
Tolerance of Cultivated Plants to Cadmium
and their Utilization in Polluted Farmland Soils
W
ANG
*
, K. R.
Chinese Academy of Sciences
*
Corresponding author
Changsha Institute of Agricultural Modernization
Phone: + 86 731 461 52 44
410125, Changsha
Fax: + 86 731 461 26 85
Hunan, P.R. China
E-mail: krwang@ms.csiam.ac.cn
Summary
For the purpose of agro-ecological regulation and safe and efficient utilization of cadmium-polluted
farmlands, a 7-year micro-plot experiment was conducted to evaluate the Cd tolerance of several
main cultivated plants in Southern China. The study revealed that cereals such as Oryza sativa and
Zea mays had a strong physiological tolerance of Cd toxicity. Nevertheless, their products (grains) are
easily polluted and hence lose their edible value. As a consequence, they are inappropriate to be
planted in the polluted soils. Other plants such as Brassica napus, Arachis hypogaea and Saccharum
officinarum also had a strong physiological tolerance to Cd pollution. Meanwhile, Cd stocks in their
products were very small. When soil Cd content was less than 50 mg/kg, Cd concentrations in vege-
table oils and cane juice were less than 0.05 mg/kg and 0.15 mg/kg, respectively. This should have
little effects on the edible quality. Therefore, these crops could be cultivated in some slightly Cd pol-
luted farmlands, but the straw and dregs of oil crops, and sugarcane bagasse are not suitable to be
used as manure or stock food as a result of their high Cd contents and should be properly treated as
pollutants. Fibre crops like Gossypium hirsutum, Hibiscus cannabinus, Boehmeria nivea and Morus
alba are tolerant towards soil Cd pollution to different degrees. Basically, soil Cd pollution has no
unfavourable effects on the products of fibre crops. Moreover, there was scarcely any Cd entering the
human food chain through these crops. Therefore, these fibre crops would be a good replacement for
those sensitive crops in the polluted region.
Introduction
Ameliorating strategy of soils polluted with heavy metals in the world mainly stresses
on reforming and purifying the polluted soils. The techniques applied include physical,
chemical and biological methods [1]. Most of the physical and chemical measures that
© WILEY-VCH Verlag Berlin GmbH, 13086 Berlin, 2002 0138-4988/02/01-205-0189 $ 17.50+.50/0
Acta Biotechnol. 22 (2002) 1--2,
189--198

have negative or damaging effects on the native characteristics of the soil should not be
considered as proper ameliorating methods of mending the polluted farmland soil.
Biological methods mainly focus on phytoaccumulation and clearing up of the heavy
metals using plants. However, until now, few super-accumulators have been put into
practice for their small biomass and low degree of soil cadmium uptake [2, 3].
Covering the polluted land with clean soil is at present regarded to be the best way in
ameliorating technology, but it is too expensive and is very difficult to be employed in
developing countries. Applying chemical reagents such as lime, phosphorous fertil-
izers, etc., is therefore still the most common technique used world-wide. The main
drawback of this method is the short duration of its effectiveness. Also, it does not
guarantee the safety of common crops, when soils are heavily polluted by Cd. Based
on the inherent differences of various biotic species to environmental changes,
W
ANG
[4] suggested a new strategy of agro-ecological regulation and safe and efficient
utilization of cadmium-polluted farmland. The key technology of this strategy is select-
ing Cd-tolerant plants, which adapt to the special regional environment where their
products meet the needs of the regional population. From this point of view, a 7-year
comparative study was made on the cadmium tolerance of several plants mainly
cultivated in Southern China, with respect to biology and eco-economics.
Experiments and Methods
Experimental Facilities and Tested Soils
Micro-plot experiments were conducted in 60 quadratic pools constructed of bricks and cements. The
volume of the pool was 100 cm
× 100 cm × 110 cm filled with 1 m
3
red soil which derived from the
Quaternary red earth. The soil was collected from a dry land field near a highway and amended with
organic composts and chemical N, P and K fertilizers before being placed in the pools. The basic
characteristics of the amended soil are shown in Tab. 1.
