
A typical endo-xylanase from Streptomyces rameus L2001 and its unique
characteristics in xylooligosaccharide production
Xiuting Li
, E. Li
, Yunping Zhu
, Chao Teng
, Baoguo Sun
, Huanlu Song
, Ran Yang
a
Department of Food Science, School of Food and Chemical Engineering, Beijing Technology and Business University, No. 33 Fucheng Road, Beijing 100048, PR China
b
Beijing Higher Institution Engineering Research Center of Food Additives and Ingredients, No. 33 Fucheng Road, Beijing 100048, PR China
c
Beijing Key Laboratory of Flavor Chemistry, No. 33, Fucheng Road, Beijing 100048, PR China
a r t i c l e
i n f o
Article history:
Received 12 March 2012
Received in revised form 2 May 2012
Accepted 3 May 2012
Available online 11 May 2012
Keywords:
Streptomyces rameus
endo-Xylanase
Agro-industrial residues
Enzymatic hydrolysis
Xylooligosaccharides
a b s t r a c t
The activity of the extracellular xylanase produced by Streptomyces rameus L2001 against different xylans
and xylooligosaccharides (XOS) was investigated. The main products of hydrolysis of birchwood xylan and
oat-spelt xylan by the S. rameus L2001 xylanase were xylobiose (X2) and xylotriose (X3), suggesting that
this is an endo-acting xylanase. This was confirmed by analysis of XOS degradation products. The enzyme
hardly hydrolyzed X2 and X3, but hydrolyzed xylotetraose (X4) and xylopentaose (X5) producing mainly
X2 and X3 through transglycosylation. Depending on the substrate, different quantities of reducing sugars
were produced by the xylanase: 150 mg/g from corncob, 105 mg/g from bean culms, and 133 mg/g from
bagasse. With the bagasse substrate, the xylanase yielded 2.36, 2.76, 2.03, and 2.17 mg/mL of X2, X3, X4,
and X5, respectively. The structure of xylobiose and xylotriose from the hydrolysis of corncob xylan was
identified by MS and NMR. The production of XOS from various agricultural wastes has potential industrial
applications. This is the first report of XOS production by S. rameus L2001.
Ó 2012 Elsevier Ltd. All rights reserved.
1. Introduction
There is increasing interest in the use of xylooligosaccharides
(XOS) as ingredients in functional foods, because prebiotic oligosac-
charides (OS) have various beneficial health effects. These include
their selective metabolism by Bifidobacteria, their ability to increase
production of volatile fatty acids, and their ability to diminish ulcer
lesions in the stomach. They are associated with a decreased risk of
colon cancer, and they are stable over a wide range of pH and
temperatures.
XOS have stimulatory effects on the selective
growth of human intestinal Bifidobacteria, which lower blood sugar
levels and blood pressure.
XOS can be produced by xylanases
(1,4-b-
D
-xylan xylanohydrolase, EC3.2.1.8) that hydrolyze lignocel-
lulosic materials containing substituted xylan as the major
hemicellulose component.
Xylanis a polysaccharide with a back-
bone consisting of b-1,4-linked xylopyranose with branches of
other residues such as arabinofuranosyl, acetyl, and glucuronosyl.
Complete hydrolysis of xylan requires the orchestrated actions of
various
enzymes
including
endo-xylanase,
b
-
D
-xylosidase,
a
-glucuronidase, acetyl esterase, and
a
-
L
-arabinofuranosidase;
endo-xylanases are the crucial enzyme components of microbial
xylanolytic systems.
Enzymes with high endo-xylanase but low
exo-xylanase and/or b-xylosidase activity are desirable for
enzymatic XOS production.
In recent years, xylanases have at-
tracted considerable research interest because of their potential
industrial applications. Fungal xylanases from Aspergillus sp. and
Trichoderma sp., and bacterial xylanases from Bacillus sp.,
Streptomyces sp., and Clostridium sp. have been intensively studied.
However, for economical production of XOS, it is important to find
highly efficient endo-xylanases. Nowadays, many researchers
working on xylanase spend considerable time and energy obtaining
XOS. The increasing number of industrial applications encourages a
search for renewable and cheap xylan sources for the preparation of
XOS. Agricultural wastes of a lignocellulosic nature are widely
available in China, and contain 15–25% xylan.
Some widely distrib-
uted and abundant agricultural wastes are corncobs, cottonseed
hulls, oat bran, bean culms, and bagasse. These could potentially
be appropriate substrates for the production of XOS. In addition,
since they are usually left to rot or are burned in the field after
harvesting, utilization of these materials for industrial purposes
provides a suitable disposal method, and some additional income
and employment for farmers.
In our previous paper, we described
the selection of Streptomyces rameus L2001 as a strain that pro-
duced high levels of xylan-degrading enzymes. The endo-xylanase
was purified from the strain and comparatively characterized.
However, its enzymatic activity against XOS and various xylans
had not been studied in detail. The S. rameus L2001 xylanase dis-
cussed here is a typical endo-xylanase. This is the first report of
its ability to produce XOS. Furthermore, this enzyme was able to
0008-6215/$ - see front matter Ó 2012 Elsevier Ltd. All rights reserved.
http://dx.doi.org/10.1016/j.carres.2012.05.005
⇑
Corresponding author. Tel.: +86 10 68985378; fax: +86 10 68985456.
