
Correlation of in planta endo-beta-1,4-xylanase activity
with the necrotrophic phase of the hemibiotrophic fungus
Mycosphaerella graminicola
A. Siah
a
, C. Deweer
a
, F. Duyme
c
, J. Sanssene´
d
, R. Durand
b
, P. Halama
a
and P. Reignault
b
*
a
Laboratoire Biotechnologie des Micro-organismes, GIS PhyNoPi, Institut Supe´rieur d’Agriculture, 48 Boulevard Vauban, F-59046,
Lille Cedex;
b
Laboratoire Mycologie-Phytopathologie-Environnement, GIS PhyNoPi, Universite´ du Littoral Coˆte d’Opale, 17 Avenue
Louis Ble´riot, BP 699, F-62228 Calais Cedex;
c
Arvalis-Institut du Ve´ge´tal, Station Expe´rimentale, F-91720, Boigneville; and
d
Laboratoire Pathologie Ve´ge´tale, Institut Polytechnique Lasalle-Beauvais, Rue Pierre Waguet, BP 30313, F-60026, GIS PhyNoPi,
Beauvais Cedex, France
The association of the cell wall degrading enzyme endo-beta-1,4-xylanase (EC 3.2.1.8) with pathogenicity of Mycosphaerella
graminicola was examined in planta. The enzyme production of two M. graminicola isolates (T0372 and T0491), as well as
their ability to infect seedlings of susceptible wheat cv. Scorpion, was first compared. No significant difference was found
between the two isolates regarding spore germination rates, mycelial growth on the leaf surface or direct and stomatal pene-
trations. However, restricted hyphal growth was observed inside leaves inoculated with T0372, whereas successful meso-
phyll colonization with a strong intercellular fungal growth was found in leaves infected with T0491. Likewise, T0372 was
unable to induce lesions bearing pycnidia and to produce endo-beta-1,4-xylanase activity until 22 days post-inoculation
(d.p.i.). On the other hand, significant high increases of both diseased leaf area bearing pycnidia and endo-beta-1,4-xylanase
activity were observed between 16 and 22 d.p.i. for T0491 (r = 0Æ98). The investigation of 24 additional isolates, including
the IPO323 and IPO94269 reference isolates, highlighted a strong correlation between endo-beta-1,4-xylanase activity and
disease development levels (r = 0Æ94). This study demonstrates that differences in pathogenicity in M. graminicola are not
linked to events on the leaf surface or to frequency of leaf penetration, but to the ability of the fungus to colonize the meso-
phyll and to produce the cell wall degrading enzyme endo-1,4-beta-xylanase during the necrotrophic phase.
Keywords: cell wall degrading enzymes, pathogenicity, septoria tritici blotch, Triticum aestivum
Introduction
Septoria tritici blotch caused by the hemibiotrophic
fungus Mycosphaerella graminicola (anamorph: Septoria
tritici) is one of the most serious foliar diseases of wheat
in many parts of the world, capable of reducing yields by
40% (Eyal, 1999). Because of its experimental amenabil-
ity and strong economic significance, M. graminicola is
currently being established as a model, not only for the
Mycosphaerellaceae, but also for the order Dothideales
(Kema et al., 2008). During recent years, knowledge of
this fungus, including the molecular basis of its patho-
genicity, has significantly improved (Mehrabi et al.,
2006a,b; Kema et al., 2008). However, the biochemical
bases of its pathogenicity and infection process are still
poorly understood (Palmer & Skinner, 2002; Douaiher
et al., 2007b).
The infection biology of M. graminicola has been
examined in the past at the cytological level. High humid-
ity and moderate temperature conditions favour spore
germination and disease establishment (Kema et al.,
1996). In contrast to some other plant pathogenic fungi
such as Phaeosphaeria nodorum, the causal agent
of glume blotch of wheat (Solomon et al., 2006),
M. graminicola does not produce typical appressoria
when it actively penetrates its host (Kema et al., 1996;
Duncan & Howard, 2000; Mehrabi et al., 2006b). How-
ever, appressorium-like structures were observed at
both direct (Dancer et al., 1999; Rohel et al., 2001)
and stomatal penetration sites (Cohen & Eyal, 1993;
Kema et al., 1996). Leaf tissue colonization by the infec-
tious hyphae remains strictly intercellular, although it
*E-mail: philippe.reignault@univ-littoral.fr
Published online 9 May 2010
No claim to original US government works
Journal compilation
ª 2010 BSPP
661
Plant Pathology (2010) 59, 661–670
Doi: 10.1111/j.1365-3059.2010.02303.x

occurs in close contact with mesophyll cells (Cohen &
Eyal, 1993; Kema et al., 1996). Throughout the latent
phase, M. graminicola uptakes nutrients such as soluble
carbohydrates available in the apoplast and does not
appear to secrete toxins or compounds which are able to
kill the host (Rohel et al., 2001). The transition from bio-
trophy to necrotrophy (possibly triggered by critical
amounts of fungal biomass and ⁄ or altered host physiol-
ogy) takes place suddenly around 10 days after the infec-
tion process is initiated (Kema et al., 1996, 2008;
Mehrabi et al., 2006a,b), but this time lapse may vary
depending both on the cultivar infected and environmen-
tal conditions (Lovell et al., 2004). This transition eventu-
ally results in typical disease symptoms, consisting of
visible irregular chlorotic lesions containing necrotic
blotches within which the fungus ultimately sporulates
by producing dark brown to black pycnidia (Kema et al.,
1996; Duncan & Howard, 2000).
During a successful invasion, most necrotrophic plant-
pathogenic fungi such as P. nodorum (Magro, 1984;
Lalaoui et al., 2000) produce a battery of cell wall degrad-
ing enzymes (CWDE) capable of degrading cell-wall
polymers (Belie¨n et al., 2006), although biotrophic fungi
also produce CWDE (Komiya et al., 2003). Unlike cell
walls from dicotyledonous plants, where pectin is a major
polysaccharide polymer, the cell wall matrix from grami-
naceous monocotyledonous plants consists mainly of
hemicellulose (Labavitch & Ray, 1978), with xylan as
the predominant hemicellulosic component and the most
abundant polysaccharide in cereal cell walls (Cooper
et al., 1988). This polymer consists of a b-1,4-linked
D
-xylopyranosyl backbone that can be substituted by
different side groups such as
L
-arabinose,
D
-galactose,
acetyl, feruloyl, p-coumaroyl and glucuronic acid
residues. Complete hydrolysis of such a polysaccharide
complex requires the synergistic action of several xylan-
degrading enzymes (Collins et al., 2005). Among these,
endo-b-1,4-xylanase (EC 3.2.1.8) (hereafter referred to
as xylanase) is of potentially crucial importance since it
cleaves internal bonds in the xylan backbone, leading to
the production of xylo-oligomers (Collins et al., 2005).
Xylanase is associated with pathogenicity in number
of plant pathogenic fungi, including cereal pathogens,
where it is the most abundant CWDE (Magro, 1984;
Cooper et al., 1988; Lalaoui et al., 2000; Douaiher et al.,
2007a,b). In addition, xylanase is known to induce host
defence responses in certain host–pathogen pathosystems
(Belie¨n et al., 2006).
