
Journal of Cereal Science 33 (2001) 223–229
doi:10.1006/jcrs.2000.0362, available online at http://www.idealibrary.com on
Analysis of Headspace Compounds of
Distillers Grains using SPME in Conjunction
with GC/MS and TGA
Sumana Biswas and Charlie Staff
University of Louisville Food Processing Program, Speed Research Administration, KY 40292, U.S.A.
Received 20 September 2000
ABSTRACT
Chemical composition of headspace compounds of distillers grains was investigated using solid phase
microextraction (SPME) along with GC/MS and TGA. Dryer feed (DF) and distillers dried grains
(DDG) from a local distillery were utilised for this study. A SUPELCO 75
m Carboxen/PDMS
SPME fibre was found suitable for extraction of analytes from grains. Samples were placed in small
vials (40 mL from SUPELCO) filled to one-third of their capacity. SPME was exposed to the
headspace for 1 to 2 h. The fibre was then thermally desorbed in GC injection port for separation
and identification. The gas chromatogram showed as many as 64 compounds eluting out of the
column, the number being greater in DF as compared to DDG. Thermogravimetric analysis (TGA)
was performed on the grains to study the oxidative stability of the compounds. SPME fiber was
inserted directly into TGA purge gas outlet to study the compounds being evolved. Three weight
loss transitions were observed in the TGA curve of DF and DDG. The components from the three
transitions were captured by SPME and analysed by GC/MS for identification. TGA helped in
determining the degradation stages of grains thereby indicating the temperature limits in drying
grains. Di
fference between the heated and unheated DF and DDG have been discussed in detail.
2001 Academic Press
Keywords: distillers grains, SPME, GC/MS, TGA, grain drying.
to analyse oil, protein and moisture content of
INTRODUCTION
cereal grains. Cereal grains have been subjected
Distillers grains—a co-product when cereal grains,
to pressure digestion for mineral analysis by atomic
predominantly corn, are used in the production
absorption spectroscopy.
Fluorescence micro-
of fuel or beverage ethanol, have long been re-
has been reported to detect niacin, aromatic
cognised as a potential animal feed ingredient,
amines and phytin in mature cereal grains. How-
particularly for dairy and beef cattle. They contain
ever, no systematic study has been reported so
a highly desirable combination of by-pass protein,
far about this nutritionally rich co-product called
digestible fibre and fat. Research is being con-
‘distillers grains’. To ensure that the highest quality
ducted worldwide to e
ffectively utilise this nu-
distillers grains are provided to customers, it is
tritionally rich co-product from the distilleries for
necessary that there be proper investigation re-
human consumption.
There are many factors
garding its chemical composition. Besides nutrients
that contribute to the quality of distillers dried
like protein, minerals and fat, the presence of
grains (DDG) including initial grain quality, pro-
trace compounds cannot be overlooked which are
cess conditions and drying technology.
critical in producing proper taste and aroma of
Technology has been developed to study and
food and feed products. These trace quantities of
analyse di
fferent aspects of cereal grains. Near
compounds are also responsible for the ‘o
ff’ flavour
infrared reflectance spectroscopy
has been applied
or taste often detected in food. Therefore it is
0733–5210/01/020223
+07 $35.00/0
2001 Academic Press

S. Biswas and C. Staff
224
Table I
SPME-GC/MS results of DF and DDG
Dryer feed
DDG
Compound
R.T.
