
Improved menthol production from
chitosan-elicited suspension culture of
Mentha piperita
Jun Hyong Chang
1
, Joong Han Shin
1
, In Sik Chung
2
and Hyong Joo Lee
1
*
1
Department of Food Science and Technology and Research Center for New-Biomaterials in Agriculture,
Seoul National University, Suwon, 441–744 Korea
2
Department of Genetic Engineering, Kyung Hee University, Suwon, 449–701 Korea
The optimum concentration of chitosan to menthol production by Mentha piperita cells cultured in shake flasks was
200 mg/l, which gave 166 mg menthol/l after 12 days. Chitosan elicitation may activate the conversion of pulegone to
menthol.
Keywords: Chitosan, elicitation, Mentha piperita, menthol
Introduction
In cell cultures of Mentha piperita, the effects of medium
composition and bioreactor operation on cell growth and
menthol formation have been investigated (Chung et al.,
1994; Kim and Lee, 1992; Oh et al., 1993; Park et al.,
1993; Park and Chae, 1990). However, some problems still
remain unresolved in improving menthol production. A
wide variety of elicitors have been employed to alter cell
metabolism in order to enhance the production of second-
ary metabolites in plant cell cultures (Eilert, 1987; Funk
and Brodelius, 1990; Kohle et al., 1985; Cline and Coscia,
1988; Payne et al., 1991). Among these elicitors, chitosan
(
b-1,4-linked glucosamine) proved to be very effective in a
suspension culture of Vanilla planifolia, Glycine max, and
Polygonum tinctorium cells (Funk and Brodelius, 1990;
Kohle et al., 1985; Kim et al., 1997). This suggested that
menthol production could be enhanced if such an elicitor
was used in a suspension culture of M. piperita cells. To the
best of our knowledge, we first report the effect of chitosan
elicitation on a suspension culture of M. piperita.
Materials and methods
Cell line and suspension culture
Peppermint cell line was derived from the leaves of Mentha
piperita L. Suspension cultures of M. piperita were estab-
lished and maintained in Lin-Staba (LS) medium supple-
mented with 2 mg 2,4-dichlorophenoxyacetic acid (2,4-D)
and 20 g sucrose per litre. Suspension cultures were grown
in shaking incubators at 120 rpm, 27°C, with 16 h
illumination per day.
Elicitation with chitosan
Chitosan was purchased from Sigma Chemical Co. and
purified by the method of Young et al. (1982). Briefly, it
was dissolved in 90 ml of 0.1 M acetic acid and the
solution was centrifuged for 30 min. The insoluble frac-
tions were then discarded. This procedure was performed
four times. After centrifugation, the supernatant was pre-
cipitated by adjusting of its pH to 8.0 with 5 M NaOH.
The precipitates were washed extensively with distilled
water and then freeze-dried. The purified chitosan was
dissolved in 0.1 M acetic acid (1 g chitosan/90 ml acetic
acid) and the pH of the solution was adjusted to 5.0. For
the determination of the optimum concentration, chitosan
concentration was varied from 50 to 300 mg/l. To deter-
mine the effect of growth regulators on elicitation, 2,4-D,
a-naphthaleneacetic acid (NAA), or kinetin was added to
the culture medium at a concentration of 2 mg/l. Suspen-
sion cultures of M. piperita were performed for 20 days
under the conditions as described above except that chitosan
was added to cell cultures unless otherwise specified.
Analysis
The cell suspension was centrifuged in a 15 ml tube at
1100
3g for 20 min. Centrifuged cells were washed two
times with distilled water and dried at 80°C for 24 h to
analyze dry cell weight (DCW). Essential oil analysis was
performed as follows. Culture medium was collected after
centrifugation (2000
3g, 20 min). Peppermint oleoresin
was extracted with a mixture solution of pentane and
dichloromethane (2:1) for 8 h in a continuous liquid-liquid
extractor. Menthone, menthol and pulegone were analyzed
Biotechnology Letters, Vol 20, No 12, December 1998, pp. 1097–1099
© 1998 Chapman & Hall
Biotechnology Letters
⋅
Vol 20
⋅
No 12
⋅
1998
1097
using a gas chromatograph (Hewlett Packard 5890 series
II) fitted with a FID detector and an UItra-1 capillary
column (Hewlett-Packard) packed with 100% dimethyl
polysiloxane. The flow rate of the carrier gas was 2 ml/min.