The involvement of CWDE during M. graminicola
pathogenesis has been suggested by several reports
(Cohen & Eyal, 1993; Kema et al., 1996; Duncan &
Howard, 2000; Shetty et al., 2003, 2007). More recently,
an examination of expressed sequenced tag (EST)
libraries generated from different stages of pathogenesis
allowed the identification of a range of genes expressed
during the necrotrophic phase, including genes showing
high similarity levels to CWDE-encoding genes in other
fungi (Kema et al., 2008). One of the most abundant
CWDE mRNAs was that encoding for xylanase, the
enzyme for which in vitro activity was previously shown
to correlate with necrosis development by M. graminicola
(Douaiher et al., 2007b). Despite this knowledge, the
M. graminicola in planta xylanase activity produced
during infection, as well as its relationship with patho-
genicity of the fungus, are still unknown. Hence, the pres-
ent study investigated in planta xylanase production, as
well as its association with different aspects of
M. graminicola pathogenesis, including infection and
disease establishment. First, the in planta abilities of two
M. graminicola isolates differing in pathogenicity levels
(i) to induce different cytological events, (ii) to cause leaf
lesion areas bearing pycnidia and (iii) to produce xylan-
ase activity were compared. Thereafter, the correlation
between xylanase activity and disease development was
investigated by using 24 additional isolates with different
pathogenicity levels in order to confirm the association of
xylanase activity with pathogenicity within the fungus.
Materials and methods
Fungal isolates and inoculum production
Mycosphaerella graminicola isolates used in this study,
including the two reference isolates from the Netherlands
IPO323 and IPO94269 (Kema et al., 2000), are listed
in Table 1. Isolates T0372 and T0491, used for both
infection and xylanase time-course assays, were found
to be weakly pathogenic and pathogenic, respectively,
on the susceptible wheat cv. Scorpion in preliminary
experiments. The other 22 isolates were a subset of a
Table 1 Isolates of Mycosphaerella graminicola used in this study
Isolate
Sampling location
Year of isolation
T021
Nord
2005
T032
Nord
2005
T049
Nord
2005
T055
Nord
2005
T083
Nord
2005
T0148
Eure-et-Loir
2005
T0242
Aube
2005
T0251
Aube
2005
T0254
Aube
2005
T0262
Aube
2005
T0278
Cher
2005
T0290
Somme
2005
T0293
Somme
2005
T0345
Seine-et-Marne
2005
T0365
Loire-Atlantique
2005
T0372
Loire-Atlantique
2005
T0384
Loire-Atlantique
2005
T0403
Essonne
2005
T0414
Essonne
2005
T0451
Eure-et-Loir
2005
T0466
Gers
2005
T0473
Gers
2005
T0485
Meuse
2005
T0491
Meuse
2005
IPO323
Netherlands
1981
IPO94269
Netherlands
1994
662
A. Siah et al.
Plant Pathology (2010) 59, 661–670

collection of 363 French isolates previously genotyped
using microsatellites, actine and b-tubulin markers (data
not shown). These isolates were chosen on the basis of
their maximum Euclidean genetic distance to each other
calculated with
XLSTAT
. They were grown on potato dex-
trose agar (PDA) medium (Sigma) for 10 days at 18C
with a 12 ⁄ 12-h day ⁄ night cycle. Inocula were prepared
by washing cultures with 10 mL sterile distilled water.
The obtained suspensions were then adjusted to
10
6
spores mL
)1
.
Plant growth conditions and pathogenicity tests
Wheat grains of the susceptible cv. Scorpion were preger-
minated in Petri dishes (12 · 12 cm) on moist filter paper
in darkness at 20C for 24 h, at 4C for 48 h and then at
20C for 24 h. Scorpion is not known to have any Stb
resistance genes, therefore a compatible interaction
between M. graminicola and wheat was investigated in
this study. Germinated grains were then placed into
15-cm-diameter pots. Six pots of 12 grains were used as
replicates for each isolate. The pots were placed in the
glasshouse at 18C (±2C) with a day ⁄ night cycle of
16 ⁄ 8 h with supplementary illumination. After 3 weeks
(when third leaves from the base of plants were fully
expanded), plants of each pot were inoculated using hand
sprayer until runoff with 30 mL spore suspension
(10
6
spores mL
)1
) amended with 0Æ05% polyoxyethyl-
ene-sorbitan monolaurate (Tween 20, Sigma) surfactant.
Control plants were treated with sterile water containing
Tween 20 surfactant alone. Immediately after inocula-
tion, each pot was covered with a clear polyethylene bag
for 6 days in order to ensure a water-saturated atmo-
sphere compatible with good fungal germination. The
disease was scored every 2 days for 22 days post-inocula-
tion (d.p.i.) by noting the percentage of the area of the
third leaf covered by lesions (chlorosis or necrosis) bear-
ing pycnidia.
Histopathological analysis
Monitoring of spore germination and leaf penetration by
M. graminicola was performed using Fluorescence
Brightener 28 (Calcofluor, Sigma). Wheat leaf segments
(2 cm) were harvested 1, 2, 4 and 6 d.p.i. from control
and inoculated plants and then immersed for 5 min in a
solution of 0Æ1% (w ⁄ v) Calcofluor, 0Æ1
M
Tris–HCl buf-
fer pH 8Æ5. Leaf segments were then washed for 2 min
with sterile distilled water. After drying in darkness at
laboratory temperature, they were placed on a glass slide,
covered with a cover slip and observed microscopically
(Nikon, Eclipse 80i) under UV illumination. Pictures
were taken with a digital camera (DXM1200C) using
image capture software (
NIS-ELEMENTS BR
).
Mesophyll colonization was investigated using Trypan
blue according to Shetty et al. (2003) with few modifica-
tions. Leaf segments (2 cm) were harvested from both
control and inoculated leaves and destained in a absolute
ethanol:glacial acetic acid (3:1, v ⁄ v) mixture for 1 night.
The solution was refreshed twice. Cleared leaves were
rehydrated for 4 h in distilled water and then fixed for
20 min in lactoglycerol (lactic acid:glycerol:water, 1:1:1,
v ⁄ v ⁄ v). Staining of the fungus was carried out at 70C by
immersing the leaf segments in 0Æ1% Trypan blue dis-
solved in lactophenol-ethanol (1:2, v ⁄ v) for 2 h. After
washing, leaf segments were fixed in lactoglycerol, placed
on a glass slide, covered with a cover slip and then
observed microscopically under white light illumination
in the same conditions as above. An additional Trypan
blue assay was performed in order to reveal the fungal
growth on the leaf surface. This staining was carried out
using a Trypan blue solution at 50C for 20 min rather
than 70C for 2 h.
Protein extraction and enzyme assay
Total protein extractions were carried out every 2 days
up to 22 d.p.i. as previously described by Magro (1984)
with few modifications. Briefly, 10 g inoculated and con-
trol third leaves were removed and immediately frozen in
liquid nitrogen prior to grinding in a mortar. The result-
ing powder was then dispersed in 10 mL 0Æ05
M
Tris–HCl buffer pH 7Æ8 containing 0Æ1
M
KCl and 0Æ5%
cysteine. After cooling for 30 min at 4C, the mixture
was squeezed through three layers of cheesecloth and
centrifuged at 13 000 g for 20 min at 4C. From the
supernatant, 100 lL were transferred to an Eppendorf
tube and stored at 4C for total protein quantification.