b
Unheated
Heated
Unheated
Heated
Ethanol
2·23
Present
Present
Present
Present
2-methyl propanal
3·31
Present
Present
Present
Present
2,3-butanedione
3·9
Present
Present
Present
Present
Formic acid
4·46
Present
Present
Present
Present
Acetic acid
5·05
Present
Present
Present
Present
Pentanal
5·83
Present
Present
Present
Present
Dimethyl disulphide
6·72
Present
Present
Present
Present
Propanoic acid
6·87
Present
Present
Present
1-pentanol
7·46
Present
Present
Propylene glycol
7·9
Present
Present
Present
Present
Hexanal
7·96
Present
Present
Present
Present
Methyl pyrazine
8·42
Present
Present
Present
2,3-butanediol
8·64
Present
Present
Present
Present
1,2-dimethylbenzene
9·17
Present
Furfural
9·27
Present
Present
Present
Present
2-hexenal
9·4
Present
Present
3-methyl butanoic acid
9·68
Present
Tetrahydrothiophene
9·68
Present
2-furanmethanol
9·8
Present
Present
Present
Present
5-methylhexanone
9·91
Present
Present
Present
Present
Heptanal
10·07
Present
Present
Present
Present
2,6-dimethylpyrazine
10·21
Present
Present
Present
Present
2,6-dimethylphenol
10·41
Present
3-methylthio propanal
10·7
Present
Present
Present
1,2-furanylethanone
10·77
Present
Present
Present
Present
2-pentylfuran
11·41
Present
Present
Present
Present
2-heptenal
11·5
Present
Present
Present
Present
1-octene-3-one/3-ol
a
11·62
Present
Present
Present
Present
Benzaldehyde
11·79
Present
Present
Present
Present
2-hydroxypropanoic acid
11·86
Present
Present
Present
2-furancarboxaldehyde
11·93
Present
Ethyl acetate
12·05
Present
Present
Present
Present
Hexanoic acid
12·35
Present
Present
Present
Present
2,4-heptadienal
12·71
Present
Present
3-ethyl2-methylhexadiene
12·96
Present
Undecane
13·04
Present
Present
Present
Present
Benzeneacetaldehyde
13·44
Present
Glycerin
13·68
Present
Present
Present
Present
Nonanal
13·97
Present
Present
Present
Present
Heptanoic acid
14·04
Present
Triacontane
14·3
Present
Present
Dodecane
14·8
Present
Present
Present
Phenylethyl alcohol
14·95
Present
Present
Present
Present
Octanoic acid ethylester
15·21
Present
Present
Present
Present
Octanoic acid
15·65
Present
Present
Present
Present
Decanal
15·72
Present
Present
Ethyl hydrogen succinate
16·11
Present
Present
Benzoic acid
16·19
Present
Present
Present
Present
Tridecane
16·43
Present
Present
Present
Present
2-decenal
16·94
Present
Present
Nonanoic acid
17·18
Present
Present
Present
Present
2,3-dihydro benzofuran
17·35
Present
Present
Phenylpropanedioicacid
17·57
Present
Tetradecane
17·97
Present
Present
Present
Present
1,3-methoxy phenyl ethanol
18·19
Present
Present
Decanoic acid ethyl ester
18·34
Present
Present
Present
Present
continued

Distillers grains headspace compounds
225
Table I
SPME-GC/MS results of DF and DDG—continued
Dryer feed
DDG
Compound
R.T.
b
Unheated
Heated
Unheated
Heated
n-decanoic acid
18·65
Present
Present
Propanoic acid,2-methyl heptyl ester
18·97
Present
Present
Octanoic acid,3-methylbutyl ester
19·12
Present
Present
Pentadecane
19·43
Present
Present
2,5-cyclohexadiene-1,4-dione2,6-bis
20·34
Present
Present
Eicosane
21·01
Present
Present
Phenol bis-1,1-dimethylethyl
21·38
Present
Present
Dodecanoic acidethyl ester
21·44
Present
Present
Nonacosane
22·86
Present
Present
2,4-di-t-butyl6-nitrophenol
24·76
Present
Pentadecanoic acid ethyl ester
21·4
Present
Present
Tetradecanoicacid ethyl ester
25·956
Present
Present
Hexadecanoic acid ethyl ester
33·57
Present
Present
a
1-octene-3-ol in unheated grains.
b
Retention time.
essential to develop a procedure for assay of these
Equipment Specifications
minor but important constituents. The objective
SPME
of the present study is to determine the composition
For SPME extraction, SUPELCO 75
m Car-
of the compounds present in the headspace of
boxen/PDMS (polydimethylsiloxane) was used.
distillers grains (dryer feed (DF) & distillers dried
grains (DDG)), using a simple, fast and inexpensive
method which would benefit the cereal food in-
GC/MS Conditions
dustry in their quality control operations. SPME
The extractives were analysed using a Hewlett-
(solid phase microextraction)
unit consists of a
Packard (Palo Alto, U.S.A.) gas chromatograph
fused silica fiber coated with a polymeric material.
