
Applications of solid-phase microextraction to
chemical analysis of live biological samples
Fabio Augusto*, Antonio Luiz Pires Valente
y
Institute of Chemistry, State University of Campinas, CP 6154, Campinas, Sa˜o Paulo, Brazil
This work reviews some recent applications of solid-
phase microextraction (SPME) for the chemical
analysis of live biological samples. Application of
SPME to microbiological analysis, organic volatile
compounds emitted by vegetables and insect semi-
ochemicals will be discussed. A short discussion on
the principles and the basic parameters of SPME is
also included. # 2002 Published by Elsevier Science
B.V. All rights reserved.
Keywords:
Biochemical analysis; In-vivo analysis; Solid-phase
microextraction; Sample preparation
1. Introduction
Sensitive, precise and accurate methodologies
for chemical analysis are fundamental tools in
biochemical research. However, the develop-
ment of bioanalytical procedures is one of the
most formidable challenges faced by analytical
chemists.
Typical biological samples are very complex
mixtures, in which the analytes of interest can
be present in reduced amounts. Also, samples
obtained in in-vitro conditions usually do not
correspond to the blend of compounds pro-
duced and/or released by an undisturbed live
organism. For example, it has been shown that
the composition of the volatile extracts collected
from detached or damaged plants can differ
significantly from the mixture emitted by the
live, undamaged specimen [1–3].
Additional analytical difficulties arise from the
intrinsic dynamic behavior of live biological
systems–for the above-mentioned case of plant
volatiles, production and emission can be affec-
ted or triggered by factors such as light condi-
tions, environmental temperature, stress and
presence of trace atmospheric pollutants [4,5].
Therefore, chemical analysis of live biological
samples customarily requires state-of-the-art
techniques for sampling and sample preparation,
analyte separation, detection and quantitation.
For the sample-preparation step, solid-phase
microextraction (SPME) has gained increasing
popularity. This simple, fast and reliable extrac-
tion technique was introduced in 1990 and has
been widely employed in a large range of appli-
cations, such as environmental, food, clinical
and forensic analysis [6].
The aim of this review is to describe the
application of SPME for in-vivo analysis of
compounds
produced
by
microorganisms,
plants and animals. The basics of SPME
method development for live samples will be
addressed and some recent applications will be
presented and discussed.
2. A SPME primer
SPME is based on the sorption of analytes
present in the sample or in its headspace by a
thin film of an extracting phase immobilized
over the surface of a fused-silica fiber. Fibers
are available coated with pure liquid polymeric
phases or with porous solid phases (more accu-
rately, porous solids dispersed in liquid polymer
matrixes). For liquid polymeric phases such as
polydimethylsiloxane (PDMS), the physico-che-
mical mechanism responsible for the extraction
is partition, and, when porous solid coatings like
Carboxen/PDMS are used, the process also
0165-9936/02/$ - see front matter
# 2002 Published by Elsevier Science B.V. All rights reserved.
P I I : S 0 1 6 5 - 9 9 3 6 ( 0 2 ) 0 0 6 0 2 - 7
*Corresponding author.
Tel.: +55-19-3788-83057; Fax: +55-19-3788-3023.
E-mail: augusto@iqm.unicamp.br
y
In memoriam (1944–2002)
428
trends in analytical chemistry, vol. 21, no. 6+7, 2002
involves adsorption of the analytes by the fiber
coating.
The theoretical foundations of SPME have
been extensively addressed in the literature [7].
Under practical conditions, the extracted mass,
n, of an analyte is proportional to its con-
centration, C
0
, in the sample- which is the basis
of the quantitative use of SPME. Extracted
masses also depend on several other con-
trollable and/or measurable parameters: the
coating/sample distribution constant, K
fs
; the
headspace/sample distribution constant K
hs
(when a headspace is present); sample and
coating volumes; temperature; etc. K
fs
is a
function of the affinity between analyte and
coating, and the proper choice of coating phase
can lead to selective extractions.