Total protein extracts were then dialysed against 2 L dis-
tilled water for 24 h at 4C (SpectrumLab Inc.). Water
was changed once. The dialysed solution was then sub-
jected immediately to xylanase activity quantification
using a modified 3,5-dinitrosalicylic acid (DNS) method
(Miller, 1959; Douaiher et al., 2007b). As xylan is a
heteropolymer, accessory enzymes, especially arabinosi-
dase, would also give a positive result as they can release
reducing sugars during enzyme assay. Xylanase activity
was measured at pH 4Æ8 [sodium citrate buffer (100 m
M
)]
and 45C using xylan (1%, w ⁄ v) from oat spelt (Sigma)
as a substrate and xylose (10 m
M
) (Sigma) as the reducing
control sugar. Both enzyme and substrate controls were
included in each assay. The reaction mixture was incu-
bated for 30 min. Absorbancies were measured at
540 nm. Total protein concentrations were determined
at 595 nm using bovine serum albumin as a standard
(Bradford, 1976).
Statistical analysis
Comparisons between rates of spore germination, leaf
penetration, leaf area with lesions bearing pycnidia and
xylanase activity for the two isolates T0372 and T0491
were carried out using the Tukey test at a significance
level of P = 0Æ05 (
STATISTICA
8 software, StatSoft). Corre-
lations between levels of xylanase activity and leaf area
with lesions bearing pycnidia within the assessed iso-
lates were performed using the Pearson correlation test
(
STATISTICA
8 software).
Xylanase and septoria tritici blotch
663
Plant Pathology (2010) 59, 661–670
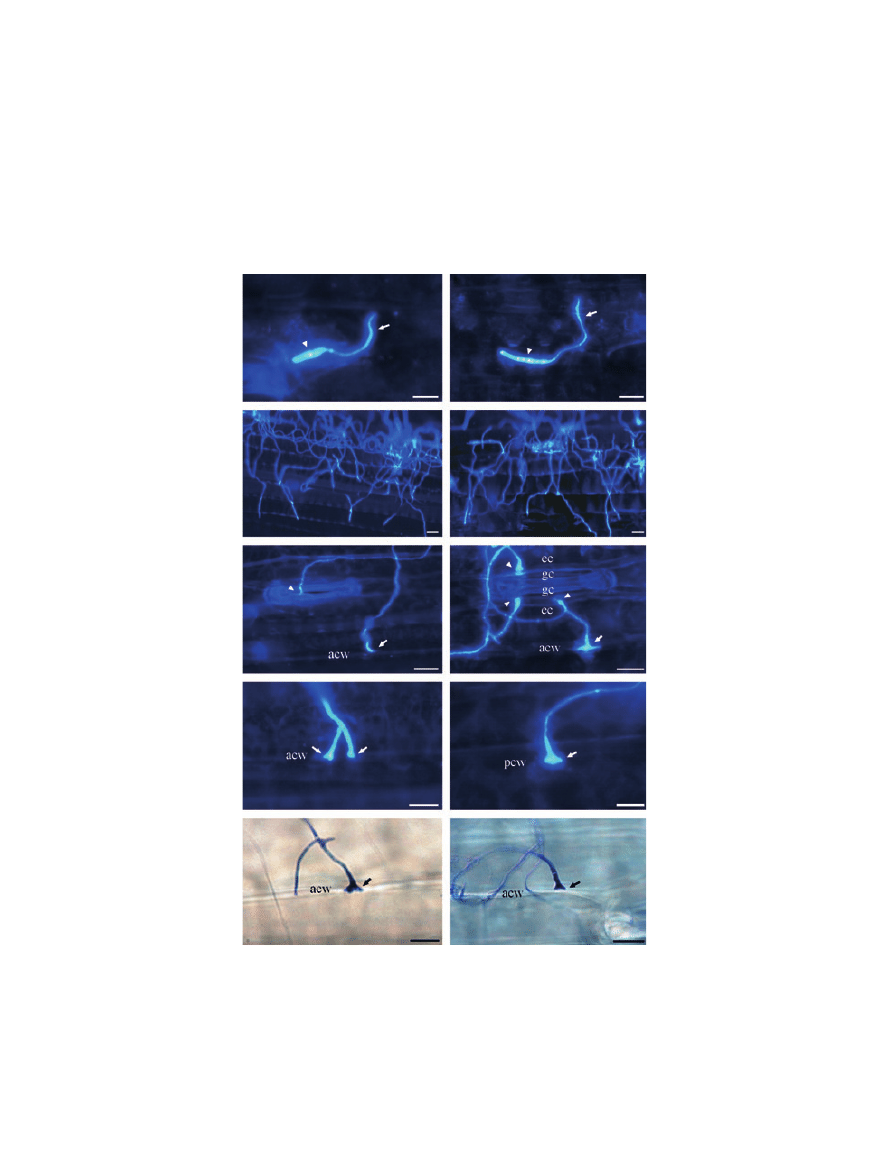
Results
Spore germination of, and leaf penetration by,
isolates T0372 and T0491
Fungal growth of isolates T0372 and T0491 was revealed
on the leaf surface using Calcofluor. Spores from both
isolates formed germ tubes from their ends (Fig. 1a,b). At
1 d.p.i., 54Æ3 and 57Æ8% of spores were germinated for
T0372 and T0491, respectively, and these rates did not
further increase significantly over time and were not sig-
nificantly different between the two isolates at any time
point (56Æ9, 56Æ2 and 56Æ9% for T0372 and 58Æ2, 58Æ3
and 54Æ0% for T0491 at 2, 4 and 6 d.p.i., respectively).
Germ tubes were often branched and oriented randomly
T0372
T0491
1 d.p.i.
6 d.p.i.
6 d.p.i.
6 d.p.i.
6 d.p.i.
(a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
(i)
(j)
Figure 1 Similar prepenetration growth and penetration behaviour of the weakly pathogenic Mycosphaerella graminicola isolate T0372 (a, c, e,
g and i) and the pathogenic isolate T0491 (b, d, f, h and j) on the leaf surface of susceptible wheat cv. Scorpion. The fungus was revealed
using Calcofluor (a–h) or Trypan blue (i–j). Germinating spores (a and b) (arrowheads) generating infectious germ tubes (arrows) at 1 day
post-inoculation (d.p.i.). Extensive hyphal growth (c and d) on the leaf surface at 6 d.p.i. (e, f, g, h, i and j). Appressorium-like structures
formed by infectious germ tubes over stomata (e, arrowhead) as well as over the epidermis (arrow), over both anticlinal (e, f, g, i and j) and
periclinal cell walls (h). Note the formation of multiple appressorium-like structures (f, arrowheads) between stomatal guard cells and
epidermal cells. acw, anticlinal call wall; pcw, periclinal cell wall; gc, guard cell; ec, epidermic cell. Scale bar = 10 lm.
