres (90).
Besides the collagen biocompatibility and nontoxicity for most tissues
(91), several factors including the possible occurrence of antigenic responses,
tissue irritation due to residual aldehyde cross-linking agents, and poor patient
tolerance of ocular inserts have adversely influenced its use as a drug delivery
vehicle (90). For example, 5-fluorouracil and bleomycin cross-linked sponges
made from purified bovine skin collagen were implanted in rabbit eyes to test
their posssible use in preventing fibroblast proliferation following ophthalmic
surgery, resulting in a chronic inflammatory reaction elicited by the sponges even
in the absence of drug (92).
Noncollagenous proteins, particularly albumin and to a lesser extent gelatin,
continue to be developed as drug delivery vehicles. The exploitable features of albu-
min include its reported biodegradation into natural products, its lack of toxicity,
and noninmunogenicity (93).

278
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
Vol. 5
Medical Applications
Over the past decade the use of polymeric materials for the administration of
pharmaceuticals and as biomedical devices has increased dramatically. Important
biomedical application of biodegradable polymers is in the areas of controlled drug
delivery systems (92–96) and in the form of implants and devices for fracture
repairs (97–100), ligament reconstruction (101), surgical dressings (102), dental
repairs, artificial heart valves, contact lenses, cardiac pacemakers, vascular grafts
(103), tracheal replacements (104), and organ regeneration (105).
Biomaterials in general are used for the following purposes:
(1) to replace tissues that are diseased or otherwise nonfunctional, as in-joint
replacements, artificial heart valves and arteries, tooth reconstruction, and
intraocular lenses;
(2) to assist in the repair of tissue, including the obvious sutures, but also bone
fracture plates and ligament and tendon repair devices;
(3) to replace all or part of the functions of the major organs, such as in
haemodialysis, oxygenation (lungs), left ventricular or whole heart assis-
tance (heart), perfusion (liver), and insulin delivery (pancreas);
(4) to deliver drugs to the body, either to targeted sites (eg, directly to a tumor)
or sustained delivery (insulin, pilocarpine, contraceptives).
Biodegradable plastics have been developed as surgical implants in vascu-
lar and orthopedic surgery, as implantable matrices for the controlled long-term
release of drugs inside the body, as absorbable surgical sutures, and for use in the
eye (106,107).
Surgical Sutures.
Tissue damage that results in a loss of structural in-
tegrity, for example, a deep cut in soft tissue or a fracture of a bone, may not
be capable of unassisted self-healing. The insertion of a device to hold the tissue
together may facilitate the healing process. The classic examples are the use of
sutures to hold both deep and superficial wounds together. Once the healing is
complete, the suture becomes redundant and can impose undesirable constraints
on the healing tissues. It is preferable to remove the material from the site, either
physically or by self-elimination.
Synthetic absorbable sutures were developed in the 1960s, and because of
their good biocompatibility in tissues they are now widely used in tracheobronchial
surgery, as well as general surgery. They are multifilament-type sutures, which
have good handle ability (106). However, for continuous suturing, braided sutures
with nonsmooth surfaces are not useful. Monofilament sutures have smooth sur-
faces and are adequate for continuous suturing. For a monofilament suture, PGA
or PLA are too stiff and inflexible. The more flexible polydioxanones and polyg-
lyconates can be used as sutures because of their lower bending moduli (108).
Furthermore, copolymers of l-lactide and
ε-caprolactone-poly(caprolactone-lactic
acid) are bioabsorbable elastic materials and their clinical applications have been
studied (109).
Bone Fixation Devices.
Although metal fixation in fracture treatment for
undisturbed bone healing is a successful procedure, cortical bone and steel have

Vol. 5
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
279
very different mechanical properties. The elasticity constant of bone is only 1/10
of implanted steel while tensile strength is 10 times lower (110). Thus, the re-
moval of metal implants can result in weakened bone with a danger of refracture.
Biodegradable implants can meet the dynamic processes of bone healing, decreas-
ing the mechanical strength of the material. After months, the entire material will
disappear and no secondary surgery will be required. PGA, PLA, polydioxanone,
and PHB have potential roles in this area. For clinical applications, polydioxanone
was recommended for ligament augmentation, for securing a ligament suture, as
a kind of internal splinting suture, and as a kind of internal splinting to allow for
early motion of the extremities after an operation (108,109).