E-mail addresses:
(X. Li).
Carbohydrate Research 359 (2012) 30–36
Contents lists available at
Carbohydrate Research
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / c a r r e s

hydrolyze several kinds of agricultural wastes. Therefore, we eval-
uated the potential use of this endo-xylanase to produce XOS via
hydrolysis of xylan from agricultural wastes that are cheap and
abundant biomass resources in China.
2. Material and methods
2.1. Production of xylanase by S. rameus L2001 and enzyme
assay
Xylanase was produced by S. rameus L2001 under submerged
fermentation conditions using a corncob xylan preparation (2.5%,
w/v) as the inducer. The culture was incubated at 140 rpm for
7 days at 40 °C. The crude enzyme solution was recovered by cen-
trifuging the culture broth at 8000g for 10 min at 4 °C. The super-
natant was stored at 4 °C for further purification. The pure xylanase
used for enzymatic hydrolysis was produced as described previ-
ously.
The dialyzed culture supernatant was loaded on a DEAE-
52 column (1.0 10 cm) using a loading buffer (20 mM Tris–HCl,
pH 7.0) at a flow rate of 1.0 mL/min. The xylanase eluted from
the DEAE-52 column was further purified on a CM Sepharose Fast
Flow column (1.0 10 cm). The bound proteins were eluted with a
0–0.05 M NaCl gradient at a flow rate of 1.0 mL/min. The homoge-
neity of the active xylanase fraction was checked by SDS–poly-
acrylamide gel electrophoresis. The xylanase activity was assayed
using birch xylan as the substrate, as described previously.
2.2. Main reagents
Xylose, xylobiose, xylotriose, xylotetraose, and xylopentaose
(X1, X2, X3, X4, and X5, respectively) were purchased from Mega-
zyme (Megazyme International Ireland Ltd, Ireland). Birchwood
xylan, oat-spelt xylan were purchased from Sigma (Sigma–Aldrich
Co. Ltd, Germany). Xylans were obtained from the alkali extraction
of corncob. Water-soluble and water-insoluble xylans from corn-
cobs, and whole soluble xylans from corncobs, cottonseed hulls,
oat bran, bean culms, and bagasse, were obtained from the alkali
extraction of agricultural wastes as described elsewhere,
with
minor modifications. Locally obtained corncob, cottonseed hulls,
oat bran, bean culms, and bagasse were chopped and milled into
fine powders. The powders were treated with 10% NaOH with a so-
lid–liquid ratio of 1:15 (w/v) at 100 °C for 60 min. The resultant
soluble fraction was recovered by filtration and adjusted to pH
7.0 with dilute hydrochloric acid. Then, three volumes of 95% eth-
anol were added and the resultant mixture was incubated for
60 min at room temperature. Xylans were obtained from the
recovered precipitate. All other chemicals were of analytical grade
and were obtained locally.
2.3. Enzymatic hydrolysis of xylans and xylooligosaccharides as
substrates
For enzymatic hydrolysis of xylan, the reaction mixture con-
sisted of 2% of each xylan in 50 mM of acetic acid buffer with
4 U/mL of the enzyme. The mixture was incubated at 50 °C for
12 h. For enzymatic hydrolysis of different XOS, 2% of each xylool-
igosaccharide was incubated at 50 °C for 24 h, with 2 U/mL of
xylanase.
At selected time intervals, samples were analyzed for hydro-
lyzed products using thin layer chromatography (TLC) and silica
gel plates. The plates were developed with butanol, acetic acid,
and water (2:1:1), followed by heating for few minutes at 105 °C;
the plates were then sprayed with a methanol and sulfuric acid
mixture (20:1). A XOS mixture consisting of X1, X2, X3, X4, and
X5 was used as the standard.
2.4. Enzymatic hydrolysis of other xylans from different kinds
of agricultural waste
For enzymatic hydrolysis of xylans from different kinds of agri-
cultural waste, the reaction mixture consisted of 2% of each xylan
in 50 mM acetic acid buffer with 20 U/mL of the enzyme. The mix-
ture was incubated at 50 °C for 2 h. Reducing sugars were quanti-
fied using the 3,5-dinitrosalicylic acid(DNS) method,
with xylose
as a standard. XOS were separated by chromatography using a
Waters 1525 HPLC system equipped with a refractive index detec-
tor (Waters 2707) and a column oven (Waters 1500). The hydro-
lyzed products were separated by HPLC on a Shodex KS-800
column (8 300 mm, 6
l
m) after hydrolysis. The column oven
was set at 60 °C, the temperature of the refractive index detector
was 40 °C, the flow rate was 0.6 mL/min, and the injection volume
was 10
l
L.
2.5. Purification of xylobiose and xylotriose
The enzymatic hydrolysis products of corncob xylan were puri-
fied by column chromatography. After the hydrolysis reaction, the
reaction mixture was separated by column chromatography on a
silica gel column with butanol, acetic acid, and water (2:1:1) as
eluent. The xylobiose and xylotriose were collected and evaporated
in vacuum and lyophilized for further analysis.