For practical reasons, in commercial SPME
apparatus (such as that available from Supelco
Inc., Bellefonte, PA, USA, since 1993), the
fused-silica fiber is mounted in an assembly, as
shown in Fig. 1. This fiber assembly can be fit-
ted to a syringe-like holder (Fig. 2), which
ensures its easy handling: when the plunger is
pushed down the fiber is exposed, allowing
sample extraction or analyte desorption; and,
when the plunger is retracted, the fiber is pro-
tected from the environment inside the needle
of the assembly.
Fig. 3 shows a scheme of the basic SPME
experimental procedure. Most proposed SPME
methods use either GC or HPLC for separation
and detection of the extracted materials. For
GC analysis, the extracts can be thermally des-
orbed after introducing the fiber inside the
injection port of the chromatograph [9]. When
HPLC is used for separation and detection,
analytes can be removed from the fiber and
introduced in the column by redissolution in
adequate organic solvents using a simple
SPME-HPLC interface [10].
3. SPME method development for
in-vivo sampling
The general principles of SPME method
development have been discussed in the litera-
ture [7]. Consequently, we will focus the fol-
lowing
discussion
on
the
problems
and
particularities found in the optimization of
SPME for in-vivo bioanalysis.
3.1. Extraction technique
SPME can be applied to both direct (fiber
directly immersed in the liquid or gaseous sam-
ple) and headspace analysis (fiber exposed to
the headspace of a liquid or solid sample). The
main factor for consideration in selecting an
extraction technique is the volatility of the target
analytes– high and moderately volatile species
are preferably determined in the headspace [12].
However, the nature of the matrix should also
be considered, and this factor seems to be
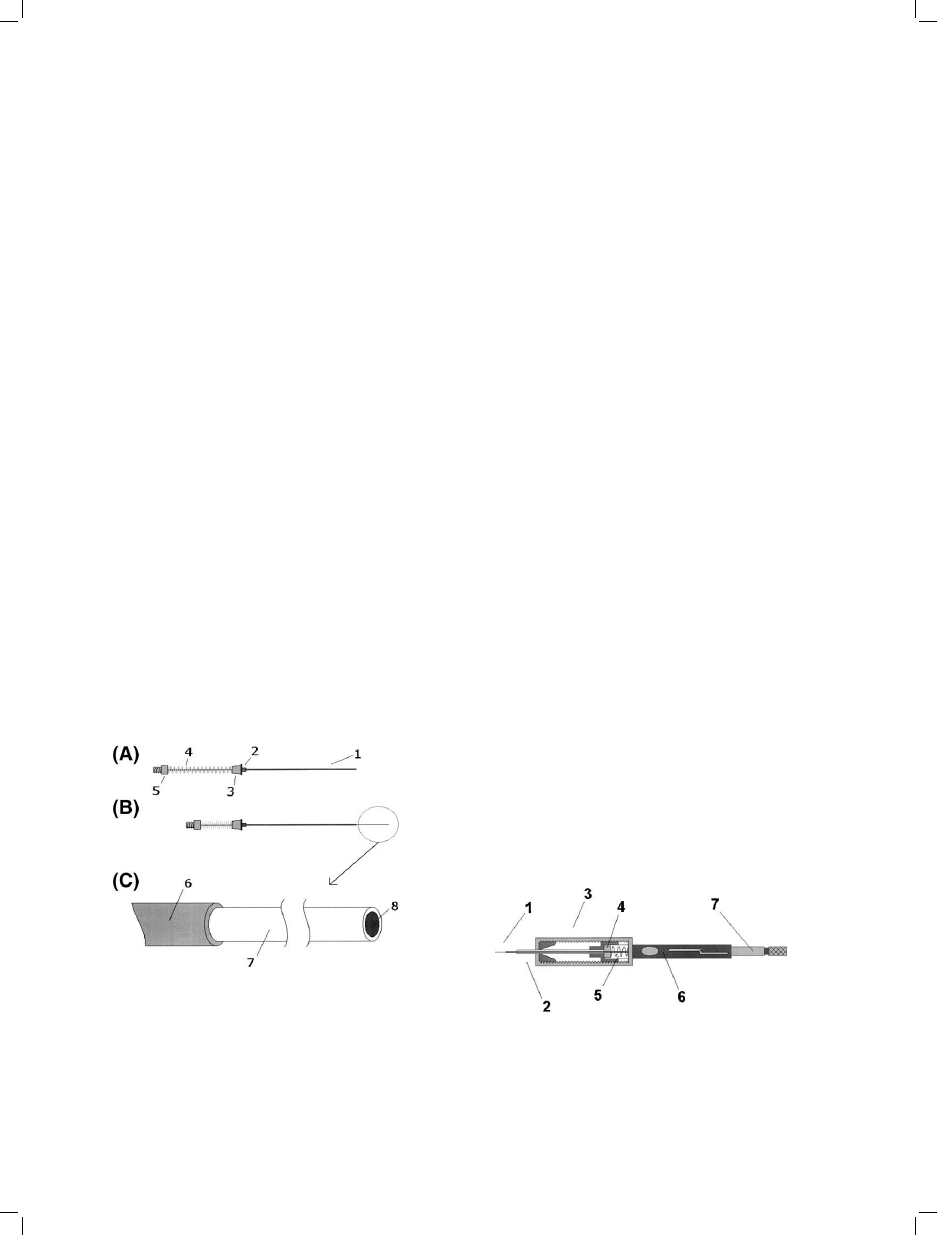
Fig. 1. Commercial solid-phase microextraction (SPME)
fiber assembly. A=Fiber protected inside needle; B=Fiber
exposed; and, C=Magnified detail of the SPME fiber.