Samples were injected at a 25:l split ratio via an injection
port at 250°C with l
ml aliquot and a temperature program
of 80
,l50°C at 5°C/min and 150,210°C at 20°C/min.
All the data were represented as the average of duplicate
experiments.
Results and discussion
The effect of chitosan concentrations (0, 50, 100, 150, 200,
250 and 300 mg chitosan per litre) on cell growth and
menthol production was investigated for 5 days using a
suspension culture of M. piperita. The chitosan did not
inhibit the growth of M. piperita cells and menthol reached
a maximum value (20 mg/l) at 200 mg chitosan/1. This
result is in agreement with previous findings on the opti-
mum concentration of chitosan (200 mg/l) in indirubin
production using suspension cultures of P. tinctorium (Kim
et al., 1997).
To determine the optimum period of elicitation, 200 mg
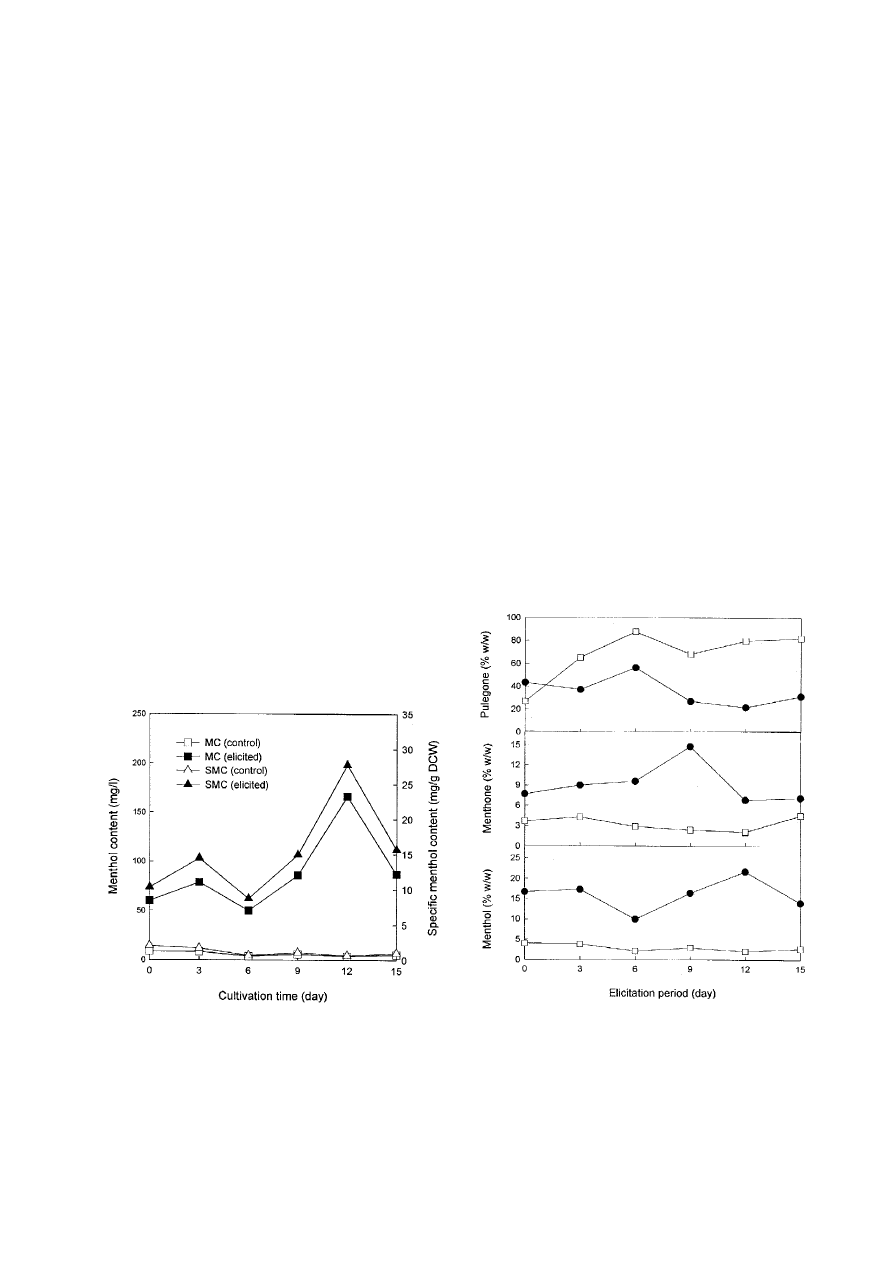
chitosan/l was added to suspension cultures of M. piperita.
In elicited cells, the specific menthol content increased up
to 27.8 mg/g DCW at 12 days and then decreased, whereas
the control specific menthol content remained low through-
out the 15-day period (Fig. 1). The maximum menthol
concentration reached 166.4 mg/l at 12 days of elicitation.
This value is a 40 fold increase compared to the control.
This result indicates that the optimum period of elicitation
for menthol production is 12 days. In our experiments,
menthol concentration decreased after 15 days. This
decrease is unlikely due to cell death caused by the
depletion of substrate in medium since the elicited cells at
15 days are in the exponential phase of cell growth. Rather,
this could be due to metabolism by extracellular enzymes
such as peroxidases. Similar results have been reported for
the production of monoterpenes by shoot cultures of