2.6. Identification of the structure of xylobiose and xylotriose
2.6.1. Positive mass spectrometry (MS)
ESI-MS was performed (LTQ XL ion trap MS; Thermo Scientific,
San Jose, CA) on lyophilized xylobiose and xylotriose. Samples
were dissolved in milliQ water (100
l
g/mL) and applied to a Ther-
mo
Accela
UHPLC
system
equipped
with
a
Hypercarb
(100 2.1 mm, 3 lm) column (Thermo Scientific). Elution was per-
formed with a gradient of deionized water/acetonitrile and 0.2%
trifluoroacetic acid (0.4 mL/min; 70 °C). MS detection was per-
formed in the positive mode using a spray voltage of 4.5 kV and
a capillary temperature of 260 °C and auto-tuned on glucohexaose.
2.6.2. The nuclear magnetic resonance (NMR) spectrometry
The structures of xylobiose and xylotriose were also analyzed
by the proton nuclear magnetic resonance (
1
H NMR). Spectra were
recorded at 22 °C with a Bruker Advance DPX300 MHz spectrome-
ter operating at 300.13 MHz for
1
H and are referenced to the sol-
vent peak (D
2
O, 4.7 ppm).
3. Results and discussion
3.1. Enzymatic hydrolysis of xylans
To test whether the xylanase was an endo-xylanase, TLC was
used to analyze the products of hydrolysis of birchwood xylan,
oat-spelt xylan, and water-soluble and water-insoluble xylans.
Analysis of the degradation products of birchwood xylan (
a)
showed that X2 and X3 were the main end products. Xylose was
not detected as an end product of the enzymatic hydrolysis reac-
tion. As the reaction time increased, both X4 and X5 were readily
degraded. Production of X3 was greater than that of X2. This pat-
tern of hydrolysis differs from that of xylanases from other bacte-
ria. For example, the xylanase of Thermotoga maritima exclusively
liberated X2 from birchwood xylan.
X2 and trace amounts of xy-
lose were liberated from birchwood xylan by the purified xylanase
from strains SSBP after 24 h of incubation.
The enzyme from
Talaromyces thermophilus released mainly X2; the major product
of xylan degradation.
X. Li et al. / Carbohydrate Research 359 (2012) 30–36
31
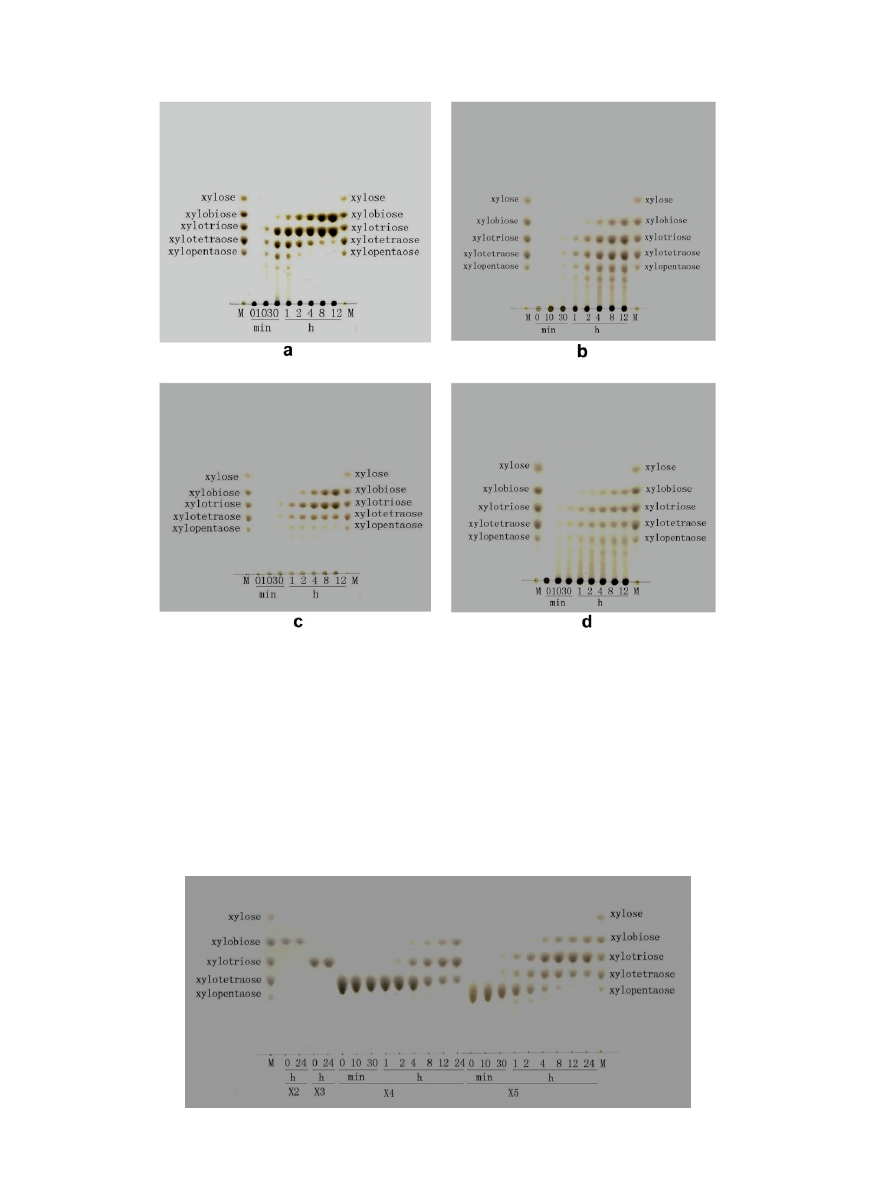
The degradation products of oat-spelt xylan (
b) were a
variety of XOS, including X2, X3, X4, and X5. Within 12 h, there
were increased concentrations of all XOS. With longer hydrolysis
time, the levels of X4 and X5 decreased gradually. Hydrolysis of
X4 and X5 slowed after 12 h of incubation, indicating that endo-
xylanase activity decreased because of inhibition by the end
products.