1=Stainless steel needle; 2=Brass base; 3=Sealing silicone
septa; 4=Tensioning spring; 5=Hub; 6=Fiber attachment
steel tube; 7=Sorbent coating; and, 8=Fused silica fiber
core.
Fig. 2. Commercial solid-phase microextraction (SPME)
holder device (based on [9]). 1=SPME fiber; 2=Stainless
steel needle; 3=Holder body; 4=Silicone septa; 5=Spring;
6=Barrel; and, 7=Plunger.
trends in analytical chemistry, vol. 21, no. 6+7, 2002
429
especially relevant for bioanalytical applications.
For example, in the monitoring of compounds
produced by live plants and insects, headspace
SPME (HS-SPME) is imperative to avoid
damage and to minimize stress in the live
sample
specimens.
Furthermore,
biological
materials are usually ‘‘dirty’’ samples, and direct
immersion of SPME fibers in these matrixes
can lead to extensive fiber contamination and
even fiber damage [13].
HS-SPME of volatile substances released by
live insects and other small animals has been
performed by enclosing an adequate number of
individuals in conventional septum-sealed glass
vials and exposing a SPME fiber to the air
inside the vial [14]. Nonetheless, specially-
designed devices have also been described as
improving the isolation of the specimens from
environment and allowing extractions with
minimum disturbance to them. An example of
such apparatus is the glass chamber described
by Zini et al [15], utilized to monitor biogenic
volatile organic compounds (BVOCs) emitted
by leaves of Eucalyptus plants (Fig. 4). For HS-
SPME determination of BVOCs released by
Penicillium
fungi, Nilsson et al. [16] used a simple
flow-through device (Fig. 5), which isolates the
culture media containing the fungi from the
environment, providing proper conditions both
for fungal growth (constant renewal of interior
air and removal of produced CO
2
), as well as
easy access for SPME sampling.
3.2. Selection of fiber coating
Table 1 provides an up-to-date (January 2001)
list of the commercially available SPME fibers.