5890 equipped with a 5970 mass selective detector,
The coated fiber is attached to a stainless steel
and a HP G1034C MS chemstation. The column
plunger inside a protective needle. It requires no
used was a 30 m SPB-624 SUPELCO, 0·25 mm
solvent or complicated apparatus. It can con-
id and 1·4
m film thickness. An SPME injection
centrate volatiles and non-volatiles in both liquid
sleeve (0·75 mm id for Hewlett-Packard) was in-
and gaseous samples for analysis by GC/MS or
serted into the GC injection port. The carrier gas
HPLC. Use of SPME has been extended to food
was helium maintained at 32 psi. The splitless
flavour
injection port was heated at 220
°C. Temperature
and countless other applications. The grains were
ramping was adjusted from 40
°C to 250 °C at the
also subjected to TGA to study their oxidative
rate of 10
°C/min. All mass spectra were acquired
stability at high temperature.
in the electron impact (EI) mode at 70 eV. The
electron multiplier was set at 1490 V. Compound
identification was done using NIST 98 library.
MATERIALS AND METHOD
Materials
TGA Conditions
Thermogravimetric analysis was performed using
Dryer feed (DF) and distillers dried grains (DDG)
were obtained from a local distillery that pre-
Hi-Res. TGA 2950, TA Instruments. The tem-
perature range of the instrument was from ambient
dominantly uses corn as a feed stock. DF is the
concentrated mixture (after distillation of alcohol)
to 1000
°C. Balance sensitivity and accuracy were
0·1
g and ±0·1% respectively. Air was used as
that enters the final stage of grain drying. DDG
is the final product of the drying process. The
purge gas in the experiments. Purge gas flow rates
in the furnace and balance were 60 mL/min and
moisture contents of DF and DDG are 31% and
11·6% respectively.
40 mL/min respectively. Temperature ramping
S. Biswas and C. Staff
226
Table II
TGA-SPME-GC/MS results of DF and DDG
60–70
°C
250–300
°C
510–550
°C
Compounds
DF
DDG
DF
DDG
DF
DDG
2-propenal
Present
Present
Present
Acetone
Present
Present
2,3-butanedione
Present
Present
Cyclobutylamine
Formic acid
Present
Present
Present
Present
Present
Present
Acetic acid
Present
Present
Present
Present
Present
Present
Pyridine
Present
Present
Pentanal
Present
Present
Hexanal
Present
Present
Present
Dimethyl disulphide
Present
2-propanoic acid
Present
Present
Furfural
Present
Present
Present
Present
Present
Present
3-methyl butanoic acid
Present
2-furanmethanol
Present
Present
Present
Present
2-propanone,1-acetyloxy,
Present
Present
Heptanal
Present
Present
2,6-dimethylpyrazine
Present
2-cyclopentene-1,4-dione
Present
Present
Present
Present
2-pentyl furan
Present
Present
Present
Heptenal
Present
Present
Dimethyl trisulfide
Present
Present
Benzaldehyde
Present
Present
Present
Present
2-furanone
Present
Present
5-methylfurancarboxaldehyde
Present
Present
Present
Present
Octanal
Present
Present
Present
2,5-furandione,3-methylene
Present
Present
Benzofuran
Present
Present
Hexanoic acid
Present
Benzonitrile
Present
Present
Butyl benzene
Present
Present
Present
Present
Phenol
Present
Present
Acetophenone
Present
Present
Nonanal
Present
Present
Present
Present
2-methoxy phenol
Present
Pentyl benzene
Present
Present
Dodecane
Present
Present
Hexyl benzene
Decadienal
Present
Present
Phthallic anhydride
Present
6-undecylamine
Present
Present
Present
Present
Furaltadone
Present
Present
Present
Present
Present
Present
Decanamine
Present
Present
Present
Present
Present
Present
was set from ambient to 800
°C at the rate of
order to facilitate analyte concentration in the
10
°C/min.