Analysis of different water-soluble xylans (
c and d) as sub-
strates for enzymatic hydrolysis showed that product composition
and production trends showed few differences; the main
difference was in the quantity of hydrolysis. When the same sub-
strate concentration, enzyme concentration, and time were used,
greater quantities of XOS were produced with water-soluble xylan
as the substrate than water-insoluble xylan, which indicated that
the water-soluble xylan was degraded more easily than the
water-insoluble xylan.
This indicated that the xylanase was a true endo-b-1,4-xylanase
that only cleaves internal b-1,4-xylosidic linkages on the backbone
of various xylosic compounds consisting of three or more b-
D
-xylopyranoside units.
Figure 2. Thin layer chromatography (TLC) analysis of hydrolyzed products of xylooligosaccharides by xylanase from Streptomyces rameus L2001.
Figure 1. Thin layer chromatography (TLC) analysis of hydrolyzed products of different xylans by xylanase from Streptomyces rameus L2001. (a) Birchwood xylan; (b) oat-
spelt xylan; (c) water-insoluble xylan form corncobs; (d) water-soluble xylan from corncobs.
32
X. Li et al. / Carbohydrate Research 359 (2012) 30–36

3.2. Enzymatic hydrolysis of XOS
The mode of action of the xylanase was determined using X2,
X3, X4, and X5 as substrates (
). The Streptomyces L2001 en-
zyme could not hydrolyze X2 or X3, but it could hydrolyze X4
and X5. During the early course of hydrolysis of X5, the main prod-
ucts formed were X2, X3, and X4. A small amount of XOS with
higher degree of polymerization (DP >5) was observed at 1 h
(
). As the incubation time increased, X4 and higher xylooligo-
saccharides were further hydrolyzed into X2 and X3. Although X4
was slowly hydrolyzed by the Streptomyces L2001 xylanase, it was
ultimately hydrolyzed to X2 and X3. The high production of X2 and
X3 in the X4 and X5 reaction mixtures confirmed that the Strepto-
myces L2001 xylanase was a typical endo-b-1,4-xylanase that only
cleaves internal b-1,4-xylosidic linkages on the backbone of
various xylosic compounds consisting of four or more b-
D
-xylopyranoside units.
Similar production of X2 and X3 has been obtained from xylan
hydrolysis with the endo-acting xylanase from Chaetomium sp.
CQ31.
An enzyme from Streptomyces matensis DW67 converted
X4 to X5.
It has been reported that xylose and X2 were the end
products obtained from hydrolysis of X3, X4, and X5, via a transgly-
cosylation mechanism. Kumar et al. found that xylose and X2 were
the main end products, and that the conversion of X3 to X2 might
have occurred through a glycosyl transfer reaction.
Previously, it
was reported that the L. sulphureus xylanase is an endo-xylanase
that hydrolyzes X3, X4, and X5, but not X2.
Li et al. found that
X2 and a small amount of xylose and X3 were the main products
when X3 and X4 were converted to X2.
3.3. Enzymatic hydrolysis of xylans from various agricultural
wastes
A sample of pretreated xylan (2%) was hydrolyzed by the xylan-
ase (20 U/mL) at pH 7.0, 50 °C for 2 h. The products of the hydroly-
sis (X1, X2, X3, X4, and X5) were analyzed by HPLC.
Different xylans from various agricultural residues hydrolyzed
by the xylanase from S. rameus L2001 gave different quantities of
reducing sugars: 150 mg/g from corncobs, 105 mg/g from bean
culms, and 133 mg/g from bagasse (
). These quantities are
similar to those produced from birchwood xylan by the
T. thermophilus xylanase.
shows the quantities of oligosaccharides produced from
each xylan. Akpinar et al. reported that the Aspergillus niger xylan-
ase can hydrolyze several kinds of agricultural wastes, but the
quantities of X2, X3, X4, and X5 obtained were lower than those
obtained in the present study.