Biodegradable polymers are useful for many other applications. A marrow
spacer can help to save autologous bone material. A plug for closing the bone
marrow is employed for endoprosthetic joint replacement. Fibers are used for
filling large bone defects without mechanical loads (110).
Vascular Grafts.
Many studies have been undertaken to develop accept-
able small diameter vascular prostheses. Niu and co-workers (111) designed small
diameter vascular prostheses with incorporated matrices that can be absorbed into
a growing anastomotic neointima. It was pointed out that a gelatin–heparin com-
plex when adequately cross-linked, could simultaneously function as a temporary
antithrombogenic surface and as an excellent substructure for an anastomotic
neointima.
Adhesion Prevention.
Tissue adhesion after surgery occasionally causes
serious complications. Materials that prevent tissue adhesion should be flexible
and tough enough to provide a tight cover over the traumatized soft tissues,
and should be biodegradable and reabsorbable after the injured tissue is com-
pletely regenerated. Matsuda and co-workers (112,113) developed photocurable
mucopolysaccharides for a newly designed tissue adhesion prevention material
that meets numerous requirements such as nonadherent surface characteristics,
biocompatibility, biodegradability in accordance with the wound healing rate, and
nontoxicity. Mucopolysaccharides (hyaluronic acid and chondroitin sulphate) par-
tially functionalized with photoreactive groups, such as cinnamate or thyamine,
were subjected to UV irradiation to produce water-insoluble gels via intermolec-
ular photodimerization of the photoreactive groups (113). Photocured films with
lower degrees of substitution, which had high water swellability and flexibility,
prevented tissue adhesion and exhibited enhanced biodegradability. It was sug-
gested that these newly designed mucopolysaccharide gels may aid injured tissue
repair in a bioactive manner.
Artificial Skin.
For healing burns, skin substitutes or wound dressings
made of biodegradable polymeric materials have recently been developed. Until
now, most of the commercially developed artificial skins have utilized biodegrad-
able polymers such as collagen (114), chitin, and poly-l-leucine (115,116) which
are enzymatically degradable polymers. Recently, Koide and co-workers (117) de-
veloped a new type of biomaterial for artificial skin, in the form of a sponge,
by combining fibrillar collagen (F-collagen) with gelatin. The sponge was phys-
ically and metabolically stabilized by introducing cross-links. Although several
types of collagen-based artificial skins have been developed (118–120), some un-
desirable characteristics of native collagen were noticed (121), such as inducing
rod-like shapes in fibroblasts and enhancing the expression of collagenase genes

280
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
Vol. 5
in fibroblasts. F-collagen with gelatin was found to overcome the above prob-
lems. Yasutomi and co-workers (122) developed a biosynthetic wound dressing
with a drug delivery capability. This medicated wound dressing is composed of a
spongy mixture sheet of chitosan-derivatized collagen, which is laminated with a
gentamycin sulphate impregnated polyurethane membrane. From In vitro eval-
uation, it was shown that this wound dressing is capable of suppressing bacte-
rial growth and minimizing cellular damage. Evaluation of this wound dress-
ing was conducted in 80 clinical cases including superficial second-degree burns,
deep second-degree burns, donor sites, and pressure sores, and achieved excel-
lent results. The development of hybrid artificial skins is also another important
target in biomedical engineering. Here, synthetic polymers and cell cultures are
combined to form a synthetic–biological composite. In this case, a biodegradable
polymer may be required as the template for growing cells and tissue cultures
in vivo.
Drug Delivery Systems.