headspace. Two vials each of DF and DDG were
prepared and left to equilibrate overnight at room
temperature. After equilibration, one vial each of
Sample Preparation and Analysis
DF and DDG were exposed to heat at 60
°C for
40 min. SPME analysis was performed on both
DF and DDG were finely ground in a cyclone
the 60
°C and room temperature vials on each DF
sample mill (Udy Corp., 1 mm screen), and placed
and DDG sample, by piercing the needle into the
in screw-capped vials (40 mL) fitted with rubber
vial septum and coated fibre exposed for sample
septa. The vials were filled to one-third of their
capacity (
≈3·20 g) and then shaken vigorously in
adsorption. The heated vials were subjected to
Distillers grains headspace compounds
227
0
800
100
Temperature (
°C)
1
W
eight (%)
20
40
60
80
200
400
600
2
3
Derivative weight
Thermal degradation
curve
–2
6
0
2
4
Deriv
. weight (%/min)
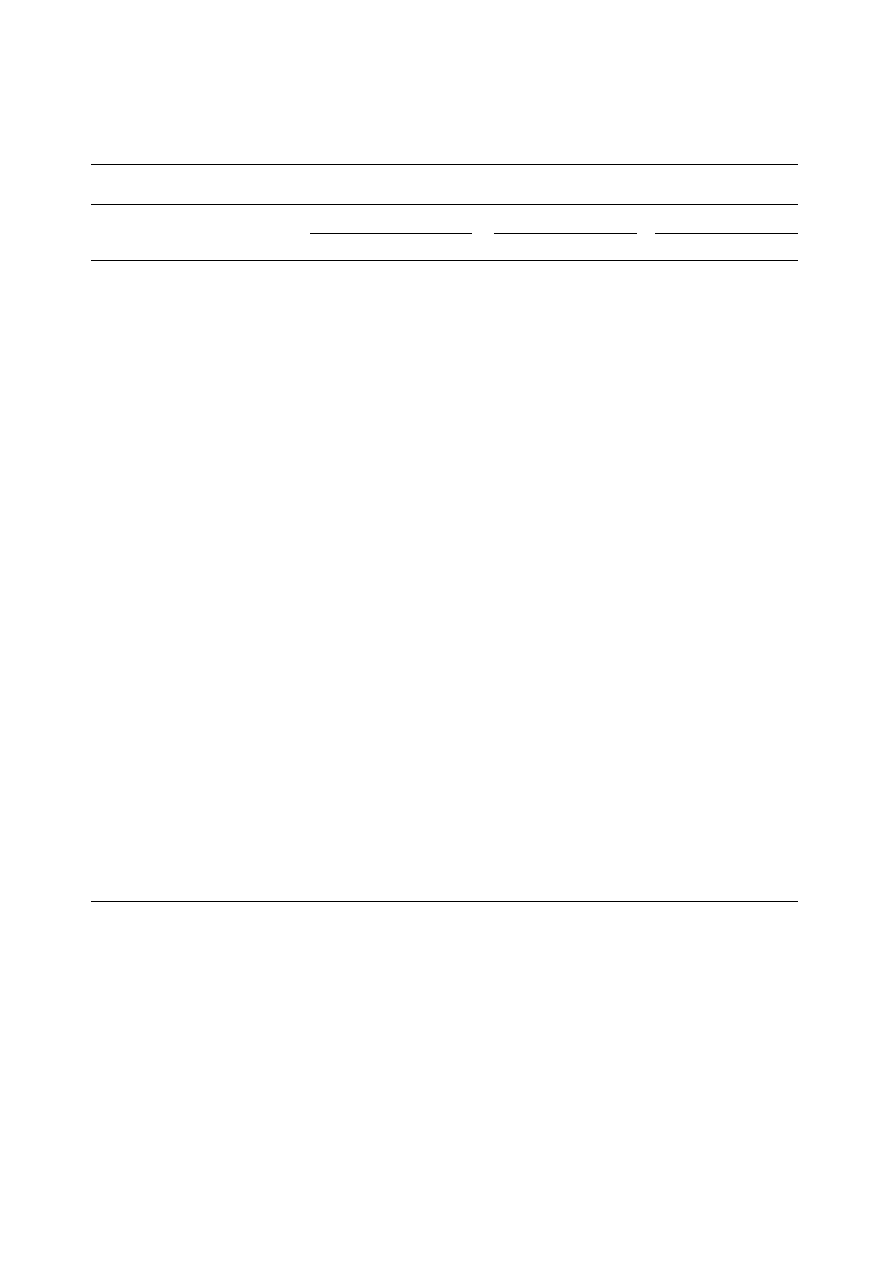
Figure 1
TGA curve of DF.
0
800
100
Temperature (
°C)
1
W
eight (%)
20
40
60
80
200
400
600
2
3
Derivative weight
Thermal degradation
curve
–2
8
0
2
4
Deriv
. weight (%/min)
6
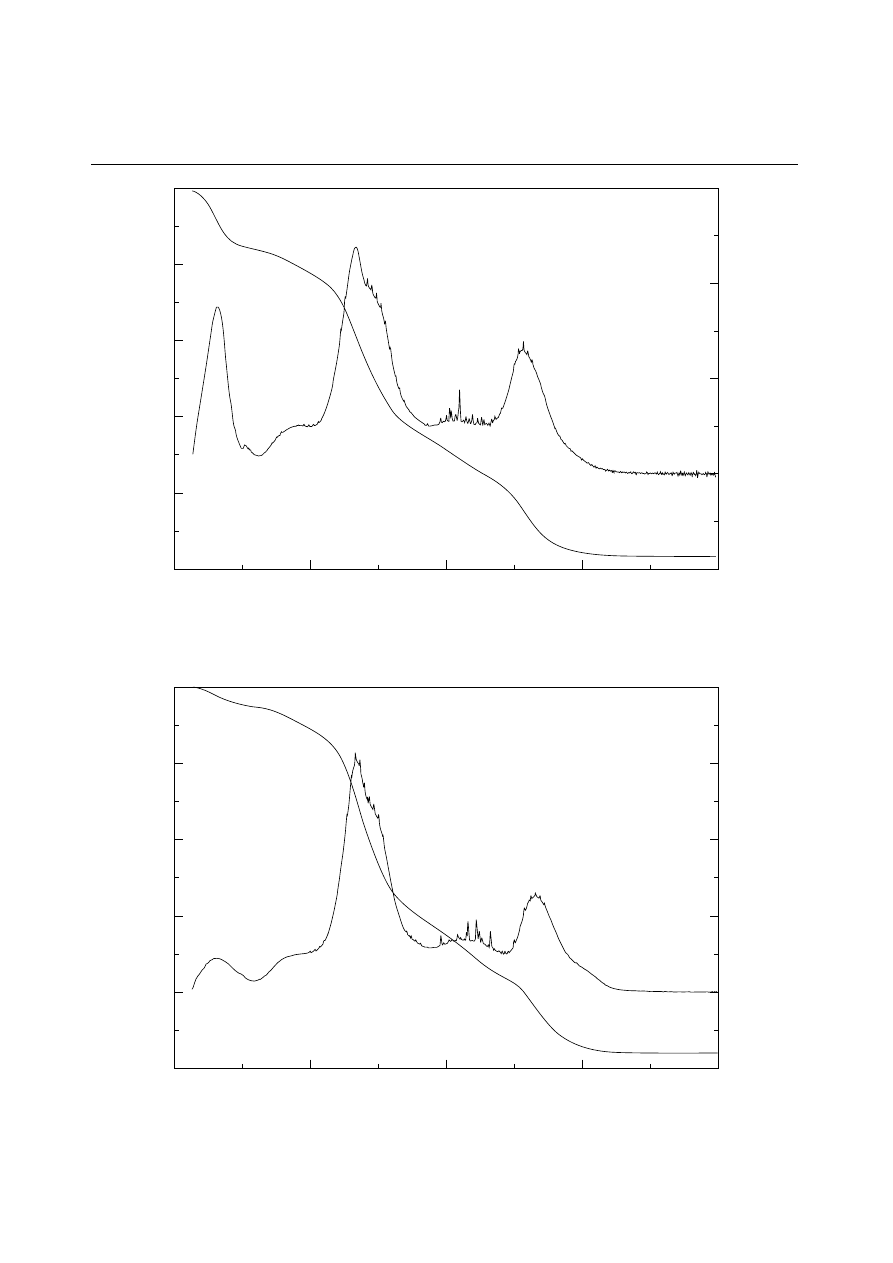
Figure 2
TGA curve of DDG.

S. Biswas and C. Staff
228
SPME extraction for 1 h and the unheated vials-
Difference between Unheated and Heated
(room temperature) for 2 h. The SPME fibre con-
Grains
taining the volatiles was injected and thermally
A larger number of constituents were observed in
desorbed in GC injection port maintained at