As shown in
, X2, X3 or both
of them could be detected in the hydrolysis of all the agricultural
residues tested. The highest amount of X2 and X3 was 87.2% of
sugars, obtained from hydrolysis of the birchwood xylan bought
from Sigma Ltd. Among all the other agricultural residues, hydroly-
sis of water-insoluble xylan from corncob exhibited high amount
of X2 and X3 (76.7% of sugars). In addition, the amount of X2
and X3 in hydrolysis of whole soluble xylan from bagasse and
water-soluble xylan from corncob also accounted for 63.6% and
56.2%, respectively. To the best of our knowledge, this is the first
report of enzymatic hydrolysis of bagasse xylan by S. rameus xylan-
ase producing XOS.
Xylobiose and xylotriose have been found to have a stimulatory
effect on the selective growth of human intestinal Bifidobacteria,
Table 2
Hydrolysis products from different xylans produced by S. rameus L2001 xylanase
Substrates
Total sugar (peak area)
X2–5 (peak area)
X1/total sugars (%)
X2/total sugars (%)
X3/total sugars (%)
Birchwood xylan
8.9 10
5
8.8 10
5
0
40.2
47.0
Water-soluble xylan from corncob
3.4 10
5
2.6 10
5
0
24.3
31.9
Water-insoluble xylan from corncob
3.4 10
5
3.3 10
5
0
32.8
43.9
Whole soluble xylan from corncob
2.5 10
5
4.2 10
4
0
—
10.4
Whole soluble xylan from cottonseed hulls
4.3 10
5
4.0 10
4
0
—
6.3
Whole soluble xylan from oat bran
—
—
0
—
—
Whole soluble xylan from bean culm
1.0 10
5
1.0 10
5
0
—
48.4
Whole soluble xylan from bagasse
2.8 10
5
2.7 10
5
0
24.7
38.9
0
20
40
60
80
100
120
140
160
180
1
2
3
4
5
6
7
Reducing sugars (mg/g)
Different xylans of agricultural wastes
Figure 3. Reducing sugars from enzymatic hydrolysis of various agricultural
wastes. (1) Water-soluble xylan from corncobs; (2) water-insoluble xylan from
corncobs; (3) whole soluble xylan from corncobs; (4) whole soluble xylan from
cottonseed hulls; (5) whole soluble xylan from oat bran; (6) whole soluble xylan
from bean culms; (7) whole soluble xylan from bagasse.
Table 1
HPLC analysis of XOS produced by xylanase of S. rameus L2001 from various agricultural wastes
Different xylans from agricultural wastes
Oligosaccharides (mg/mL)
X2
X3
X4
X5
Water-soluble xylan from corncob
2.50
2.76
1.92
2.08
Water-insoluble xylan from corncob
2.79
3.17
2.02
1.97
Whole soluble xylan from corncob
—
1.92
1.83
—
Whole soluble xylan from cottonseed hulls
—
1.94
1.80
—
Whole soluble xylan from oat bran
—
—
—
—
Whole soluble xylan from bean culm
—
2.15
2.03
1.82
Whole soluble xylan from bagasse
2.36
2.76
2.03
2.17
X2: xylobiose; X3: xylotriose; X4: xylotetraose; X5: xylopentaose.
X. Li et al. / Carbohydrate Research 359 (2012) 30–36
33
which are important for the maintenance of a healthy intestinal
microflora. But, the production of analytical grade xylobiose and
xylotriose is a time consuming and expensive process. The current
results suggest that the enzyme from S. rameus L2001 is a highly
efficient endo-xylanase to convert agro-industrial residues to xylo-
biose and xylotriose. Actually, the materials tested as substrates in
the present study are cheap, renewable, and readily available
sources of sugars. Therefore, the production cost of XOS could be
hopefully reduced significantly if agricultural residues could be
used instead of xylan. The high yield of xylobiose and xylotriose
in the hydrolysis of agricultural residues by xylanase from S. ram-
eus indicates the application prosperity in functional food industry.