664
A. Siah et al.
Plant Pathology (2010) 59, 661–670
on the leaf surface without specific orientation towards
stomata and no possible chemotropism or thigmotropism
could be noticed. No visible difference was observed
between the two isolates regarding hyphal growth on the
leaf surface at 6 d.p.i. (Fig. 1c,d) throughout the duration
of the experiment.
Both direct and stomatal leaf penetrations were
observed for the two isolates. They occurred from 2 d.p.i.
and their rates increased over time for both isolates
(Table 2). For T0372, stomatal and direct penetrations
increased from 6Æ1 and 9Æ5%, respectively, at 2 d.p.i., to
22Æ3 and 32Æ4%, respectively, at 10 d.p.i., whilst for
T0491, these penetration events increased from 8Æ5 and
10Æ5%, respectively, at 2 d.p.i., to 24Æ9 and 31Æ1%,
respectively, at 10 d.p.i. The rates of penetration events
were not significantly different between the two isolates
at any time point (Table 2). Both isolates formed appres-
sorium-like structures by swelling of the hyphae at the tip
of germ tubes (Fig. 1e–j). These structures occurred over
stomata in contact with the ridges of guard cell lips
(Fig. 1e), as well as above epidermal cells on both anticli-
nal (Fig. 1e,f,g,i,j) and periclinal (Fig. 1h) cell walls.
Occasionally, these appressorium-like structures were
observed at the junction between stomatal guard cells
and epidermic cells (Fig. 1f). No difference was observed
between the two isolates regarding the frequencies of
these infection structures.
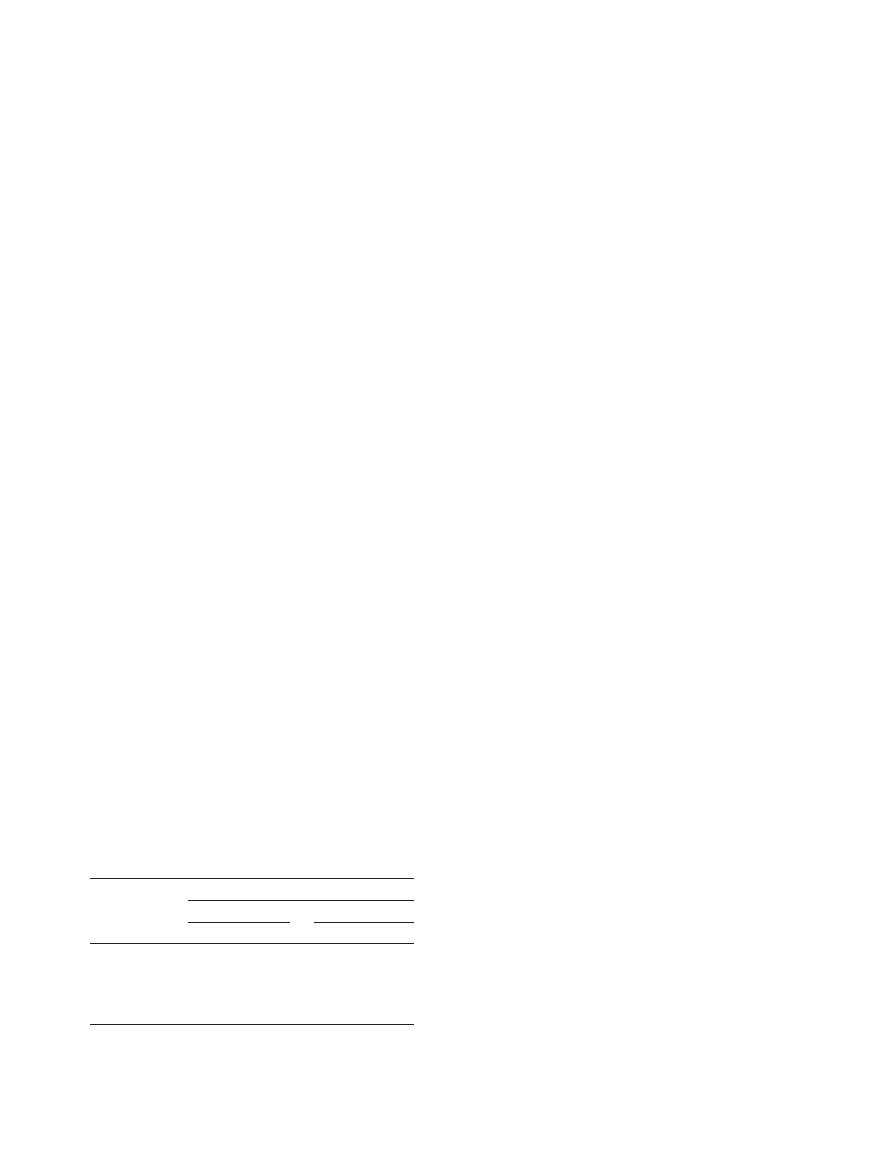
Mesophyll colonization by isolates T0372 and
T0491
The assessment of host colonization at 22 d.p.i. using
Trypan blue revealed clear differences between isolates
T0372 and T0491. Intercellular hyphae with longitudi-
nal and transversal growth between mesophyll cells were
observed in leaves infected with the pathogenic isolate
T0491 (Fig. 2b,d). Hyphae usually aggregated in the sub-
stomatal cavities (Fig. 2f) and eventually led to mature
pycnidia bearing pycniospores (Fig. 2h). By contrast, very
little hyphal growth, without the formation of any pycni-
dia, was found inside leaves inoculated with T0372
(Fig. 2a,c,e,g). At 22 d.p.i., T0372 had colonized only
11Æ02 ± 4Æ63% of the host substomatal cavities, whereas
T0491
colonized
85Æ54 ± 7Æ50%
of
them,
with
35Æ13 ± 3Æ07% bearing pycnidia (Fig. 3).