the heated (60
°C) grain samples as compared to
220
°C. SPME was held in the port for 10–15 s
the unheated ones. The low molecular weight
and retracted. Desorption of the SPME for 45–60 s
compound profile was somewhat similar in both
was tried but it did not show any di
fference in the
the heated and unheated ones, but it was in the
overall compound profile in the chromatogram.
higher retention time region, that showed marked
To test the reproducibility of the procedure and
di
fference. Heated samples showed the presence
confirm the retention times, about 10–15 replicates
of esters of higher fatty acids as dodecanoic, tetra-
were analysed.
decanoic and hexadecanoic acid. This increase in
In TGA analysis, the grains were subjected to
number of components in the heated samples was
an air-purged environment during the experiment.
reasonable due to increased mass transfer of larger
They were heated from ambient to 800
°C, heating
molecules in the headspace during heating.
rate being 10
°C/min. SPME fibre was used to
adsorb the gases evolved from the distillers grains
at the TGA outlet and then the fiber was injected
Difference between DF and DDG
in GC injection port for analysis by GC/MS. This
Mass chromatograms of DF show slightly in-
process was followed at each weight loss transition
creased number of components than for DDG. As
of DF and DDG.
explained earlier, DF is the wet concentrated dryer
feed before the final drying process and DDG is