Biodegradable and non-degradable poly-
mers have been used for controlled delivery of drugs (see C
ONTROLLED
R
ELEASE
T
ECHNOLOGY
). The limitations of conventional methods of drug delivery,
by tablet or injection for example, are well known. As a dose is applied, the plasma
levels will be raised, but these will be rapidly decreased as the drug is metabo-
lized and will soon be below therapeutic levels. The next dose takes the plasma
level high again and a cyclic pattern may be established, with most of the drug
plasma levels possibly being outside the optimal range. In addition, the drug usu-
ally permeates throughout the body and it is not targeted to the location where
it is specifically required. Among the many possible solutions to these problems
is the use of controlled drug delivery systems (123,124), from which the drug is
released at a constant, predetermined rate, and possibly targeted to a particular
site. One of the most prominent approaches is that in which the drug is contained
within a polymer membrane or is otherwise encapsulated in a polymer matrix,
and where the drug diffuses out into the tissues following implantation, and ero-
sion or dissolution of the polymer contributes to the release mechanism. It sounds,
therefore, feasible to produce systems that allow easy and safe processing and can
be injected into a body cavity without the need for surgical retrieval after com-
pletion of the release. Furthermore, the differential rates of drug delivery might
be of profound interest for cases where elevated drug doses are necessary in the
beginning of treatment.
BIBLIOGRAPHY
“Microbiological Degradation” in EPST 1st ed., Vol. 4, pp. 716–725, by W. M. Bejuki, Bio-
Sciences Information Service of Biological Abstracts; “Biodegradable Polymers” in EPSE
2nd ed., Vol. 2, pp. 220–243, by S. J. Huang, The University of Connecticut.
1. J. H. Han, Food Technol. 54, 56 (2000).
2. I. S. Arvanitoyannis, J. Macromol. Sci., Rev. Macromol. Chem. Phys. C 39, 205
(1999).
3. C. L. McCormick, Encycl. Polym. Sci. Eng. 1, 611 (1985).
4. K. J. Sugimoto, Ferment. Assoc. Jpn. 36, 98 (1990).

Vol. 5
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
281
5. C. G. Pitt and A. Schindler, Prog. Contracept. Delivery Syst. 1, 17 (1980).
6. T. Hayashi, Prog. Polym. Sci. 19, 663 (1994).
7. R. J. Mumper and S. L. Klakamp, Drug Targeting Delivery 10, 143 (1999).
8. A. Nagarsekar and H. Ghandehari, J. Drug Targeting 7, 11 (1999).
9. H. W. T. Matthew, Polymeric Biomaterials, 2nd ed., Marcel Dekker, Inc., New York,
2002, p. 167.
10. B.-S. Kim and D. J. Mooney, Trends Biotechnol. 16, 224 (1998).
11. S. J. Huang, M. Bitritto, K. W. Leong, J. Pavlisko, M. Roby, and J. R. Knox, Adv. Chem.
Ser. 169, 205 (1978).
12. J. E. Potts, in M. Grayson, ed., Kirk-Othmer Encyclopeida of Chemical Technology,
(Suppl. Vol.), Wiley-Interscience, New York, 1984, p. 626.
13. Y. Udagawa, J. Polym. Sci., Part A2 9, 1807 (1971).
14. G. N. Patel and A. Keller, J. Polym Sci., Polym. Phys. Ed. 13, 323 (1975).
15. F. B. Khambatta, F. Warner, T. Russell, and R. S. Stein, J. Polym. Sci., Polym. Phys.
Ed. 14, 1391 (1976).
16. W. D. Varnell, I. R. Harnson, and S. J. J. Kosnioski, J. Polym. Sci., Polym. Phys. Ed.
10, 1237 (1981).
17. C. V. Benedict, C. V. Cook, P. Jarrett, J. A. Cameron, S. J. Huang, and P. Bell, J. Appl.
Polym. Sci. 28, 327 (1983).
18. C. V. Benedict, J. A. Cameron, and S. J. Huang, J. Appl. Polym. Sci. 28, 335
(1983).
19. G. E. Zeikov and U. S. Livshitz, Polym. Degrad. Stab. 17, 65 (1987).
20. A. C. Albertsson, S. O. Anderson, and S. Karlsson, Polym. Degrad. Stab. 18, 73
(1987).
21. C. C. Chu, Polymer 26, 591 (1985).
22. D. F. Williams, C. C. Chu, and J. Dwyer, J. Appl. Polym. Sci. 29, 1865 (1984).
23. S. W. Shalaby, ed., Biomedical Polymers, Designed to Degrade Systems, Hanser Pub-
lishers, New York, 1994.
24. A. J. Domb, J. Kost, and D. M. Wiseman, Handbook of Biodegradable Polymers, Har-
wood Academic Publishers, London, 1997.