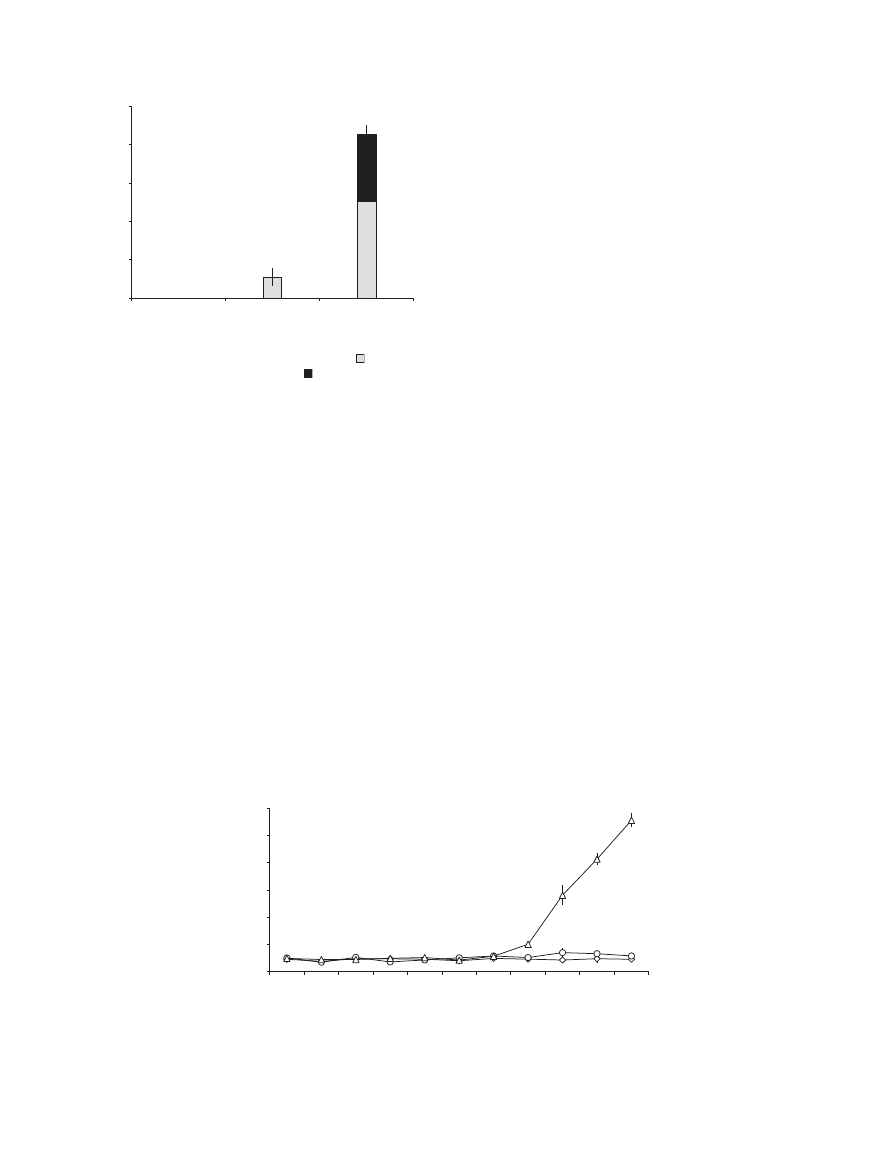
Correlation of in planta xylanase activity with
disease establishment in isolates T0372 and T0491
Both xylanase activity and disease level were quantified
every 2 days for 22 days in leaves infected with T0372 or
T0491. In the case of the weakly pathogenic isolate
T0372, xylanase activity levels remained low and not sig-
nificantly different from those of control leaves until
22 d.p.i. (Fig. 4). Only limited chlorotic spots containing
no pycnidia were observed on the leaves inoculated with
this isolate (Figs 2i & 5). With the pathogenic isolate
T0491, a significant high increase of xylanase activity
from 100Æ5 ± 9Æ4 to 555Æ7 ± 25Æ7 mU lg
)1
total proteins
was observed between 16 and 22 d.p.i. (Fig. 4). Early
chlorotic and necrotic lesions covered with numerous
pycnidia became visible at 16 d.p.i. on the leaves inocu-
lated with this isolate. Symptoms expanded thereafter
across the leaf surfaces, reaching 75Æ83 ± 7Æ78% of the
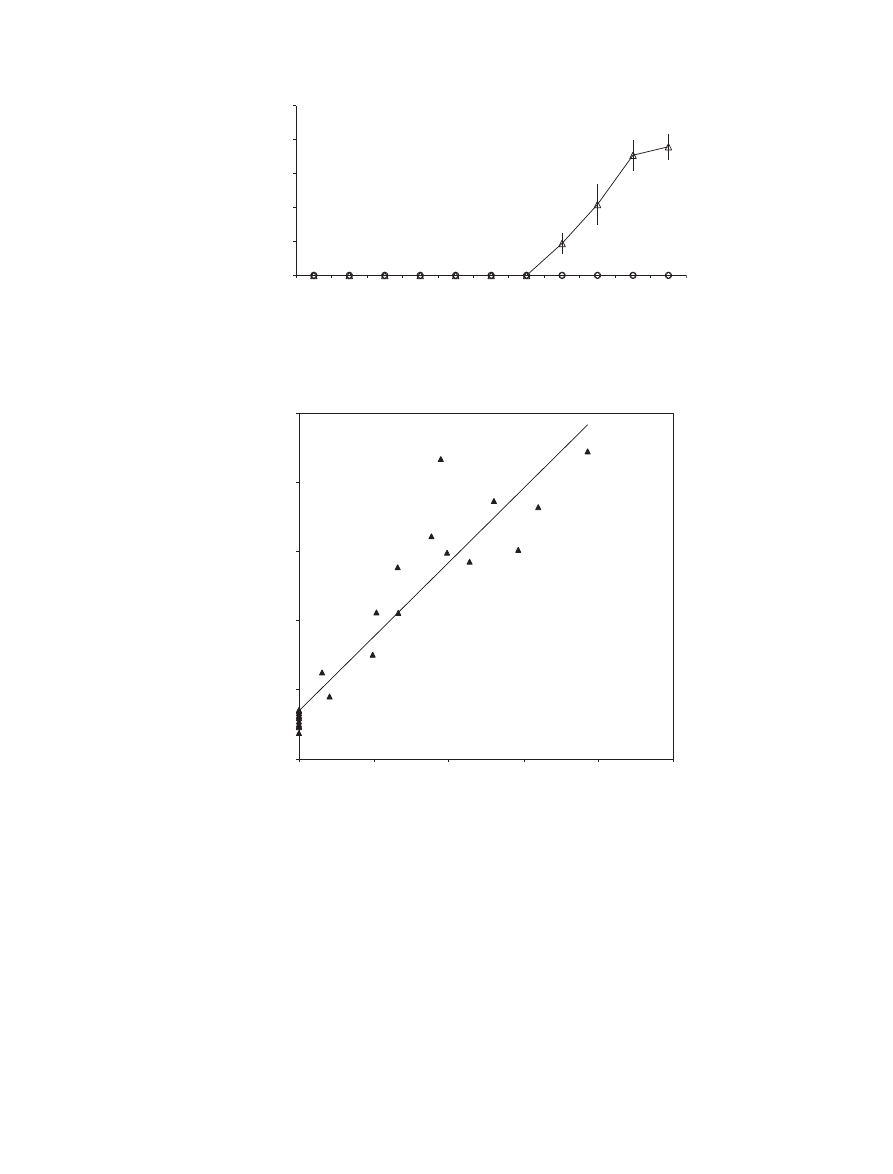
leaf area at 22 d.p.i. (Figs 2j & 5). The Pearson correla-
tion test used to measure the association between xylan-
ase activity and leaf area with lesions bearing pycnidia
within the isolate T0491 revealed a strong correlation
between these two parameters (r = 0Æ98). The whole
experiment was replicated three times and all assays dis-
played a similar enzymatic production pattern for each
isolate and for controls. Control plants remained free of
disease throughout the experiment.