the final dried product. So it can be inferred that
some of the volatiles are driven o
ff from DDG
during drying. A number of higher alkanes like
RESULTS AND DISCUSSION
undecane, dodecane, pentadecane, eicosane etc.
are observed in both DF and DDG which could
GC/MS results showed the presence of as many
be coming from natural gas used in direct fired
as 62 compounds in the distillers grains. These
plant dryers. The aldehydes detected could be the
included the volatile compounds as well as the
result of fatty acid breakdown of the corn oil from
semi-volatile ones. SPME extracted volatile com-
the original grain. Ethyl acetate and glycerin was
pounds like ethanol, formic and acetic acid to
found in the samples. 2,6-dimethyl and methyl
aldehydes such as pentanal, hexanal, heptanal,
pyrazine was detected which are fairly common
furfural and several unsaturated aldehydes as hep-
chemicals found in grain and feed that has been
tenal and decenal from the grain headspace (Table
heated to toasted levels. Lactic or 2-hydroxy pro-
I). Acetic acid was detected in significantly high
panoic acid was seen in GC that could have
amount in all the grain samples. A major source
come from Lactobacillus bacterial growth during
of this chemical could be microbial contamination
fermentation.
during yeast fermentation. These compounds were
identified by library search (NIST 98). SPME has
earlier been used for measurement of hexanal and
Thermogravimetric analysis of grains
pentanal in cooked turkey.