25. K. A. Walter, R. Tamargo, A. Olivi, P. C. Burger, and H. Brem, Neurosurgery 37, 1129
(1995).
26. L. Montanari, M. Costantini, E. C. Sigoretti, L. Valvo, M. Santucci, M. Bartolomei,
P. Fattibene, S. Onori, A. Faucitano, B. Conti, and I. Genta, J. Controlled Release 56,
219 (1998).
27. U. Siemann, in Proc. Int. Symp. Control. Rel. Bioact. Mater., Vol. 12, 1985, p. 53.
28. I. Engelberg and J. Kohn, Biomaterials 12, 292 (1991).
29. U. Daniels, M. K. O. Chang, K. P. Andriano, and J. Heller, J. Appl. Biomater. 1, 57
(1990).
30. D. E. Perrin and J. P. English, in A. J. Domb, J. Kost, and D. M. Weiseman, eds., Hand-
book of Biodegradable Polymers, Vol. 7, Hardwood Academic Publishers, Amsterdam,
the Netherland, 1997, p. 63.
31. A. Schindler, R. Jeffcoat, G. L. Kimmel, C. G. Pitt, M. E. Wall, and R. Zweidinger,
Contemp. Top. Polym. Sci. 2, 251 (1977).
32. C. G. Pitt, F. I. Chasalow, Y. M. Hibionada, D. M. Klimas, and A. Schindler, J. Appl.
Polym. Sci. 26, 3779 (1981).
33. Y. Tokiwa and T. Suzuki, Nature 270, 76 (1977).
34. X. D. Feng, C. X. Song, and W. Y. Chen, J. Polym. Sci., Polym. Lett. Ed. 21, 593
(1983).
35. S. R. Amor, T. Rayment, and J. K. M. Sanders, Macromolecules 24, 4583
(1991).

282
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
Vol. 5
36. H. Brandl, R. A. Gross, R. W. Lenz, and R. C. Fuller, Appl. Environ. Microbiol. 54,
1977 (1988).
37. K. W. Leong, Connect. Tissue Res. 31, S69 (1995).
38. K. W. Leong, Z. Zhao, and B. I. Dahiyat, Controlled Drug Delivery 469 (1997) (CA 127:
23619).
39. T. Kawaguchi, M. Nakano, K. Juni, S. Inoue, and Y. Yoshida, Chem. Pharm. Bull. 30,
1517 (1982).
40. R. S. Bezwada, S. W. Shalaby, H. D. Newman, and A. Kafrauy, Trans. Soc. Biomater.
13, 194 (1990).
41. R. W. Lenz and P. Guerin, in E. Chiellini and P. Giusti, eds., Polymers in Medicine:
Functional Polymers and Polyamides for Medical Applications of Biodegradable Poly-
mers, Plenum Press, New York, 1993.
42. J. Kemnitzer and J. Kohn, in A. J. Domb, J. Kost, and D. M. Weiseman, eds., Handbook
of Biodegradable Polymers, Vol. 7, Hardwood Academic Publishers, Amsterdam, the
Netherlands, 1997, p. 251.
43. S. Bogdansky, Drugs Pharm. Sci. 45, 231 (1990).
44. S. J. Huang and L.-H. Ho, Macromol. Symp. 144, 7 (1999).
45. K. W. Leong, Polym. Controlled Drug Delivery 127 (1991) (CA 114: 192314).
46. A. Nathan and J. Kohn, in S. W. Salaby, ed., Biomedical Polymers: Designed to Degrade
Systems, Hanser/Gardner, Cincinnati, Ohio., 1994, p. 117.
47. J. Kohn, Trends Polym. Sci. 1, 206 (1993).
48. J. Kohn and R. Langer, J. Am. Chem. Soc. 109, 817 (1987).
49. J. Vandorpe, E. Schacht, S. Dejardin, and Y. Lemmouchi, in A. J. Domb, J. Kost, and
D. M. Weiseman, eds., Handbook of Biodegradable Polymers, Vol. 7, Hardwood Aca-
demic Publishers, Amsterdam, the Netherlands, 1997, p. 161.
50. C. W. R. Wade, S. Gourlay, R. Rice, A. Hegyeli, R. Singler, and J. White, in C. E.
Carraher, J. E. Sheats, and C. U. Pittman, eds., Organometallic Polymers, Academic
Press, New York, 1978, p. 289.