The choice of the fiber coating depends mainly
on the nature of the target analytes. The princi-
ple ‘‘like dissolves like’’ serves as a general rule
for coating selection; also, thicker coatings pro-
vide higher recoveries but slower equilibration
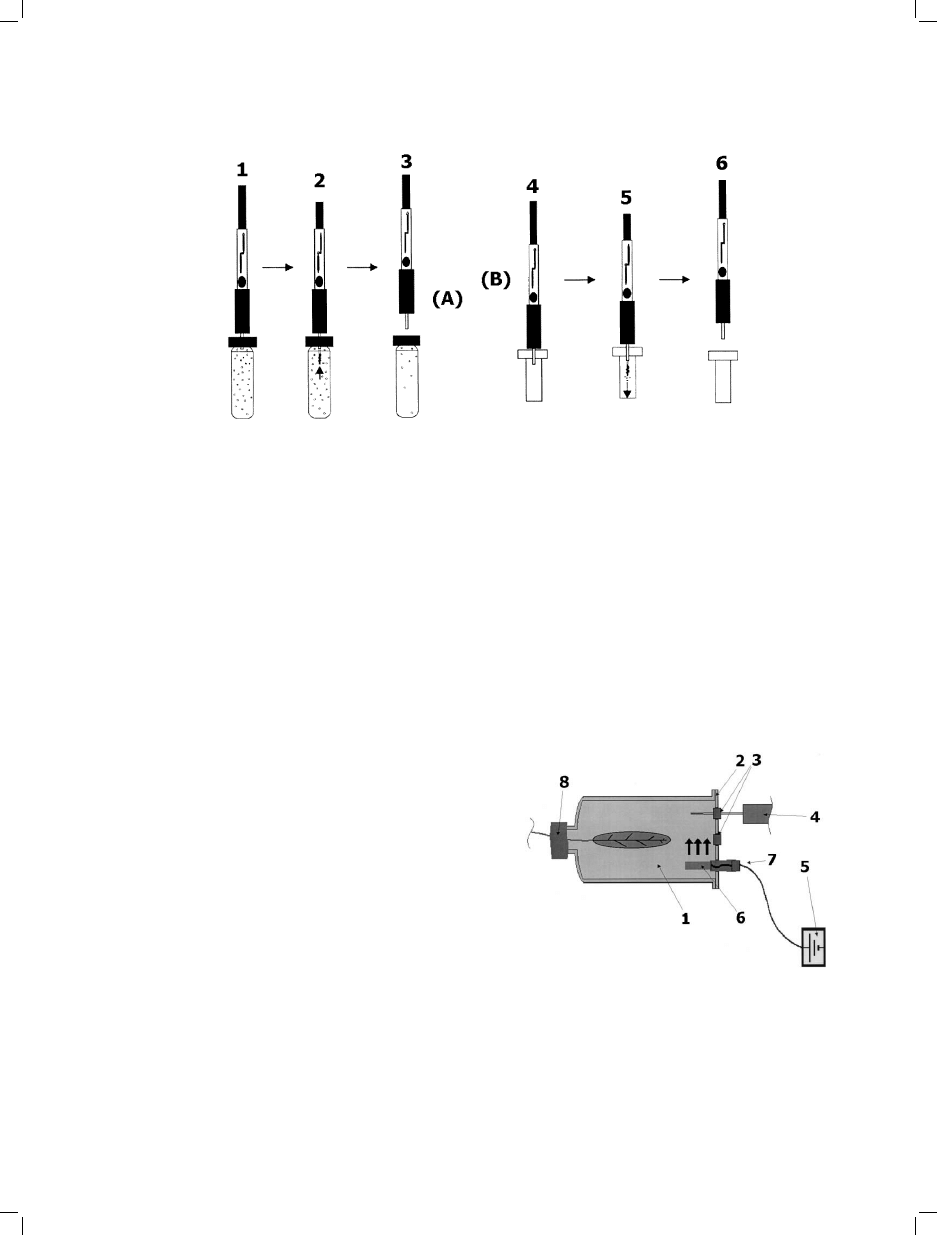
Fig. 3. Basic solid-phase microextraction (SPME) procedure (modified from [8]). A=Extraction; and, B=Desorption. 1=Pierce
septum on sample container; 2=Expose SPME fiber/extract analytes; 3=Retract fiber/withdraw needle; 4=Pierce septum in GC
inlet (or introduce needle into SPME/HPLC interface); 5=Expose fiber/desorb analytes; and, 6=Retract fiber/withdraw needle.
Fig. 4. Glass chamber for solid-phase microextraction
(SPME) sampling of volatiles emitted from single live leaves
(based on [15]). 1=Silanized glass cylindrical body; 2=Sila-
nized glass lid; 3=SPME sampling holes topped with sili-
cone septa; 4=SPME holder plus fiber; 5=DC power supply
for microfan; 6=Microfan; 7=Teflon support for microfan;
and, 8 - Teflon tape seal.