Correlation of in planta xylanase activity with
leaf area with lesions bearing pycnidia in 24
M. graminicola isolates with varying pathogenicity
levels
In order to confirm the relationship observed for the iso-
late T0491 between xylanase activity and leaf area with
lesions bearing pycnidia, both parameters were quanti-
fied at 20 d.p.i. for 24 additional isolates of the fungus
(Fig. 6). Ten out of the 24 isolates, including the reference
isolate IPO94269, were unable to induce any lesions
bearing pycnidia on the inoculated plants and produced
non-significant levels of xylanase activity compared to
control plants (Fig. 6). For the remaining 14 isolates, the
percentage of leaf area with lesions bearing pycnidia ran-
ged from 6Æ2 ± 2Æ1 to 77Æ0 ± 8Æ5% and xylanase activity
levels from 37Æ5 ± 12Æ9 to 445Æ2 ± 53Æ4 mU lg
)1
total
proteins. A correlative analysis displayed a high correla-
tion level between xylanase activity and leaf area with
lesions bearing pycnidia within the 24 assessed isolates
(r = 0Æ94).
Discussion
The investigation of both prepenetration growth and
penetration-associated events revealed no significant dif-
ference between the two isolates T0372 and T0491. At
1 d.p.i., more than 50% of fungal spores had germinated;
Table 2 Rates of direct and stomatal leaf penetration events obtained for
Mycosphaerella graminicola isolates T0372 and T0491 on inoculated
plants of wheat cv. Scorpion
Days
post-inoculation
Percentage of germ tubes giving penetration
Stomatal penetration
Direct penetration
T0372
T0491
T0372
T0491
2
6Æ1a
8Æ5ab
9Æ5ab
10Æ5ab
4
9Æ6ab
13Æ6abc
13Æ7abc
11Æ8ab
6
12Æ1ab
13Æ2abc
16Æ7bcd
17Æ7bcd
8
22Æ7cdef
25Æ4def
31Æ6ef
29Æ0ef
10
22Æ3cde
24Æ9def
32Æ4f
31Æ1ef
Means tagged with the same letter are not significantly different
using the Tukey test (P = 0Æ05).
Xylanase and septoria tritici blotch
665
Plant Pathology (2010) 59, 661–670
after this time it is considered that ungerminated spores
will be unable to germinate (Cohen & Eyal, 1993; Shetty
et al., 2003). The strong branching of infectious germ
tubes at the leaf surfaces probably increases the like-
lihood of penetration.
The mode of penetration by M. graminicola has been
controversial. It has been reported that leaf penetration
occurs either through the leaf cuticle (Weber, 1922),
through stomata (Kema et al., 1996; Duncan & Howard,
2000; Mehrabi et al., 2006b) or via both stomata and epi-
dermis (Cohen & Eyal, 1993; Dancer et al., 1999; Rohel
et al., 2001). The results obtained here confirm these lat-
ter observations and show that M. graminicola penetrates
its host through both stomatal and direct routes. The effi-
ciency of direct penetrations was confirmed for both iso-
lates by the observation of continuous growth of
T0372
T0491
22 d.p.i.
22 d.p.i.
22 d.p.i.
22 d.p.i.
22 d.p.i.
(a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
(i)
(j)
Figure 2 Reduced mesophyll colonization and sporulation by the weakly pathogenic Mycosphaerella graminicola isolate T0372 (a, c, e, g and i)
in comparison with the pathogenic isolate T0491 (b, d, f, h and j) on susceptible wheat cv. Scorpion at 22 days post-inoculation. The fungus was
stained by heating cleared leaf segments in Trypan blue at 70
C for 2 h. Note: absence of hyphal growth in the mesophyll for T0372 (a and c) vs.
longitudinal intercellular growth of infection hyphae of T0491 (arrows) growing around mesophyll cells (b) and transverse growth of infection hyphae
(black arrows) occurring in close contact with mesophyll cells (d, arrowhead); limited colonization of substomatal cavity for T0372 (e) vs. abundant
hyphal growth within substomatal cavity for T0491 (f); absence of pycnidia in stomatal cavities for T0372 (g) vs. formation of pycnidia (p) bearing
pycniospores (arrow) for T0491 (h); limited chlorotic spots for T0372 (i) vs. necrotic lesions bearing numerous pycnidia for T0491 (j). Black scale
bar = 10 lm. White scale bar = 1 mm.
666
A. Siah et al.
Plant Pathology (2010) 59, 661–670
penetrating germ tubes from the leaf surface to the meso-
phyll (data not shown). As described here, appressorium-
like structures occur at the tip of penetrating germ tubes,
in accordance with several previous reports (Cohen &
Eyal, 1993; Dancer et al., 1999; Rohel et al., 2001).
In contrast to what was observed at the leaf surface
level, substantial differences were obtained regarding the
hyphal growth patterns of the two isolates inside the host
tissues. Only the pathogenic isolate T0491 was able to
fully colonize the mesophyll and to differentiate pycnidia
within substomatal cavities. The colonization of the
mesophyll is a crucial step for the disease establishment: it
allows further expansion of the fungus via the apoplast
and colonization of neighbouring substomatal cavities
(Cohen & Eyal, 1993; Duncan & Howard, 2000).
The transition from biotrophy to necrotrophy in the
pathogenic isolate T0491 was associated with the secre-
tion of xylanase activity, which was then highly corre-
lated with disease severity. The investigation of 24 other
isolates confirmed this finding and showed that only iso-
lates which induce the disease produce xylanase activity,
strongly suggesting the involvement of xylanase activity
in pathogenesis of M. graminicola, especially in the cell
wall degradation and host tissue maceration occurring
during the necrotrophic phase (Kema et al., 1996, 2008).
A screening of filtered transcript models released from the
whole M. graminicola genome (versions v1.0 and v2.0)
revealed four putative xylanase genes (MgXYL1,
MgXYN1, MgXYN2 and BXL) and one gene expected
to be a xylanase transcriptional regulator (MgXLR1)
(http://genome.jgi-psf.org/Mycgr3/Mycgr3.home.html).
Two of these genes (MgXYL1 and MgXYN2) were
recently shown, by using an in planta EST approach, to
be the most expressed CWDE sequences during the necro-
trophic phase of M. graminicola (Kema et al., 2008),
coinciding with the results obtained here for xylanase
activity. The production of CWDE by M. graminicola in
association with pathogenicity was first suggested by the
observation of disorganized cell walls during later stages
of pathogenesis without the presence of mycelium in the
vicinity (Kema et al., 1996). Furthermore, Douaiher et al.
(2007b) highlighted a range of CWDE produced in vitro
by M. graminicola, among which xylanase activity was
the enzyme most correlated with necrosis development in
a detached-leaf pathogenicity assay.
The in planta xylanase activity highlighted here for
M. graminicola is in accordance with previous studies
involving other fungi infecting cereals. For instance, it
has been shown that xylanase is the most-produced
CWDE by P. nodorum when growing in pure culture with
host cell walls as carbon source (Cooper et al., 1988;
Lalaoui et al., 2000) or when invading plant tissues
(Magro, 1984; Carlile et al., 2000). In addition to
xylanase, P. nodorum produces other CWDE, such as
proteases, during infection (Bindschedler et al., 2003).