Volatile fatty acids
like formic, acetic, propanoic and lactic acid were
Thermogravimetric analysis or TGA was per-
formed on distillers grains to study their oxidative
detected. Long chain fatty acids were found in the
heated samples. Rosenberg et al.
has reported
stability and volatile compounds evolved during
heating. The GC/MS results of TGA outlet gases
detection of these fatty acids from headspace vol-
atiles of di
fferent varieties of cheese using SPME.
are given in Table II. Three TGA weight loss
transition stages were observed in the TGA curve
Dowd et al.
analysed the stillage from ethanol
distillation of yeast fermented hydrolysed corn
of both DF and DDG (Figs 1 & 2). The degradation
pattern of DF and DDG were similar except for
starch by GC/MS and HPLC. They reported the
presence of volatile low-molecular-weight com-
their first transitions, where DF shows a larger
transition change than DDG. This is due to the
pounds like lactic acid, glycerol, ethanol and some
fatty acids which were also detected in the DDG
higher moisture content of DF. The first transition
at 60–70
°C, gives off mostly moisture along with a
headspace in the present study.

Distillers grains headspace compounds
229
Dried Grains with Solubles from Soft White Winter
few volatile compounds. Use of TGA to determine
Wheat. Cereal Chemistry 64 (1987) 139–143.
moisture content of grains has been reported
2. Wu, Y.V., Youngs, V.L., Warner, K. and Bookwalter,
The second transition temperature range
G.N. Evaluation of Spaghetti Supplemented with Corn
(250–300
°C) shows compounds similar to those
Distillers’ Dried Grains. Cereal Chemistry 64 (1987) 434–
seen earlier in this work in the headspace of heated
436.
3. Bookwalter, G.N., Warner, K., Wall, J.S., Wu, Y.V. and
DF and DDG. Some amino and nitro compounds
Kwolek, W.F. Corn Distillers’ Grains and other By-
are seen which could have resulted from the break-
products of Alcohol Production in Blended Foods. II.
down of proteins/amino-acids of the grains. But
Sensory, Stability and Processing Studies. Cereal Chemistry
it is the third and last TGA transition (510–550
°C)
61 (1984) 509–513.
that shows maximum number of degradation prod-
4. Williams, P.C. Application of Near Infrared Reflectance
ucts. Compounds like butyl benzene, furanone,
Spectroscopy to Analysis of Cereal Grains and Oilseeds.
Cereal Chemistry 52 (1975) 561–576.
pyran and some diones and dienals are observed
5. Lorenz, K., MacFarland, G. and Maga, J. Research Note
which formed from breakdown of proteins, car-
on Pressure Digestion of Cereal Grains and Flours for
bohydrates and possible reaction between them in
Mineral Analysis by Atomic Absorption Spectroscopy.
the course of oxidation.
Cereal Chemistry 54 (1977) 281–286.
6. Fulcher, R.G., O’Brien, T.P. and Wong, S.I. Micro-
CONCLUSION
chemical Detection of Niacin, Aromatic Amine, and
Phytin Reserves in Cereal Bran. Cereal Chemistry 58 (1981)
From the above study it can be concluded that
130–135.
SPME in conjunction with GC/MS and TGA
7. Technology licensed exclusively to SUPELCO.
8. Pelusio, F., Nilsson, T., Tino, R., Larsen, B. and Montna-
provides a quick and convenient way to determine
rell, L. Headspace SPME Analysis of Volatile Organic
the volatiles and semi-volatiles from distillers grains
Sulfur Compounds in Black and White Tru
ffle Aroma.
headspace. The minor constituents discussed
Journal of Agriculture and Food Chemistry 43 (1995) 2138–
earlier are usually present in complex mixtures
2143.
which otherwise pose great di
fficulty during sep-
9. Ng, L., Hupe, M., Harnois, J. and Moccia, D. Char-
aration. SPME could also separate the long chain
acterisation of Commercial Vodkas by SPME-GC/MS.