Tab. 1. The basic fertility features of the test soil
___________________________________________________________________________________________________________________________________________________________________
Texture
O.M.
Total [g/kg]
Rapid available [mg/kg]
CEC
pH
____________________________
_________________________________________
[g/kg]
N
P
K
N
P
K
[Cmol(+)/kg]
___________________________________________________________________________________________________________________________________________________________________
Clay
8.39
0.68
0.47
169
77.2
9.0
188.7
8.15
6.43
___________________________________________________________________________________________________________________________________________________________________
Cd Amendment
CdCl
2
. 2 H
2
O solution was first added to small amounts of soil (about 3 kg) and then mixed with the
upper layer (0–50 cm) of each pool. After about 2 months of amendment, crops were planted or
seeded. Soil Cd was prepared in 5–7 pollution levels. The total Cd content of the upper layer soil of
each pool was measured 2 weeks after planting or seeding. The highest level of Cd amendment
exceeded the extreme Cd content (228 mg/kg) of the heavy polluted farming soil near a non-ferrous
Acta Biotechnol. 22 (2
002) 1--2
190

metal refinery in Southern China [4]. In the control plots, the contents of soil Cd ranged from
0.59 mg/kg to 1.1 mg/kg. High background was probably caused by the pollution of highway
transportation and contamination during the experiments, such as sampling, tillage and fertilizer
application.
Plant Species and Varieties
Eleven plant species and varieties were tested in the experiments, including maize (Zea mays),
var. “xiangyu 4”;
soybean (Glycine max), var. “ zechun 2”; peanut (Arachis hypogaea) ,
var. “luhua 8”; rape (Brassica napus), var. “xiangyou 10”; cucumber (Cucumis sativus),
var. “jinyan 4”; sugarcane (Saccharum officinarum), var. “nayin 310”; bluish dogbane (Hibiscus
cannabinus), var. “917”; roundpod jute (Corchorus olitorius), var. “075-22”; cotton (Gossypium),
var “simian 2”; ramie (Boehmeria nivea), var. “xiangzhu 5”; and mulberry (Morus alba),
var. “husang 197”. Spray irrigation was employed in the experiments. At each sampling time, the
harvested plants were immediately returned to the original plots after measurement in order to keep a
relatively stable soil Cd level in the pools.
Analytical Methods
Soils sampled from a layer of 0–25 cm of each pool were air-dried, sieved and analyzed for their
physical and chemical properties according to the methods suggested by the Specialized Commission
of Agro-Chemistry, the Chinese Society of Soil Sciences (SCAC) [5]. For soil Cd analysis, samples
were digested with aqua regia and HClO
4
and determined using a flame atomic absorption
spectrophotometer (PE-4000). Vegetable oil was extracted by ligroin and oil Cd was detected
according to the method suggested by C
ALAPAJ
et al. (1988) [6]. Plant tissues were dried at 85 °C to a
constant weight and weighed for dry matter (biomass) yields. Plant tissue and cane juice samples
were wet-digested in HNO
3
-HClO
4
solution and Cd concentrations were determined by either flame
or graphite furnace (HGA-400) atomic absorption spectrophotometry depending on the concentration.
SAS software was employed for the data processing.
Results and Discussion
Response of Cereal Crops to Cd Pollution
In Southern China, rice and maize are the main cereal crops. There is much research to
suggest that rice has a strong physiological tolerance to Cd [7, 8]. The main effect is
the accumulation of Cd in rice kernels, hence reducing the grain’s hygienic quality and
their edibility [9]. Study results show that more than 70% of the Cd consumed by hu-
mans comes from vegetables. In Asia, rice is the main contributor of Cd in the human
body [1, 10]. Consequently, in view of public health, rice is a sensitive crop that needs
to be free of Cd pollution.