Evidence supporting a role for xylanase in pathogenicity
has been reported for several other plant pathogenic
fungi. Giesbert et al. (1998) demonstrated using tissue
printing immunostaining analysis that xylanase was
exclusively present in rye ovaries infected with Claviceps
a
a
a
a
ab
ab
a
a
a
a
a
a
a
a
b
c
d
e
0
100
200
300
400
500
600
2
4
6
8
10
12
14
16
18
20
22
In planta
xylanase activity
(mU
µ
g
–1
total proteins)
Days post-inoculation
Figure 4 Time course of in planta xylanase activity in wheat cv. Scorpion control leaves (e) and leaves inoculated with Mycosphaerella
graminicola isolates T0372 (s) and T0491 (D). Means tagged with the same letter are not significantly different using the Tukey test at
P = 0Æ05.
0
20
40
60
80
100
Control
T0372
T0491
Coloniz
ed substomatal ca
vities (%)
Isolate
Figure 3 Frequencies of colonized substomatal cavities
and
colonized substomatal cavities with pycnidia
on wheat cv.
Scorpion leaf segments for controls and plants inoculated with
Mycosphaerella graminicola isolates T0372 and T0491 at 22 days
post-inoculation.
Xylanase and septoria tritici blotch
667
Plant Pathology (2010) 59, 661–670
purpurea. Furthermore, deletion of a xylanase-encoding
gene in Botrytis cinerea significantly reduced ‘‘virulence’’
on grape plants and tomato leaves, delaying the appear-
ance of secondary lesions and reducing the lesion size by
more than 70% (Brito et al., 2006). In addition to their
role in the host tissue degradation, certain fungal xylanas-
es can also act as elicitors of plant defences (Belie¨n et al.,
2006). A xylanase from Trichoderma viride has been
referred as ethylene-inducing and has been used most
extensively to study elicitor activity of xylanases. When
applied to pepper and tomato plants (Avni et al., 1994), it
induces ethylene biosynthesis and the production of both
phytoalexins and pathogenesis-related proteins, and
causes symptoms associated with the hypersensitive
response (HR). In the case of the present study, the late
production of xylanase activity by M. graminicola
strongly suggests that this enzyme could be not consid-
ered as an elicitor involved in host-defence triggering.
Xylanase activity in M. graminicola is likely to act as a
pathogenicity factor involved in host tissue degradation,
secreted by the fungus in response to the xylan-rich wheat
cell wall. However, the outcome of the interaction
between wheat and M. graminicola is likely to be also
affected by other factors since virulence is multifaceted.
T0251
T0384
IPO94269
T0485
T0242
T0262
T0466
T0403
T0414
Control
T0365
T0345
T0451
IPO323
T0278
T021
T0290
T032
T083
T0293
T055
T0254
T0148
T0473
T049
(r = 0·94)
0
100
200
300
400
500
0
20
40
60
80
100
In planta
xylanase activity
Leaf area with lesions bearing pycnidia (%)
Figure 6 Correlation between in planta xylanase activity (mU lg
)1
total proteins) and disease intensity (percentage leaf area with lesions
bearing pycnidia) for 24 Mycosphaerella graminicola isolates at 20 days post-inoculation onto wheat cv. Scorpion plants.
a
a
a
a
a
a
a
a
a
a
a
b
c
d
e
0
20
40
60
80
100
2
4
6
8
10
12
14
16
18
20
22
Leaf area with lesions
bear
ing p
ycnidia (%)
Days post-inoculation
Figure 5 Time course of wheat cv. Scorpion percentage leaf area with lesions bearing pycnidia on control leaves (e) and on leaves
inoculated Mycosphaerella graminicola isolates T0372 (s) and T0491 (D). Means tagged with the same letter are not significantly different
using the Tukey test at P = 0Æ05.
668
A. Siah et al.
Plant Pathology (2010) 59, 661–670

The absence of xylanase in the early stages suggests it is
not likely to function in initial infection and an ABC
transporter is involved in the invasive growth of M. gra-
minicola by decreasing its sensitivity to plant-derived
compounds (Mehrabi et al., 2006a).
Xylanase genes have been cloned in wide range of plant
pathogenic fungi, such as Cochliobolus carbonum (Apel
et al., 1993; Apel-Birkhold & Walton, 1996) and Botrytis
cinerea (Brito et al., 2006). Cloning of the putative
xylanase genes involved here should pave the way for
complementary approaches such as gene disruption tech-
niques to determine specific roles of xylanases during
pathogenesis by M. graminicola. However, targeted dis-
ruption of individual xylanase genes from C. carbonum
(Apel et al., 1993; Apel-Birkhold & Walton, 1996) did
not dramatically affect pathogenicity. Functional redun-
dancy or the occurrence of multiple xylanase genes has
been put forward as one of the main reasons why
xylanase gene-disruption mutants remain pathogenic
(Apel-Birkhold & Walton, 1996). An alternative
approach to determine the role of multiple xylanase genes
in pathogenesis is the disruption of transcriptional regu-
lators or conserved signal transduction components,
since these proteins normally affect the whole set of
xylanase genes (Belie¨n et al., 2006). In this way, a targeted
disruption of the putative xylanase transcriptional
regulator MgXLR1 could be of particular value to clarify
the accurate roles of xylanases in the pathogenicity of M.
graminicola.
Acknowledgements
We thank Dr B. Tisserant, Dr L. Decourcelle-El Charto-
uni and Dr B. Randoux for their valuable suggestions dur-
ing this study. Part of this research was supported by the
Groupement National Interprofessionnel des Semences
(GNIS), France.
References
Apel PC, Pannacione DG, Holden FR, Walton JD, 1993. Cloning
and targeted disruption of XYL1, a beta-1,4-xylanase gene from
the maize pathogen Cochliobolus carbonum. Molecular Plant-
Microbe Interactions 6, 467–73.
Apel-Birkhold PC, Walton JD, 1996. Cloning, disruption, and
expression of two endo-beta 1, 4-xylanase genes, XYL2 and
XYL3, from Cochliobolus carbonum. Applied and
Environmental Microbiology 62, 4129–35.
Avni A, Avidan N, Eshed Y et al., 1994. The response of
Lycopersicon esculentum to a fungal xylanase is controlled by a
single dominant gene. Plant Physiology 105, S-158.
Belie¨n T, Van Campenhout S, Robben J, Volckaert G, 2006.
Microbial endoxylanases: effective weapons to breach the plant
cell wall barrier or, rather, triggers of plant defense systems?
Molecular Plant-Microbe Interactions 19, 1072–81.
Bindschedler LV, Sanchez P, Dunn S et al., 2003. Deletion of the
SNP1 trypsin protease from Stagonospora nodorum reveals
another major protease expressed during infection. Fungal
Genetics and Biology 38, 43–53.
Bradford MM, 1976. A rapid and sensitive method for
quantitation of microgram quantities of protein utilizing the
principle of protein-dye-binding. Analytical Biochemistry 72,
248–54.
Brito N, Espino JJ, Gonzalez C, 2006. The endo-beta-1,4-xylanase
xyn11A is required for virulence in Botrytis cinerea. Molecular
Plant-Microbe Interactions 19, 25–32.