51. S. Lora, G. Palma, M. Carenza, P. Caliceti, and G. Pezzin, Biomaterials 14, 430
(1993).
52. H. R. Allcock and S. Kwon, Macromolecules 19, 1502 (1986).
53. H. R. Allcock, in M. Chasin and R. Langer, eds., Biodegradable Polymers as Drug
Delivery Systems, Marcel Dekker, New York, 1990, p. 163.
54. J. Heller, in A. J. Domb, J. Kost, and D. M. Weiseman, eds., Handbook of Biodegradable
Polymers, Vol. 7, Hardwood Academic Publishers, Amsterdam, the Netherlands, 1997,
p. 99.
55. J. Heller, D. W. H. Penhale, B. K. Fritzinger, J. E. Rose, and R. F. Helwing, Contracept.
Delivery Syst. 4, 43 (1983).
56. A. J. Domb, S. Amselem, and M. Maniar, Polym. Biomater. 399 (1994).
57. A. J. Domb, O. Elmalak, V. R. Shastri, Z. Ta-Shma, D. M. Masters, I. Ringel,
D. Teomim, and R. Langer, in A. J. Domb, J. Kost, and D. M. Weiseman, eds., Hand-
book of Biodegradable Polymers, Vol. 7, Hardwood Academic Publishers, Amsterdam,
the Netherlands, 1997, p. 135.
58. J. C. Huang, A. S. Shetty, and M. S. Wang, Adv. Polym. Technol. 10, 23
(1990).
59. F. H. Otey, R. P. Westhoff, W. F. Kwolek, and C. L. Mehletter, Ind. Eng. Chem. Prod.
Res. Dev. Biol. Stand. 8, 267 (1969).
60. F. H. Otey, R. P. Westhoff, and C. L. Mehletter, Staerke 24, 267 (1972).
61. S. D. Seneker and J. E. Glass, Adv. Chem. Ser. 248, 125 (1996).
62. B. Rossall, Int. Biodeterior. Bull. 10, 95 (1974).
63. R. M. Gardener, C. M. Buchman, R. Komark, D. Dorschel, C. Boggs, and A. W. White,
J. Appl. Polym. Sci. 52, 1477 (1994).

Vol. 5
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
283
64. W. J. Bailey, Polym. J. (Tokyo) 17, 85 (1985).
65. O. C. Agboh and Y. Qin, Polym. Adv. Technol. 8, 355 (1997).
66. J. Hosokawa, M. Nishiyama, K. Yoshihara, and T. Kubo, Ind. Eng. Chem. Res. 29, 800
(1990).
67. Q. Li, E. T. Dunn, E. W. Grandmaison, and M. F. A. Goosen, J. Bioact. Compat. Polym.
7, 370 (1992).
68. S. P. C. Filho and J. Desbrieres, Natural Polymers and Agrofibers Based Composites
41 (2000).
69. S. Tokura, Y. Miura, Y. Uraki, K. Watanabe, I. Saiki, and I. Azuma, Am. Chem. Soc.
Div. Polym. Chem. 31, 627 (1990).
70. M. Tashibana, A. Yaita, H. Tamiura, K. Fukasawa, N. Nagasue, and T. Nakanura,
Jpn. J. Surg. 18, 533 (1988).
71. R. A. A. Muzzarelli, Chitin in Nature and Technology, Plenum Press, New York,
1986.
72. G. A. King, A. J. Daugulis, P. Faulkner, and M. F. A. Goosen, Biotechnol. Prog. 3, 231
(1987).
73. A. A. Badwan, A. Abumalloh, E. Sallam, A. Abukalaf, and O. Jawan, Drug Dev. Ind.
Pharm. 11, 239 (1985).
74. S. J. Huang and K.-W. Leong, Polym. Prepr., Am. Chem. Soc., Div. Polym. Chem. 20,
552 (1979).
75. Y. Tabata and Y. Ikada, J. Controlled Release 27, 79 (1993).
76. Y. Tabata and Y. Ikada, J. Pharm. Pharmacol. 39, 698 (1987).
77. N. Yui, Baiosaiensu to Indasutori 56, 537 (1998).
78. B. G. Shinde, V. S. Nithianandam, K. Kaleem, and S. Erhan, Biomed. Mater. Eng. 2,
123 (1992).