Maize is more sensitive to Cd than rice in physiological appearance. There are remark-
able differences in its sensitivity to Cd in different sowing seasons. Within the same
variety, the spring sowing maize has strong physiological tolerance to Cd, while the
summer sowing maize has a weak resistance. According to the plot experiment, the
yield of summer sowing maize “xiangyu 4” was 29% lower than that of the control
when soil Cd reached 6.1 mg/kg. As for spring sowing maize, the yield was only 10%
W
ANG
, K. R., Tolerance of Cultivated Plants to Cadmium
191
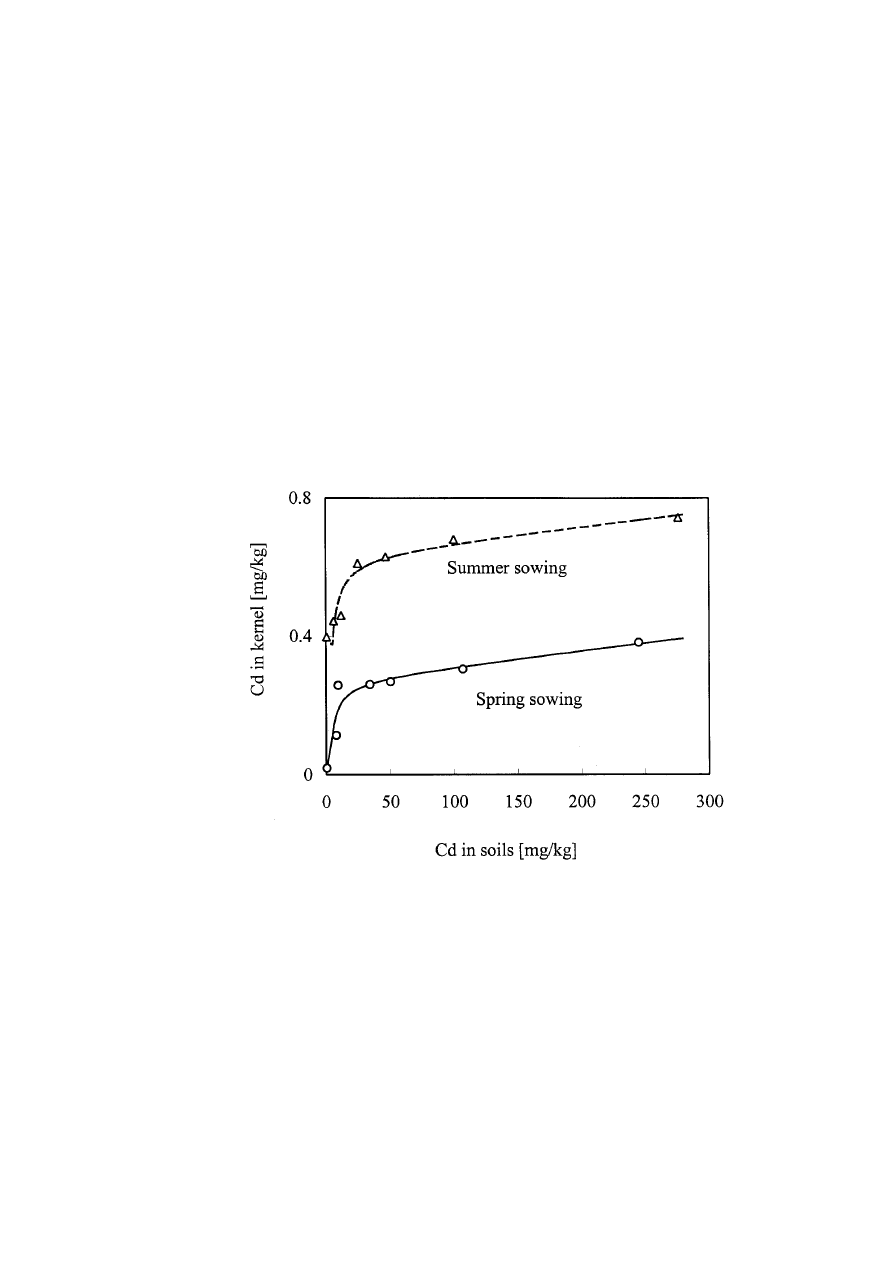
lower than that of control when soil Cd was 9.5 mg/kg and it was 28% lower than that
of control when soil Cd reached 50 mg/kg. Different sowing seasons also have remark-
able effects on the seed Cd contents. Under the same soil Cd level conditions, summer
sowing maize had a higher kernel Cd level than that of spring sowing maize (Fig. 1).