Carlile AJ, Bindschedler LV, Bailey AM, Bowyer P, Clarkson JM,
Cooper RM, 2000. Characterization of SNP1, a cell wall-
degrading trypsin, produced during infection by
Stagonospora nodorum. Molecular Plant-Microbe
Interactions 13, 538–50.
Cohen L, Eyal Z, 1993. The histology of processes associated with
the infection of resistance and susceptible wheat cultivars with
Septoria tritici. Plant Pathology 42, 737–43.
Collins T, Gerday C, Feller G, 2005. Xylanases, xylanase families
and extremophilic xylanases. Microbiological Reviews 29,
3–23.
Cooper RM, Longman D, Campbell A, Henry M, Lees PE, 1988.
Enzymic adaptation of cereal pathogens to the
monocotyledonous primary wall. Physiological and Molecular
Plant Pathology 32, 33–47.
Dancer J, Daniels A, Cooley N, Foster S, 1999. Septoria tritici and
Stagonospora nodorum as model pathogens for fungicide
discovery. In: Lucas JA, Bowyer P, Anderson HM, eds. Septoria
on Cereals: A Study of Pathosystems. Wallingford, UK: CAB
International, 316–31.
Douaiher MN, Nowak E, Dumortier V, Durand R, Reignault P,
Halama P, 2007a. Mycosphaerella graminicola produces a range
of cell wall-degrading enzyme activities in vitro that vary with
the carbon source. European Journal of Plant Pathology 117,
71–9.
Douaiher MN, Nowak E, Durand R, Halama P, Reignault Ph,
2007b. Correlative analysis of Mycosphaerella graminicola
pathogenicity and cell wall degrading enzymes produced in vitro:
the importance of xylanases and polygalacturonases. Plant
Pathology 56, 79–86.
Duncan KE, Howard RJ, 2000. Cytological analysis of wheat
infection by the leaf blotch pathogen Mycosphaerella
graminicola. Mycological Research 104, 1074–82.
Eyal Z, 1999. The septoria tritici and stagonospora nodorum
blotch diseases of wheat. European Journal of Plant Pathology
105, 629–41.
Giesbert S, Lepping HB, Tenberge KB, Tudzynski P, 1998. The
xylanolytic system of Claviceps purpurea: cytological evidence
for secretion of xylanases in infected rye tissue and molecular
characterization of two xylanase genes. Phytopathology 88,
1020–30.
Kema GHJ, Yu D, Rijkenberg FHJ, Shaw MW, Baayen R,
1996. Histology of the pathogenesis of Mycosphaerella
graminicola in wheat. Biochemistry and Cell Biology 86,
777–86.
Kema GHJ, Verstappen ECP, Waalwijk C, 2000. Avirulence in the
wheat septoria tritici leaf blotch fungus Mycosphaerella
graminicola is controlled by a single locus. Molecular Plant-
Microbe Interactions 13, 1375–9.
Kema GHJ, Van Der Lee TAJ, Mendes O et al., 2008. Large-scale
gene discovery in the septoria tritici blotch fungus
Mycosphaerella graminicola with a focus on in planta
expression. Molecular Plant-Microbe Interactions 21,
1249–60.
Xylanase and septoria tritici blotch
669
Plant Pathology (2010) 59, 661–670

Komiya Y, Suzuki S, Kunoh H, 2003. Release of xylanase from
conidia and germlings of Blumeria graminis f. sp. tritici and
expression of a xylanase gene. Journal of General Plant
Pathology 69, 109–14.
Labavitch TM, Ray PM, 1978. Structure of hemicellulosic
polysaccharides of Avena sativa coleoptiles cell walls.
Phytochemistry 17, 933–7.
Lalaoui F, Halama P, Dumortier V, Paul B, 2000. Cell wall
degrading enzymes produced in vitro by isolates of
Phaeosphaeria nodorum differing in aggressiveness. Plant
Pathology 49, 727–33.
Lovell D, Hunter T, Powers S, Parker S, Van den Bosch F, 2004. Effect
of temperature on latent period of septoria leaf blotch on winter
wheat under outdoor conditions. Plant Pathology 53, 170–81.
Magro P, 1984. Production of polysaccharide-degrading enzymes
by Septoria nodorum in culture and during pathogenesis. Plant
Science Letters 37, 63–8.
Mehrabi R, van der Lee T, Waalwijk C, Kema GHJ, 2006a.
MgSlt2, a cellular integrity MAP kinase gene of the fungal wheat
pathogen Mycosphaerella graminicola, is dispensable for
penetration but essential for invasive growth. Molecular Plant-
Microbe Interactions 19, 389–98.
Mehrabi R, Zwiers LH, De Waard M, Kema GHJ, 2006b.
MgHog1 regulates dimorphism and pathogenicity in the fungal
wheat pathogen Mycosphaerella graminicola. Molecular Plant-
Microbe Interactions 19, 1262–9.
Miller GL, 1959. Use of the dinitrosalicylic acid reagent for
determination of reducing sugar. Analytical Chemistry 31,
426–8.
Palmer CL, Skinner W, 2002. Mycosphaerella graminicola: latent
infection, crop devastation and genomics. Molecular Plant
Pathology 3, 63–70.
Rohel AE, Payne AC, Fraaije BA, Hollomon DW, 2001. Exploring
infection of wheat and carbohydrate metabolism in
Mycosphaerella graminicola transformants with differentially
regulated green fluorescent protein expression. Molecular Plant-
Microbe Interactions 14, 1–7.
Shetty NP, Kristensen BK, Newman MA, Moller K, Gregersen PL,
Jorgensen HJL, 2003. Association of hydrogen peroxide with
restriction of Septoria tritici in resistant wheat. Physiological
and Molecular Plant Pathology 62, 333–46.
Shetty NP, Mehrabi R, Lu¨tken H et al., 2007. Role of hydrogen
peroxide during the interaction between the hemibiotrophic
fungal pathogen Septoria tritici and wheat. New Phytologist
174, 637–47.
Solomon PS, Wilson TJG, Rybak K, Parker K, Lowe RGT, Oliver
RP, 2006. Structural characterisation of the interaction between
Triticum aestivum and the dothideomycete pathogen
Stagonospora nodorum. European Journal of Plant Pathology
114, 275–82.
Weber GF, 1922. Septoria diseases of wheat. Phytopathology 12,
537–85.
670
A. Siah et al.
Plant Pathology (2010) 59, 661–670

This document is a scanned copy of a printed document. No warranty is given about the accuracy of the copy.
Users should refer to the original published version of the material.
Wyszukiwarka
Podobne podstrony:
Production of xylooligosaccharides using immobilized endo xylanase of Bacillus
A typical endo xylanase from Streptomyces rameus
A typical endo xylanase from Streptomyces rameus
7 materiały endo
Agoni Ťci receptor w alfa i beta adrenergicznych
DaF Activity
malarstwo niderlandzkie XV w beta
Cómo se dice Sugerencias y soluciones a las actividades del manual de A2
Cw 2 Gin endo endokryn
Kwasy beta, Szkoła PSWIS, Kosmetologia, Semestr I, Peelingi, PEELINGI
41 Rządy Plantagenetów
student sheet activity 1 e28093 eating apples
Intelligence Support Activity
więcej podobnych podstron