79. C. M. Agrawal and K. A. Athanasiou, J. Bio. Med. Mater. Res. 38, 105 (1997).
80. P. Tormala and P. Rokkanen, Adv. Sci., Technol. 12, 639 (1995).
81. R. S. Bezwada, D. D. Jamiolkowski, I. L. Lee, A. Vishvaroop, J. Persivale,
S. Trenka-Benthin, E. Erneta, A. Y. Surydevara, and S. Liu, Biomaterials 16, 1141
(1995).
82. K. Juni and M. Nakano, Crit. Rev. Ther. Drug Carrier Syst. 3, 209 (1986).
83. C. G. Pitt, in M. Chasin and R. Langer, eds., Biodegradable Polymers as Drug Delivery
Systems, Marcel Dekker, New York, 1990, p. 71.
84. J. E. Bergshma, F. R. Rozema, R. R. M. Bos, A. W. M. Van Rozendaal, W. H.
De Jong, J. S. Teppema, and C. A. P. Joziasse, J. Mater. Sci., Mater. Med. 6, 715
(1995).
85. J. E. Bergshma, F. R. Rozema, R. R. M. Bos, G. Boering, W. C. de Brruijin, and A. J.
Pennings, Biomaterials 16, 267 (1995).
86. H. Brem, A. Kader, J. I. Epstein, R. J. Tamargo, A. Domb, R. Langer, and K. W. Leong,
Sel. Ther. 5, 55 (1989).
87. R. J. Tamargo, J. I. Epstein, C. S. Reinhard, M. Chasin, and H. Brem, J. Biomed.
Mater. Res. 23, 253 (1989).
88. L. W. Seymour, R. Duncan, J. Duffy, S. Y. Ng, and J. Heller, J. Controlled Release 31,
201 (1994).
89. J. S. Kay, B. S. Litin, M. A. Jones, A. W. Fryczkowsky, M. Chvapil, and J. Herschler,
Ophthalmic Surg. 17, 796 (1986).
90. B. A. Rhodes, I. Zolle, J. W. Buchanan, and H. N. J. Wagner, Radiology 92, 1453
(1969).
91. S. S. Davis, L. Illum, J. G. McVie, and E. E. Tomlinson, Microspheres and Drug Ther-
apy: Pharmaceutical, Immunological and Medical Aspects, Elsevier, Amsterdam, the
Netherlands, 1984.
92. J. J. Marty and R. C. Oppenheim, Aust. J. Pharm. Sci. 6, 65 (1977).

284
BIODEGRADABLE POLYMERS, MEDICAL APPLICATIONS
Vol. 5
93. H. Yu, Ph.D. dissertation, Massachusetts Institute of Technology, Cambridge, Mass.,
1988.
94. K. S. Soppimath, T. M. Aminabhavi, A. R. Kulkarni, and W. E. Rudzinski, J. Controlled
Release 70, 1 (2001).
95. M.
Chasin,
Biomed.
Appl.
Synth.
Biodegrad.
Polym.
1
(1995)
(CA
123:
296312).
96. J. Heller, CRC Crit. Rev. Ther. Drug Carrier Syst. 1, 39 (1984).
97. R. Langer, J. Controlled Release 16, 53 (1991).
98. A. K. Dash and G. C. Cudworth II, J. Pharmacol. Toxicol. Methods 40, 1
(1998).
99. O. Bostman and H. Pihlajamaki, Biomaterials 21, 2615 (2000).
100. C. Durucan and P. W. Brown, Adv. Eng. Mater. 3, 227 (2001).
101. W. L. Murphy and D. J. Mooney, J. Periodontal Res. 34, 413 (1999).
102. M. C. Wake, P. K. Gupta, and A. G. Mikos, Cell. Transplant 5, 465 (1996).
103. S. H. J. Mendak, R. J. Jensik, M. F. Haklin, and D. L. Roseman, Ann. Thorac. Surg.
38 (1984).