The different manifestations in Cd tolerance and seed Cd content between spring and
summer sowing maize were probably the result of different environmental tempera-
tures during the two seasons. During the summer season, the temperatures were
27–38 °C, much higher than those of the spring growth season (18–34 °C). Higher
temperature conditions might lead to the increases of Cd activity and phytotoxicity of
the soil. Moreover, higher temperatures might cause a higher transpiration rate, and as
a consequence, a higher transferring rate of Cd from the roots to upper-ground parts
(including the seeds) of the plants since passive sorption is a major mechanism of Cd
accumulation by higher plants.
Fig. 1. Effects of soil Cd levels on kernel Cd contents of maize sown in different sea-
sons
Responses of Oil Crops to Cd Pollution
Both rape “xiangyou 10” and peanut “luhua 8” had strong physiological tolerance to
Cd pollution. When soil Cd was below 50 mg/kg, no apparent damage symptom was
found in the plants. Only soybean “zechun 2” was relatively more sensitive to Cd.
When soil Cd was above 5 mg/kg, brown spots appeared on the leaves. The seed yield
Acta Biotechnol. 22 (2
002) 1--2
192
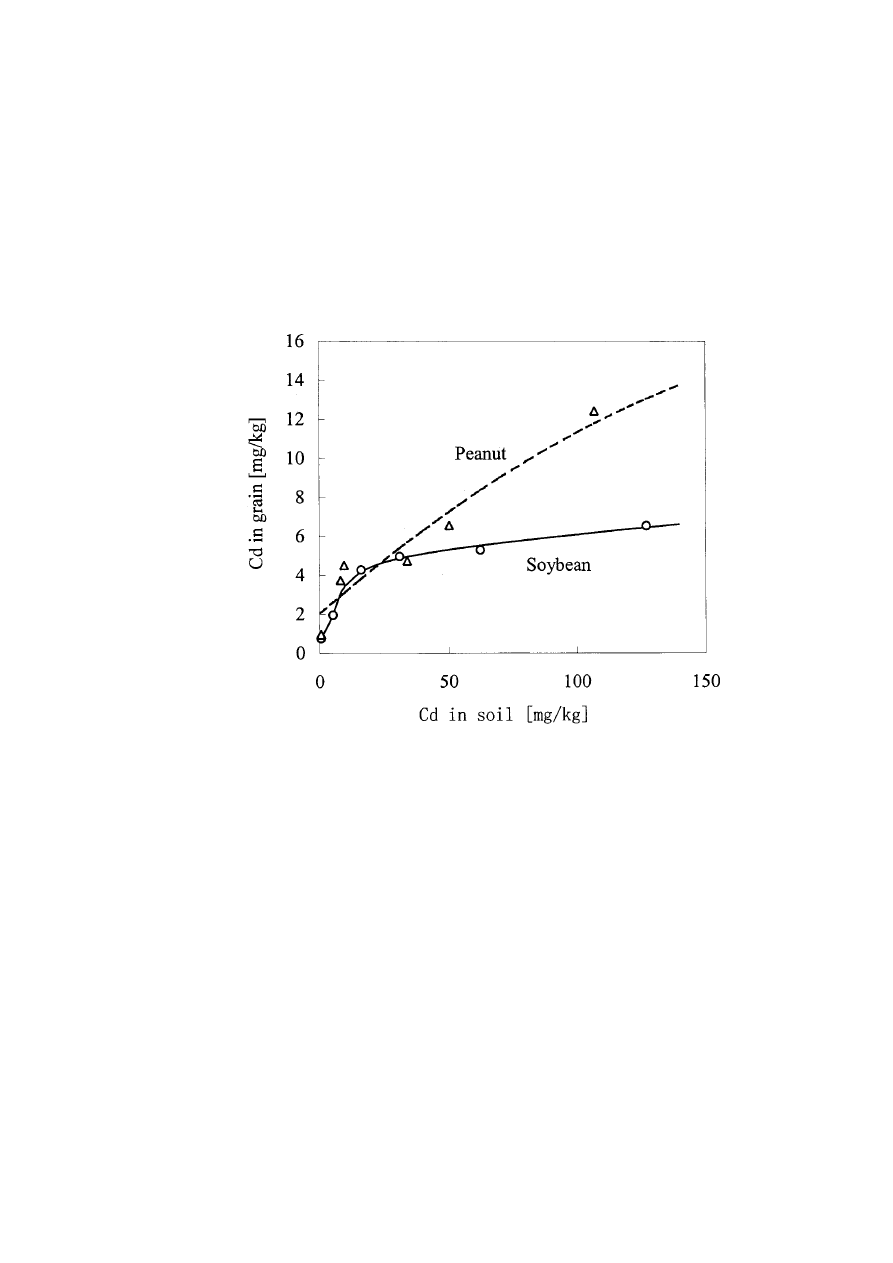
of soybean was reduced by 11% when soil Cd accounted for 16 mg/kg and reduced by
22% when soil Cd accounted for above 31 mg/kg.