peppermint (Rhodes et al., 1991).
Pulegone is metabolized to menthone and then to men-
thol. Fig. 2 shows a time course production for pulegone,
menthone, and menthol in terms of % fraction in total
oleoresin. In elicited cells, pulegone content reached a
maximum at 6 days and decreased after 6 days. Menthone
content reached a maximum at 9 days, and then declined
in elicited cells. However, menthol content in elicited cells
reached a maximum at 12 days. This result indicated that
a decrease of pulegone at 9 days coincides with an increase
of menthone in elicited cells. Moreover, menthol content
Figure 1
The effect of chitosan elicitation on the pro-
duction of menthol using suspension culture of M. piperita.
The initial cell concentration was 1 g DCW/l. Chitosan was
added at a concentration of 200 mg/l. MC, menthol con-
tent (mg/l); SMC, specific menthol content (mg/g DCW).
Figure 2
The effect of chitosan elicitation on the forma-
tion of menthol, menthone and pulegone in suspension
culture of M. piperita. The initial cell concentration was
1 g DCW/l. Chitosan was added at a concentration of
200 mg/l.
d, Elicited cells; h, non-elicited cells (control).
J.H. Chang et al.
1098
Biotechnology Letters
⋅
Vol 20
⋅
No 12
⋅
1998

increased at 12 days in elicited cells at the expense of
menthone. On the other hand, menthol and menthone
were low in the control without elicitation although
pulegone was high throughout 12-day period. This implies
that the biosynthetic pathway of pulegone to menthone
might be blocked in the control. We found that in elicited
cell an increased concentration of menthol and menthone
were observed in accordance with a decreased concentration
of pulegone during the elicitation period. This result
suggests that chitosan may activate the conversion of
pulegone into menthol via menthone.