104. J. P. Vacanti and R. Langer, Lancet 354, 132 (1999).
105. R. C. Thomson, M. C. Wake, M. J. Yaszemski, and A. G. Mikos, Adv. Polym. Sci. 122,
245 (1995).
106. R. Jain, N. H. Shah, A. W. Malick, and C. T. Rhodes, Drug Dev. Ind. Pharm. 24, 703
(1998).
107. R. A. Jain, Biomaterials 21, 2475 (2000).
108. R. S. Bezwada, D. D. Jamiolkowski, and K. Cooper, Drug Targeting Delivery 7, 29
(1997).
109. T. Nakamura, S. Hitomi, T. Shimamoto, S. H. Hyon, S. Watanabe, and Y. Shimizu,
in A. Pizzoferrato, P. G. Machetti, A. Ravaglioli, and A. J. C. Lee, eds., Biomedi-
cals and Clinical Applications, Elsevier Science, Amsterdam, the Netherlands, 1987,
p. 759.
110. K. E. Rehm, in Proc. 4th Int. Conf. on Biomaterials, Denkendorf, 1992,
p. 163.
111. S. Niu, H. Kurumatani, S. Satoh, K. Kanda, T. Oka, and K. Watanabe, ASAIO Trans.
39 M750 (1993).
112. T. Matsuda, M. J. Moghaddam, H. Miwa, K. Sakurai, and F. Iida, ASAIO Trans. 38,
M154 (1992).
113. T. Matsuda, H. Miwa, M. J. Moghaddam, and F. Iida, ASAIO Trans. 39, M327
(1993).
114. C. Artandi, CHEMTECH 476 (1981).
115. Y. Kuroyanagi, E. Kim, M. Kenmochi, K. Ui, H. Kageyama, M. Nakamura, A. Ikeda,
and N. Shioya, J. Appl. Biomater. 3, 153 (1992).
116. Y. Kuroyanagi, E. Kim, and N. Shioya, J. Burn Care Rehabil. 12, 106
(1991).
117. M. Koide, K. Osaki, J. Konishi, K. Oyamada, T. Katamura, and A. Takahasi, Biomed.
Mater. Res. 27, 79 (1993).
118. I. V. B. Annas, J. F. Bruke, J. Bimed. Mater. Res. 14, 65 (1980).
119. C. J. Doillon, C. F. Whyne, S. Brandwein, and F. H. Silver, J. Biomed. Mater. Res. 20,
1219 (1986).
120. S. T. Boyce, D. J. Christian, and J. F. Hansbrough, J. Biomed. Mater. Res. 22, 939
(1988).
121. K. Yoshizato, A. Makino, and K. Nagayoshi, Biomed. Res. 9, 33 (1988).
122. Y. Yasutomi, N. Nakakita, N. Shioya, and Y. Kuroyanagi, Burn Inj. 19, 102
(1993).

Vol. 5
BIOMOLECULES AT INTERFACES
285
123. J. Kopecek, Biomaterials (Guildford, Engl.) 5, 19 (1984).
124. M. Asano, M. Yoshida, H. Omichi, and H. Yamanaka, Maku 17, 216 (1992).
N
EERAJ
K
UMAR
A
VIVA
E
ZRA
T
IRTSA
E
HRENFROIND
M
ICHAL
Y. K
RASKO
A
BRAHAM
J. D
OMB
The Hebrew University of Jerusalem
Wyszukiwarka
Podobne podstrony:
Global Requirements for Medical Applications of Chitin and its Derivatives
Biodegradable Polymers Market Report David K Platt
medical card gp visit card application form
04 Laws of Microactuators and Potential Applications of Electroactive Polymers in MEMS
Degradable Polymers and Plastics in Landfill Sites
kompozytorklasowek gwo pl application pdfQuestions y=1339356508
Medical devices 16
Development of Carbon Nanotubes and Polymer Composites Therefrom
Applications and opportunities for ultrasound assisted extraction in the food industry — A review
FOS Medical id 179961 Nieznany
Testy biodegradacji, Studia, Politechnika
biomateriały i polimery biodegradowalne
Polymer Processing With Supercritical Fluids V Goodship, E Ogur (Rapra, 2004) Ww
Inorganic Polymers
Propylene Polymers
Lab12 Applications
Baxter Vaccine Patent Application
więcej podobnych podstron