Cadmium was easily accumulated in the kernel of the oil crops and could have reached
a high level even though the soil Cd level was relatively low (Fig. 2).
Fig. 2. Effects of soil Cd levels on kernel Cd contents of soybean and peanut
Responses of Vegetable and Sugar Crops to Cd Pollution
Further analysis revealed that kernel Cd was mainly retained in the seedcakes. The
absolute Cd content in the seed oil was very low even though the oil Cd content was
related to soil Cd (Tab. 2). The study also showed that the Cd accumulation rates in
different seedcakes were remarkably different. Peanut seedcake had a much higher Cd
concentration than soybean and rape seedcakes, although the mechanism is still an
open question.
Generally speaking, fruit vegetable crops have a relatively stronger tolerance to Cd
pollution than leaf vegetables. A number of investigations showed that it is very diffi-
cult to produce leaf vegetable products adequate for public health standards when soil
Cd is higher than 1 mg/kg. In this experiment, the Cd content in fresh cucumber fruits
(var. “Jinyan 4”) did not exceed 0.05 mg/kg, which is below the Chinese national food
hygienic standards, when the content of soil Cd was less than 5 mg/kg. The relation-
W
ANG
, K. R., Tolerance of Cultivated Plants to Cadmium
193

ship of Cd content in cucumber (F
Cd
) and Cd content in soil (S
C
) may be described
using the following model:
F
Cd
= 0.0371 + 1.74
× 10
–3
S
C
– 4.10
× 10
–6
S
C
2
(R
2
= 0.9498, n = 7).
The yield and quality of the cucumber at different soil Cd levels are shown in Tab. 3.
Tab. 2. Cd contents of the seed oil and seedcakes of three crops under the conditions
of different soil Cd levels
___________________________________________________________________________________________________________________________________________
Soil Cd levels
Peanut [mg/kg]
Soybean [mg/kg]
Rape [mg/kg]
___________________________
______________________________
_________________________________
Cake Oil Cake Oil
Cake Oil
___________________________________________________________________________________________________________________________________________
1
2.21
0.003
2.36
0.003
1.08
0.003
2
8.31
0.007
3.55
0.005
–
–
3
10.0
0.010
5.00
0.007
–
–
4
14.3
0.019
7.43
0.015
3.08
0.011
5
18.1
0.032
7.5
0.035
5.90
0.042
6
25.6
0.054
8.75
0.045
9.32
0.075
___________________________________________________________________________________________________________________________________________
The soil Cd levels [mg/kg] were:
Peanut: 1–0.59; 2–8.10; 3–9.47; 4–34.1; 5–50.3; 6–107.
Soybean: 1–0.59; 2–5.19; 3–16.1; 4–31.1; 5–62.5; 6–127.
Rape: 1–0.59; 4–38.1; 5–62.2; 6–132.