430
trends in analytical chemistry, vol. 21, no. 6+7, 2002
with analytes in the sample [12]. Apart from
affinity considerations, PDMS fibers are popular
for several bioapplications, because of their
ruggedness and stability [17].
Porous solid-coated fibers, such as CAR/
PDMS and PDMS/DVB, were introduced
more recently and are being employed for some
new bioapplications because they provide higher
extraction yields for volatile and/or polar com-
pounds–although the limited lifetime of such
fibers can be a possible drawback [17]. A com-
prehensive study of the extraction conditions
for BVOCs released by aromatic plants per-
formed by Bicchi et al [18] found that porous
solid-phase fibers allow substantially higher
recoveries for most compounds found in the
headspace of these plants. Watson et al pre-
sented similar results [19], stating that PDMS/
DVB fibers are more suitable than PDMS or
PA for monitoring odorous compounds (unsa-
turated aldehydes from 2-hexenal to 2,4-dec-
adienal) produced in surface waters by algae,
such as Synedra delicatissima.
3.3. Extraction operational conditions
A fundamental step in SPME method devel-
opment is the determination of the time, t
eq
,
needed for equilibration of the analytes between
fiber coating, sample and headspace. Fig. 6
shows a typical time profile for HS-SPME
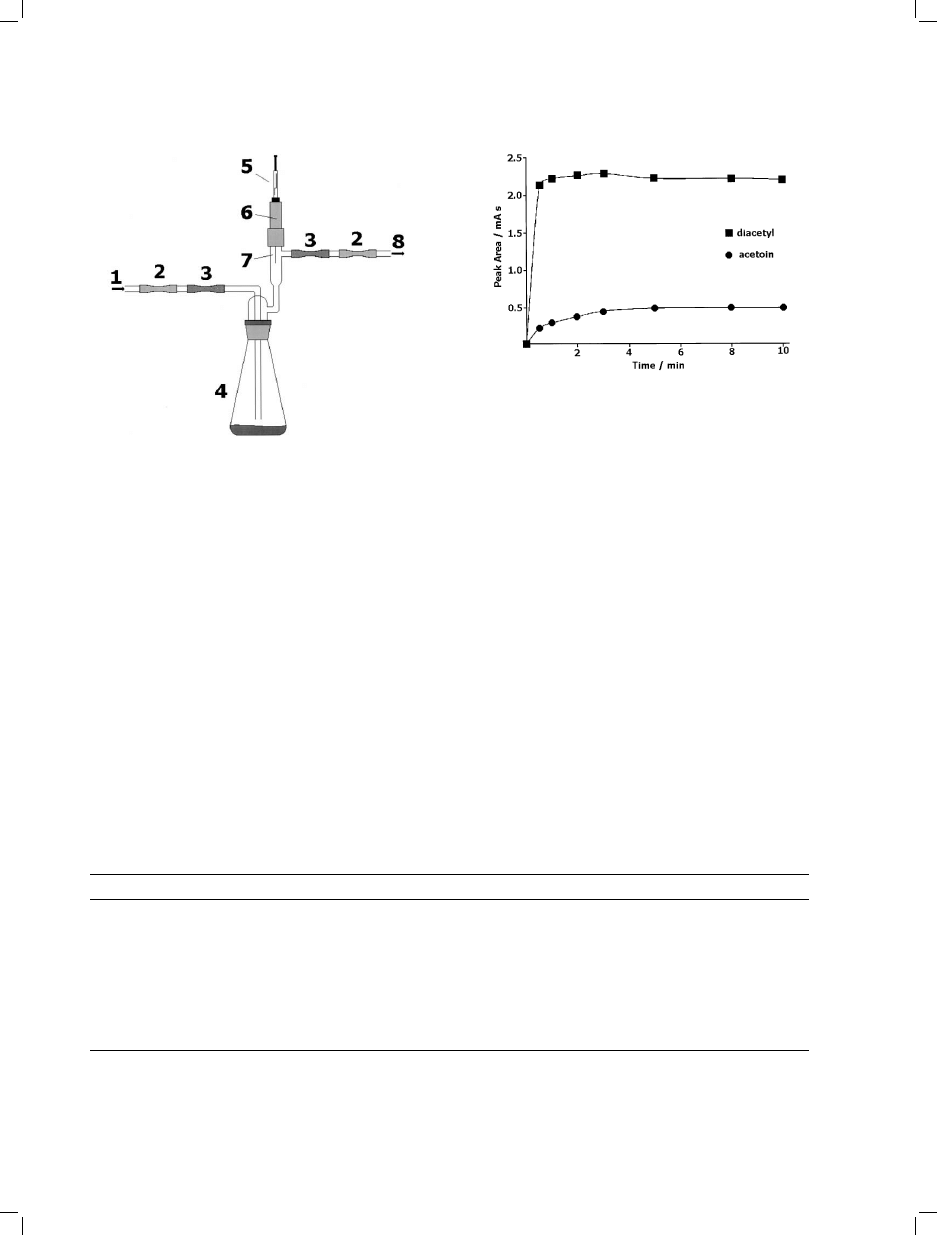
Fig. 5. Flow-through equipment for growth of fungi and
solid-phase microextraction (SPME) analysis of volatiles
(modified from [16]). 1=Purified air inlet; 2=Carbon filters;
3=Sterile filters; 4=Fungal culture on growth medium;
5=SPME device; 6=Septum-sealed support for the SPME
device; 7=SPME fiber; and, 8=Air outlet.
Table 1
Commercially available SPME fibers [11]
Coating material
Acrostic
d
F
/mm
a
Recommended use
Pure liquid polymers