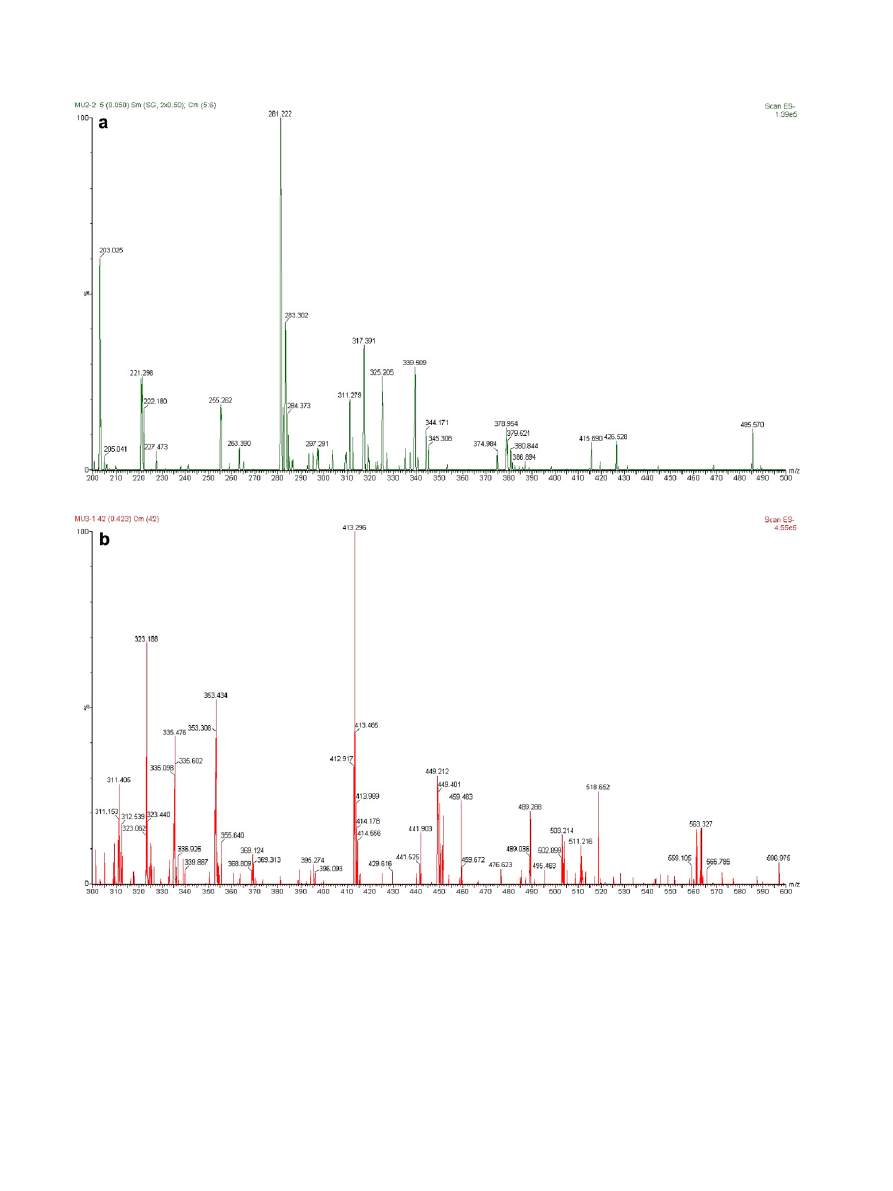
In order to confirm that the main products of the hydrolysis of
corncob xlyan were X2 and X3, the hydrolysis products from corn-
cob xylan were purified by preparative TLC and analyzed by MS
and
1
H NMR. In the full-scan MS spectrum, a main peak at m/z
283.3 (M
+
+1) was shown in
a and a main peak at m/z
413.3 (M
+
+1) was shown in
b. The chemical shifts of
1
H
NMR are shown in
. For both xylobiose and xylotriose,
the signals of H-2, H-3, and H-4 occurred at 3.25, 3.6, and 3.8,
respectively, which are accordant with the report of Haffman.
Apart from these signals, the spectra showed signals at 5.0 (H-1),
4.4 (H-2), 4.3 (H-4), and 3.4 (H-5) indicating both the X2 and X3
product were anomeric mixtures (5.05 ppm, alpha; 4.45 ppm,
Figure 4. Mass spectra of purified xylooligosaccharides: (a) xylobiose; (b) xylotriose.
34
X. Li et al. / Carbohydrate Research 359 (2012) 30–36
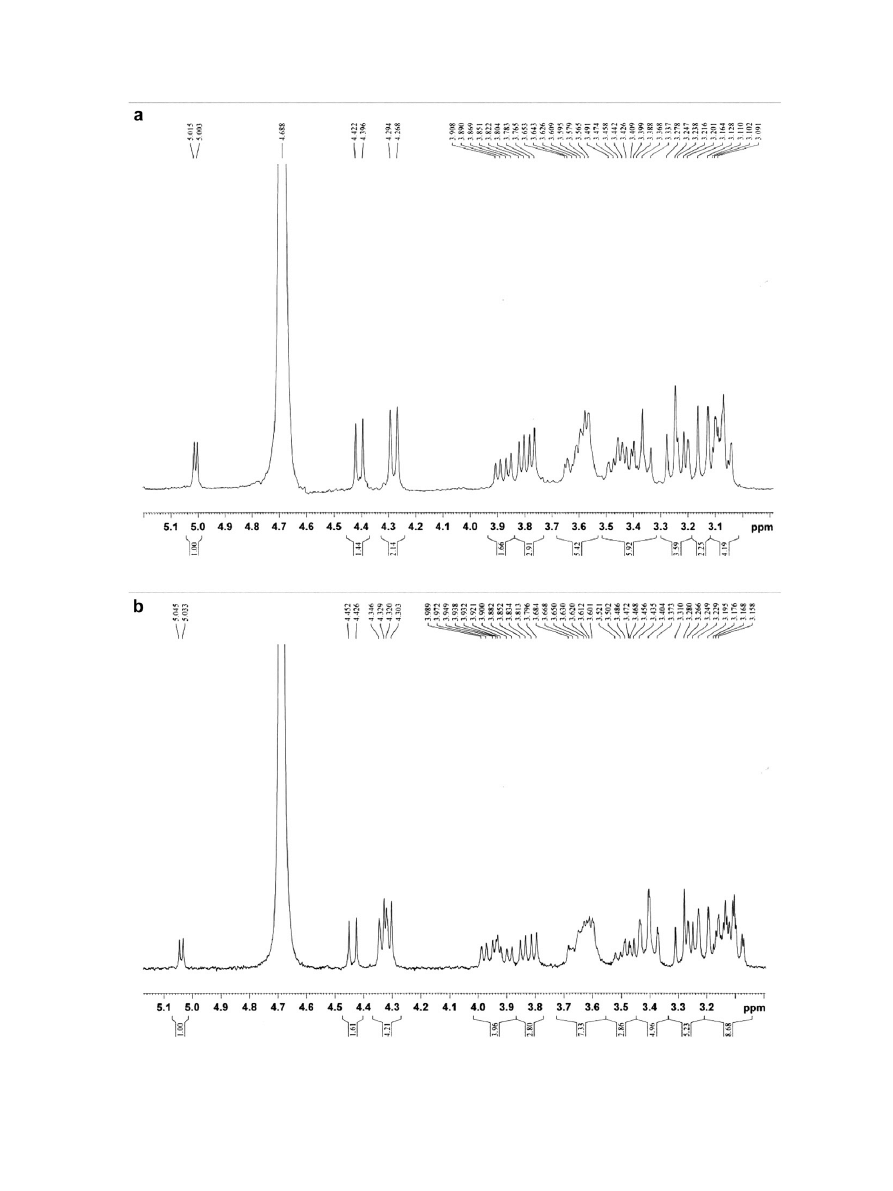
beta), which are in agreement with those reported by Drouet
et al.