Tab. 3. Effects of soil Cd on fresh cucumber fruit yield and quality
___________________________________________________________________________________________________________________________________________________________________
Soil Cd
Fruit Cd
Fruit number
Fruit length
Fruit diameter
Weight
Yield
[mg/kg]
[mg/kg]
[fruit/plant]
[cm/fruit]
[cm]
[g/fruit]
[g/plant]
___________________________________________________________________________________________________________________________________________________________________
0.59
0.015
7.0
34.0
13.0
319.5
2237
6.05
0.053
6.5
34.0
13.4
334.1
2172
11.9
0.073
5.5
34.5
13.8
351.2
1932
24.8
0.084
7.8
37.0
11.8
214.6
1663
45.1
0.108
8.0
31.3
11.8
209.8
1678
100
0.165
5.8
31.0
11.0
197.6
1136
276
0.208
2.8
34.6
12.8
185.4
510
___________________________________________________________________________________________________________________________________________________________________
Sugarcane “nayin 310” had a very strong physiological tolerance to Cd pollution.
Under the conditions of the experiment, there was nil negative effect on the yield of the
fresh sugarcane stems when soil Cd was less than 100 mg/kg. The yield was only
reduced by 13.6% compared to the control (13.8 kg/m
2
) when the soil Cd content was
up to 233 mg/kg.
The distribution of Cd in sugarcane plants showed accumulation mainly in the roots.
The content of Cd in cane juice was relatively low (Tab. 4). Further reducing the Cd
content in the refining process would have less effect on the Cd load to the human
body.
Acta Biotechnol. 22 (2
002) 1--2
194

Tab. 4. Cd distribution in sugarcane plants under different soil Cd levels
___________________________________________________________________________________________________________________________________________________________________
Soil Cd
Roots
Spear leaves
Old leaves
Stem skin
Bagasse
Cane juice
[mg/kg]
[mg/kg]
[mg/kg]
[mg/kg]
[mg/kg]
[mg/kg]
[mg/kg]
___________________________________________________________________________________________________________________________________________________________________
0.59
0.27
0.15
0.94
0.10
0.43
0.09
14.5
7.78
0.65
1.73
0.62
0.61
0.10
34.9
17.5
0.70
2.20
0.77
1.45
0.13
51.0
38.6
0.78
3.26
2.41
1.64
0.15
100
55.4
0.92
4.48
2.59
2.20
0.25
233
213
1.06
—
3.57
4.57
0.87
___________________________________________________________________________________________________________________________________________________________________
Responses of Fibrous Plants to Cd Pollution
The main fibrous plants in Southern China are cotton, bluish dogbane, roundpod jute
and mulberry, the feed crop of the silkworm and the raw material for silkworm fibber
production.
Cotton “simian 2” had a strong physiological tolerance to Cd pollution. In the plot
experiment, no negative effect on the yield of unginned cotton was measured when the
soil Cd was less than 56 mg/kg (Tab. 5).
Tab. 5. Effects of Cd pollution on the dry matter yields of different parts of cotton
___________________________________________________________________________________________________________________________________________
Soil Cd [mg/kg]
0.59
6.64 12.8
32.6
56.4
117
231
___________________________________________________________________________________________________________________________________________
Roots [g/m
2
]
18.2
20.2
25.8
27.2
29.0
27.4 40.3
Stem and leaves [g/m
2
]
163.6
173.0
221.2
279.2
308.8
262.4 398.2
Unginned cotton [g/m
2
]
115.0
112.7
156.7
157.2
134.6
63.1 15.2
___________________________________________________________________________________________________________________________________________
Stem dry matter includes cotton boll hulls.
Bluish dogbane “917” was remarkably affected by Cd pollution. There was a negative
relationship between the biomass yield of the upper ground parts of the bluish dogbane
and the soil Cd (r = – 0.907). However, the raw fibre yield was only reduced when soil
Cd content was up to 62 mg/kg (Tab. 6).