Polydimethylsiloxane
PDMS
7, 30, 100
Non-polar analytes
Polyacrylate
PA
85
Polar analytes (especially phenols)
Porous solids
Polydimethylsiloxane / Divinylbenzene
PDMS/DVB
60, 65
b
Polar analytes (especially amines)
Carboxen / Polydimethylsiloxane
CAR/PDMS
75, 85
b
Volatile / low molar mass analytes.
Carbowax / Divinylbenzene
CW/DVB
65, 70
b
Polar analytes (especially alcohols).
Carbowax / Templated Resin
CW/TPR
50
For HPLC applications.
Divinylbenzene / Carboxen / PDMS
DVB/CAR/PDMS
30
Broad range of analytes.
a
d
F
=coating thickness.
b
Highly cross-linked coating.
Fig. 6. Typical
headspace-solid-phase
microextraction
(HS-SPME) time profile: aqueous solutions of acetoin
(1.16 mmol/L) and diacetyl (1.70 mmol/L) extracted with
PDMS/DVB fiber (based on [20]).
trends in analytical chemistry, vol. 21, no. 6+7, 2002
431

analysis: the extracted mass of each analyte (and,
in consequence, the sensitivity) is maximized
after the equilibrium time. The equilibrium time
depends on several factors: sample stirring con-
ditions (faster sample agitation reduces t
eq
);
coating thickness (fast equilibrium is achieved
with thinner fiber coatings); temperature; and,
affinity between analyte and coating (t
eq
increases
with the affinity of the analyte for the coating) [7].
Despite the improved sensitivity of equili-
brium extractions due to the maximization of
extracted masses, pre-equilibrium SPME is
required in some situations. For porous solid-
coated fibers, accuracy of quantification is severely
limited because of inter-analyte competition for
the available adsorptive sites [21]. However, it
has been shown, both for gaseous [22] and
aqueous [23] samples, that inter-analyte compe-
tition can be minimized or eliminated in pre-
equilibrium extraction conditions - that is, with
very short extraction times.