and Li et al.
Several other xylanases including from Thermoascus auranti-
acu,
Trichoderma longibrachiatum,
Aspergillus oryzae MTCC
Figure 5.
1
H NMR spectra of purified xylooligosaccharides: (a) xylobiose; (b) xylotriose.
X. Li et al. / Carbohydrate Research 359 (2012) 30–36
35

Pichia stipitis,
and Geobacillus thermoleovorans
have
been previously demonstrated the capability of converting xylan
to xylose, and XOS with DPs of three or higher.
Meanwhile,
several few strains of Streptomyces sp. have been reported to pro-
duction of xylooligosaccharides. For an instant, hydrolysis of birch-
wood xylan by the xylanase from Streptomyces matensis DW67
yielded xylobiose and xylotriose as the principal products.
In
the hydrolysis of xylan by the xylanase from Streptomyces olivaceo-
viridis E-86, the xylobiose was the main products and the xylose,
xylotriose were the minor products.
In current study, xylanase
of S. rameus is the first report that hydrolyzed xylan producing
xylobiose and xylotriose.
4. Conclusions
The xylanase from S. rameus L2001 can convert X4 and X5 to X2
and X3 by transglycosation reaction. X2 and X3 are the principle
products in the hydrolysis of birchwood xylan, oat-spelt xylan,
and other xylans from agricultural residues by xylanase from S.
rameus L2001, the highest yield reached to 87.2%. We first report
how to obtain xylobiose and xylotriose using bagasse xylan as
the substrate of xylanase from S. rameus. The high yield of xylobi-
ose and xylotriose in the hydrolysis of agricultural residues by
xylanase from S. rameus indicates the application prosperity in
functional food industry. Further detailed studies on this enzyme
and its products are underway.
Acknowledgements
This research was financially supported by the Program for the
National Natural Science Foundation of China (No. 31071511), and
the Funding Project for Academic Human Resources Development
in Institutions of Higher Learning under the Jurisdiction of Beijing
Municipality (No. PHR20110872).
Supplementary data
Supplementary data associated with this article can be found, in
the online version, at
http://dx.doi.org/10.1016/j.carres.2012.05.
.
References
1. Hsu, C. K.; Liao, J. W.; Chung, Y. C.; Hsieh, C. P.; Chan, Y. C. J. Nutr. 2004, 134,
1523–1528.
2. Adsul, M. G.; Bastawde, K. B.; Gokhale, D. V. Bioresour. Technol. 2009, 100, 6488–
6495.
3. Vázquez, M. J.; Alonso, J. L.; Domínguez, H.; Parajó, J. C. Trends Food Sci. Technol.
2000, 11, 387–393.
4. Biely, P. Trends Biotechnol. 1985, 3, 286–290.
5. Beg, Q. K.; Kapoor, M.; Mahajan, L.; Hoondal, G. S. Appl. Microbiol. Biotechnol.
2001, 56, 326–338.
6. Subramaniyan, S.; Prema, P. Crit. Rev. Biotechnol. 2002, 22, 33–64.
7. Akpinar, O.; Erdogan, K.; Bostanci, S. Food Bioprod. Process. 2009, 87, 145–151.
8. Li, X. T.; She, Y. L.; Sun, B. G.; Song, H. L.; Zhu, Y. P.; Lv, Y. G.; Song, H. X. Biochem.
Eng. J. 2010, 52, 71–78.
9. Zilliox, C.; Debeire, P. Enzyme Microb. Technol. 1998, 22, 58–63.
10. Miller, G. L. Anal. Chem. 1959, 31, 426–428.
11. Jiang, Z. Q.; Deng, W.; Zhu, Y. P.; Li, L. T.; Sheng, Y. J.; Hayashi, K. J. Mol. Catal. B:
Enzym. 2004, 27, 207–213.