Tab. 6. Effects of soil Cd on the biomass and fibre productivity of Bluish dogbane
___________________________________________________________________________________________________________________________________________________________________
Soil Cd
Biomass [g/m
2
]
Raw fibre productivity
___________________________________________________________
[mg/kg]
Roots
Upper ground parts
[%]
___________________________________________________________________________________________________________________________________________________________________
0.59
296
1568
5.7
38.1
184
861
12.5
62.2
104
804
15.3
132
208
483
15.5
___________________________________________________________________________________________________________________________________________________________________
W
ANG
, K. R., Tolerance of Cultivated Plants to Cadmium
195

Compared to bluish dogbane, ramie “xiangzhu 5” was more physiologically sensitive
to soil Cd pollution. When the soil Cd content was up to 14 mg/kg, the yields of ramie
were reduced by 20%. However, cadmium pollution had less effect on the fibre quality
of ramie (Tab. 7).
Tab. 7. Effects of Cd on the fibre quality of Ramie
___________________________________________________________________________________________________________________________________________________________________
Soil Cd
Biomass
Raw
Refined
Fibre
Fibroglue
Fibre
Single fibre
fibre rate
length
fitness
strength
[mg/kg]
[g/m
2
]
[g/m
2
]
[%]
[cm]
[g]
[No./g]
[g]
_________________________________________________________________________________________
________________________________________
_
1.1
2138
323
70.3
151.3
27.9
1547
48.79
20
1508
233
69.5
130.0
27.0
1432
48.08
32
1338
204
70.2
134.0
26.5
1373
47.00
74
1210
182
70.5
134.3
26.7
1448
50.38
127
926
117
70.4
135.0
27.0
1252
57.86
___________________________________________________________________________________________________________________________________________________________________
Roundpod jute “075-22” was very sensitive to Cd pollution. When soil Cd reached
25 mg/kg, the mother roots gradually withered within a week, and the seedling rate
was below 30%.
Mulberry “husang 197” had stronger tolerance to Cd than to ramie. It even stimulated
the plant growth when the Cd level in the soil was below 10 mg/kg. But when soil Cd
exceeded 140 mg/kg, the mulberry roots could not grow regularly, and the plants
gradually died within two years of planting. The absorbed Cd in mulberry was mainly
distributed in the roots, stems and branches (Tab. 8). The Cd contents in the leaves
(fresh) kept less than 2 mg/kg, which did little harm to the silkworms, even if the soil Cd
was at a lethal concentration [11].
Tab. 8. Effect of soil Cd on the Cd contents in different parts of mulberry [dry base,
mg/kg]
______________________________________________________________________________________________________________________________________
Plant parts
Soil Cd
__________________________________________________________________________________________________________
0.92
8.49
22.3
40.6
75.8
145
___________________________________________________________________________________________________________________________________________
Fibre roots
1.07
25.0
39.4
46.5
64.1
281
Main roots
0.57
2.68
5.83
10.7
18.3
165
Main stems
0.40
0.98
1.61
2.48
3.38
33.2
First branches
0.34
0.64
0.88
1.10
1.30
7.75
Second branches
0.31
0.59
0.73
0.86
0.95
3.41
Third branches
0.30
0.47
0.69
0.87
0.97
1.21
Leaves
0.66
0.82
1.12
1.20
1.62
3.32
___________________________________________________________________________________________________________________________________________
Conclusions
According to the experiments, it may be concluded that the cereal crops are unsuitable
for planting in Cd polluted fields because they easily lose their edible value in polluted
soil conditions.
Acta Biotechnol. 22 (2
002) 1--2
196

Both rape and peanut have a strong physiological tolerance to Cd pollution, and only a
small sum of Cd is retained in the seed oil. Based on current food structures, in the
developing countries, such as China, there should not be a great impact on the load of
Cd in the human body from the edible plant oil in Cd polluted areas. So, in view of
risk-assessment, oil crops might be planted in slightly Cd polluted fields. But straws
and the seedcakes should be carefully treated and not used as organic manure or animal
fodder.
Sugarcane has a very strong physiological tolerance to Cd pollution. Cd content in
cane juice is also quite low. If Cd content in the sugar products can be further reduced
in the refining process, the effect of soil Cd pollution on the hygienic quality of the
products would be even small. So it is also a crop which could be planted in slightly
polluted fields. Again, the crop residue and bagasse should be carefully treated as Cd
pollutants.