Journal of the Science Food and Agriculture 70 (1996) 380–388.
fatty acids, which would have been a multiple
10. Potter, D. and Pawliszyn, J. Rapid Determination of
step procedure if analysed by the conventional
Polyaromatic Hydrocarbons and Polychlorinated Bi-
methods.
phenyls in Water using SPME and GC/MS. Journal of
TGA revealed many important aspects of drying
Environmental Science and Technology 28 (1994) 298–305.
temperatures of distillers grains. It is clear from
11. Cizkova, H., Voldrich, M. and Dobias, J. Determination
the study that heating and drying the grains above
of Residual Acetaldehyde in Polyethyleneterephthalate
250–300
°C would result in degradation. The com-
bottles on SPME. Czech. Journal of Food Science 36 (1998)
401–405.
bined use of TGA analysis and SPME-GC/MS
12. Frerot, B., Malosse, C. and Cain, A.SPME, A New Tool
provides a rapid means for determining the tem-
in Pheromone Identification in Lipidoptera. Journal of
perature degradation limits of cereal grains and
High Resolution Chromatography 20 (1997) 340–342.
their processed products. It also provides an in-
13. Brunton, N.P., Cronin, D.A., Monahan, F.J. and Durcan,
dication of oxidative reactions as well as identifies
R.A Comparison of Solid phase Microextraction (SPME)
volatile compounds evolved during drying.
Fibres for Measurement of Hexanal and Pentanal in
Cooked Turkey. Food Chemistry 68 (2000) 339–345.
14. Chin, H., Bernard, R. and Rosenberg, M.SPME for
Acknowledgement
Cheese Volatile Compound Analysis. Journal of Food Science
61 (1996) 1118–1123.
The authors wish to thank Distillers Grains Technology
15. Dowd, M.K., Reilly, P.J. and Trahanovsky, W.S.Low-
Council (DGTC) for financial support of this project.
molecular-weight Organic Composition of Ethanol Still-
age from Corn. Cereal Chemistry 70 (1993) 204–209.
REFERENCES
16. Neher, M.B., Pheil, R.W. and Watson, C.A. Therm-
oanalytical Study of Moisture in Grain. Cereal Chemistry
1. Rasco, B.A., Downey, S.E. and Dong, F.M. Consumer
Acceptability of Baked Goods Containing Distillers’
50 (1973) 617–628.
Document Outline
Wyszukiwarka
Podobne podstrony:
Analysis of total propionic acid in feed using headspace sol
Analysis of volatile organic compounds using gas
Analysis of soil fertility and its anomalies using an objective model
SEISMIC ANALYSIS OF THE SHEAR WALL DOMINANT BUILDING USING CONTINUOUS-DISCRETE APPROACH
Analysis of Reinforced Concrete Structures Using ANSYS Nonlinear Concrete Model
Comparative study based on exergy analysis of solar air heater collector using thermal energy storag
Solid Phase Microextraction Analyses of Flavor Compounds in
Analysis of soil fertility and its anomalies using an objective model
Rapid analysis of malathion in blood using head space solid
Malware Detection using Statistical Analysis of Byte Level File Content
Optimization of Intake System and Filter of an Automobile Using CFD Analysis
Analysis of shear wall structures of variable thickness using continuous connection method
Parallel analysis of polymorphic viral code using automated deduction system
Rapid analysis of amphetamines in blood using head space sol
An%20Analysis%20of%20the%20Data%20Obtained%20from%20Ventilat
A Contrastive Analysis of Engli Nieznany (3)
więcej podobnych podstron