12. Lin, J.; Ndlovu, L. M.; Singh, S.; Pillay, B. Biotechnol. Appl. Biochem. 1999, 30, 73–
79.
13. Jiang, Z. Q.; Cong, Q. Q.; Yan, Q. J.; Kumar, N.; Du, X. D. Food Chem. 2010, 120,
457–462.
14. Yan, Q. J.; Hao, S. S.; Jiang, Z. Q.; Zhai, Q.; Chen, W. W. J. Mol. Catal. B: Enzym.
2009, 58, 72–77.
15. Kumar, K. S.; Manimaran, A.; Permaul, K.; Singh, S. J. Biosci. Bioeng. 2009, 107,
494–498.
16. Lee, J. W.; Park, J. Y.; Kwon, M.; Choi, I. G. J. Biosci. Bioeng. 2009, 107, 33–37.
17. Li, X. T.; Jiang, Z. Q.; Li, L. T.; Feng, W. Y.; Fan, J. Y.; Kusakabe, I. Bioresour.
Technol. 2005, 96, 1370–1379.
18. Maalej-Achouri, I.; Guerfali, M.; Gargouri, A.; Belghith, H. J. Mol. Catal. B: Enzym.
2009, 59, 145–152.
19. Haffmann, R. A.; Leeflang, B. R.; de Barse, M. M. J.; Kamerling, J. P.; Vliegenthart,
J. F. G. Carbonhydr. Res. 1991, 221, 63–81.
20. Drouet, P.; Zhang, M.; Legoy, M. D. Biotechnol. Bioeng. 1994, 43, 1075–1080.
21. Li, Y. K.; Yao, H. J.; Cho, Y. Biotechnol. Appl. Biochem. 2000, 31, 119–125.
22. Kalogeris, E.; Christakopoulos, P.; Vršanská, M.; Kekos, D.; Biely, P. J. Mol. Catal.
B: Enzym. 2001, 11, 491–501.
23. Kadi, N.; Belloy, L.; Chalier, P.; Crouzet, P. C. J. Agric. Food Chem. 2002, 50, 5552–
5557.
24. Aachary, A. A.; Prapulla, S. G. Bioresour. Technol. 2009, 100, 991–995.
25. Yang, H.; Wang, K.; Song, X.; Xu, F. Bioresour. Technol. 2011, 102, 7171–7176.
26. Verma, D.; Satyanarayana, T. Bioresour. Technol. 2012, 107, 333–338.
27. Cheng, H. L.; Wang, P. M.; Chen, Y. C.; Yang, S. S.; Chen, Y. C. Bioresour. Technol.
2008, 99, 227–231.
28. Ninawe, S.; Kapoor, M.; Kuhad, R. C. Bioresour. Technol. 2008, 99, 1252–1258.
29. Yan, Q. J.; Hao, S.; Jiang, Z. Q.; Zhai, Q.; Chen, W. W. J. Mol. Catal. B: Enzym. 2009,
58, 72–77.
30. Ding, C. H.; Jiang, Z. Q.; Li, X. T.; Li, L. T.; Kusakabe, I. World J. Microbiol.
Biotechnol. 2004, 20, 7–10.
36
X. Li et al. / Carbohydrate Research 359 (2012) 30–36
Document Outline
- A typical endo-xylanase from Streptomyces rameus L2001 and its unique characteristics in xylooligosaccharide production
- 1 Introduction
- 2 Material and methods
- 2.1 Production of xylanase by S. rameus L2001 and enzyme assay
- 2.2 Main reagents
- 2.3 Enzymatic hydrolysis of xylans and xylooligosaccharides as substrates
- 2.4 Enzymatic hydrolysis of other xylans from different kinds of agricultural waste
- 2.5 Purification of xylobiose and xylotriose
- 2.6 Identification of the structure of xylobiose and xylotriose
- 3 Results and discussion
- 4 Conclusions
- Acknowledgements
- Supplementary data
- References
Wyszukiwarka
Podobne podstrony:
Production of xylooligosaccharides using immobilized endo xylanase of Bacillus
planta endo beta 1,4 xylanase activity
7 materiały endo
An%20Analysis%20of%20the%20Data%20Obtained%20from%20Ventilat
Biomass Fired Superheater for more Efficient Electr Generation From WasteIncinerationPlants025bm 422
Bleaching Water Stains from Furniture
O'Reilly How To Build A FreeBSD STABLE Firewall With IPFILTER From The O'Reilly Anthology
Estimation of Dietary Pb and Cd Intake from Pb and Cd in blood and urine
pages from xm 754sx 3
Cw 2 Gin endo endokryn
Does the number of rescuers affect the survival rate from out-of-hospital cardiac arrests, MEDYCYNA,
Test 3 notes from 'Techniques for Clasroom Interaction' by Donn Byrne Longman
06 TETRACYKLINY STREPTOGRAMINY
How to draw Donkey from Shrek
big profits from a very dirty business
Progressing from imitative to creative exercises
12 Werntges controling KNX from Linux and USB
On the Actuarial Gaze From Abu Grahib to 9 11
więcej podobnych podstron