Nevertheless, the fibrous plants, such as cotton, Bluish dogbane, Ramie and Mulberry,
should be given priority for the ecological regulation and safe and efficient utilization
of Cd polluted farmlands, because all are physiologically tolerant to soil Cd pollution
to different extents and pollution has less effects on the practical value of their prod-
ucts. Moreover, soil Cd is less likely to enter the food chain and eventually do harm to
the human being through these plants.
Acknowledgements
This study was financed by Grant No. KZCX2-407 from the Chinese Academy of Sciences. The
author expresses his sincere appreciation to Ms. H. G
ONG
and J. W
ANG
for their assistance in the plot
experiments and laboratory analysis.
Received 8 May 2001
Received in revised form 1 February 2002
Accepted 20 February 2002
References
[1] I
SKANDAR
, I. K., A
DRIANO
, D. C. (ed.): Remediation of Soils Contaminated with Metals.
Advances in Environmental Science. Science Reviews (UK), 1997.
[2] M
C
G
RATH
, S. P., S
IDOLI
, C. M. D., B
AKER
, A. J. M., R
EEVES
, R. D.: The potential for the use
of metal-accumulating plants for the in situ decontamination of metal-polluted soils. (In:
E
IJSACKERS
, H. J. P., H
AMERS
, T., eds.). Integrated Soil and Sediment Research: a Basis for
Proper Protection. Dordrecht: Kluwer Academic Publishers, 1993, 673–676.
[3] Z
AUROV
, D. E., P
E R D O M O
, P., R
ASKIN
, I.: Optimizing soil fertility and pH to maximize
cadmium removed by Indian Mustard from contaminated soils. J. Plant Nutrition. 22 (1999),
977–986.
[4] W
ANG
, K. R.: Status of Cd pollution and strategy of the treatments and utilization of Cd-
polluted farmlands in China. Agro-environmental Protection 16 (1997), 274–278.
[5] SCAC: Routine Analysis Methods of Soil and Agro-Chemistry. Science Press, China, 1984.
W
ANG
, K. R., Tolerance of Cultivated Plants to Cadmium
197

[6] C
ALAPAJ
, S., C
HIRICOSTA
, S., S
AIJA
, G., B
RUNO
, E.: Method for the determination of heavy
metals in vegetable oils by graphite furnace atomic absorption spectroscopy. Atomic Spectrosc.
9 (1988), 107–109.
[7] W
ANG
, K. R.: Comparative study on Cd phytotoxicity to different genes of rice. Rural. Eco-
Environ. 12 (1996), 18–23.
[8] K
AWADA
, T., S
UZUKI
, S.: A review on the cadmium content of rice, daily cadmium intake, and
accumulation in the kidneys. J. Occup. Health 40 (1998), 264–269.
[9] D
ONG
, K., C
HEN
, J., D
ENG
, X.: Relationship between the growth of plants polluted with
cadmium and its accumulation in them. J. Environ. Sci. 3 (1982), 31–34.
[10] W
AGNER
, G.: Accumulation of cadmium in crop plants and its consequences to human health.
Adv. Agron. 51 (1993), 173–212.
[11] G
O N G
, H., C
H E N
, C., W
A N G
, K. R., W
A N
, J.: Effect of cadmium on the growth and
development of silkworms and quality of cocoon. Chin. J. Appl. Environ. Biol. 4 (1998),
159–162.
Acta Biotechnol. 22 (2
002) 1--2
198
Wyszukiwarka
Podobne podstrony:
biotechnologia, TEST KOŃCOWY 200.., TEST KOŃCOWY 2001/2002
biotechnologia, TEST KOŃCOWY 200.., TEST KOŃCOWY 2001/2002
2 Abramowitz, Davidson BioTechniques 33 772 2002
Biotechnologia w 6
etapy i perspektywy biotechnologii
Wyklad 5 biotech2
biotechnologia
Biotechniki rozrodu 3
Biotechnologia zamkniete użycie (2012 13)
Kadm
BIOTECHNOLOGIA5
Biotechnologia w 7
Bakterie w biotech
Ustawa z 30 10 2002 r o ubezp społ z tyt wyp przy pracy i chor zawod
Biotechnologia
więcej podobnych podstron