The type of growth regulators in the culture medium can
affect the induction of secondary metabolites in cultured
cells quite dramatically (Cline and Coscia, 1988). To
investigate the effects of growth regulators on elicitation,
LS medium containing no growth regulators, 2 mg/l
2,4-D, 2 mg/l NAA, 2 mg/l kinetin was tested using
suspension cultures of M. piperita at 200 mg/l of chitosan.
In the medium with 2,4-D, the specific menthol content
was highest, indicating that 2,4-D is the best among the
regulators tested.
In summary, menthol production was improved by 40 fold
due to the elicitation of M. piperita with 200 mg/l of
chitosan. Our results also suggest that chitosan elicitation
may activate conversion of pulegone to menthol in suspen-
sion cultures of M. piperita.
Acknowledgement
This work was supported by grants from the Korea Science
and Engineering Foundation through the Research Center
for New Bio-Materials in Agriculture.
References
Chung, IS, Kang, YM, Oh, JH, Kim, T, Lee, HJ and Chae, YA
(1994). Biotechnol Tech 8:789–792
Cline, SD and Coscia, CJ (1988). Plant Physiol 86:161–165
Eilert, U (1987). Elicitation: methodology and aspects of applica-
tion. In: Cell Culture and Somatic Cell Genetics of Plants, F
Constabel and IK Vasil eds vol 4 pp 153–196, New York:
Academic Press
Funk, C and Brodelius, P (1990). Phytochemistry 29: 845–848
Kim, JH and Lee, HJ (1992). J Kor Agric Chem 35: 443–448
Kim, JH, Shin, JH, Lee, HJ. Chung, IS and Lee, HJ (1997). J
Ferment Bioeng 83:206–208
Kohle, H, Jeblick, W, Poten, F, Blaschek, W and Kauss, H
(1985). Plant Physiol 77: 544–551
Oh, JH, Kang, YM, Chung, IS, Lee, HJ and Chae, YA (1993).
Kor J Biotechnol Bioeng 8:295–299
Park, SH and Chae, YA (1990). Kor J Breed 22:53–57
Park, SH, Chae, YA, Lee, HJ and Kim, SU (1993). J Kor Agric
Chem 36:358–363
Payne, GF, Bringi, V, Prince, C and Shuler, ML (1991). Questions
and strategies for productivity improvements. In: Plant Cell
and Tissue Culture in Liquid Systems, pp 329–335, New York:
Hanser Publishers
Rhodes, MJC, Spencer, A and Hamill, JD (1991). Trans London
Biochem Soc 19: 702–706
Young, DH, Kohle, H and Kauss, H (1982). Plant Physiol 70:
1449–1454
Received: 17 August 1998
Revisions requested: 11 September 1998
Revisions received: 16 October 1998
Accepted: 19 October 1998
Menthol production by chitosan-elicited suspension culture
Biotechnology Letters
⋅
Vol 20
⋅
No 12
⋅
1998
1099
Wyszukiwarka
Podobne podstrony:
Piotr Siuda Between Production Capitalism and Consumerism The Culture of Prosumption
Biotransformation of menthol and geraniol by hairy root cultures of Anethum graveolens
Docfoc com Tubing Sizes for Improvised Firearm Barrels From
Overview of bacterial expression systems for heterologous protein production from molecular and bioc
Fuel and chemical products from biomass syngas A comparison of gas fermentation to thermochemical co
The Culture of Great Britain The Four Nations Scotland
Culture of France
Packaging Life Cultures of Everyday
Engine from an Autornobile Turbocharger Rules of Thumb
Pramod K Nayar Packaging Life, Cultures of the Everyday (2009)
Culture of India
The Culture of Great Britain The Four Nations
Kosky; Ethics as the End of Metaphysics from Levinas and the Philosophy of Religion
The Culture of the USA 11 Political System
History and Culture of Shosara
Chile The Autoritarian Culture of Chile Autorytarne kultury z Chile
PP BH&C 0 1 Introduction to the History and Culture of the B
Paul Ricoeur From Existentialism to the Philosophy of Language
więcej podobnych podstron