The development of SPME analytical meth-
ods also involves determination of several other
operational conditions, for example: sample pH
and ionic strength; sample volume; extraction
temperatures; and, desorption time and tem-
perature [17]. It needs to be considered that,
along with their effect on the extraction process,
most of these parameters also affect production
and/or emission of organic compounds by live
specimens [13]. As a result, for in-vivo bioana-
lysis by SPME (or any other technique, indeed),
free choice of some of these conditions can be
severely limited or even not possible at all. For
example, addition of inert electrolytes to the
matrix to increase analyte recovery or adjust-
ment of pH to improve extraction efficiency for
acid or basic substances are usual procedures in
HS-SPME methods [12]. However, since the
pH and ionic strength of culture media should
be rigorously controlled in microbiological
experiments because of their effect on cell
growth and metabolism [24], implementation of
SPME optimization procedures will be restric-
ted in many cases. The same considerations
apply to temperature variations: control of this
parameter should be made, taking into con-
sideration both the optimization of the analytical
method and its effect on the kinetics of production
and emission of the analytes by live organisms,
which in most cases can depend on temperature
(for example, plant BVOC emission [4]).
4. Some recent in-vivo SPME applications
Most of the literature on SPME for in-vivo
sampling is related to microbiological applica-
tions, monitoring of BVOCs emission from
plants and isolation of insect semiochemicals. It
should be pointed out that some clinical chem-
istry SPME applications could also be con-
sidered in-vivo bioanalysis; however, this subject
has been already discussed in great detail [25]
and will not be addressed here.
4.1. Microbiological applications
SPME has been applied to study the produc-
tion and the emission of food flavor and off-
flavor compounds produced by microorgan-
isms. Vergnais et al [26] monitored, using HS-
SPME and GC-FID, the production of flavor
compounds by the catabolic action of Staphylo-
coccus xylosus
and Staphylococcus carnosus (respon-
sible for some aromatic characteristics of dry
sausages) on culture media containing leucine.
The volatile metabolites were extracted for 15
min using 85 mm PA and 100 mm PDMS fibers.
Several analytes were found at nmol/L levels
(for example, 3-methylbutanal, 3-methylbutanol,
3-methylbutanoic acid, hexanal and aliphatic
esters).
Talon et al applied the same analytical proce-
dure [27] to compare the production of ethyl
esters by resting cells and extracellular con-
centrates of several Staphylococcus species; the
effects of different strains, temperatures and
media pH on the metabolism of these species
were also determined. It was stated that some
strains of S. warneri, S. xylosus and S. saprophyticus
produce mainly ethyl butanoate (200 to 300
nmol per gram of wet cells for a 4 h incubation
period); large amounts of ethyl valerate and
hexanoate were also produced by some of the
evaluated strains.
432
trends in analytical chemistry, vol. 21, no. 6+7, 2002

Nilsson et al [16] conducted a similar study on
the production of flavor substances by Peni-
cillium sp.
fungi. The volatile profiles of incu-
bated culture media, determined after 30 min
extraction with 100 mm PDMS fibers and ana-
lysed by GC-MS, were comparable to those
obtained with 14-day diffusive trapping with
Tenax cartridges and thermal desorption using a
commercial apparatus. Compounds such as 1-
octen-3-yl acetate, 3-octanol and several terpenes
(b-pinene, bornylene, terpinolene, camphor,
borneol, italicene, b-maaliene, longifolene, acor-
adiene, a-chamigrene and widdrol) were repor-
ted for the first time as metabolites for the
genus Penicillium.
Arnold and Senter [28] compared HS-SPME/
GC-MS and an ‘‘electronic nose’’ (an array of
polymer-based
microsensors
that
mimics
human olfactory sense, generating a complex
digitizable response) in assessment of off-fla-
vors released by some pathogenic bacteria (Sal-
monella enteridis
, Escherichia coli and others) found
in processed poultry. Indole and several alipha-
tic alcohols were identified in the headspace of
inoculated culture media after 30 min extrac-
tions with 100 mm PDMS fibers. The authors
suggest that the combination of HS-SPME and
‘‘electronic nose’’ data can be a powerful
tool for food-safety studies related to bacterial
contamination.
Application of HS-SPME to insect attractors
or repellers released by fungi and bacteria is
also of interest. The generation of chemical
attractors for Mexican fruit flies (Anastrepha
ludens
) in soy broth inoculated with Staphylo-
coccus aureus
[29], Klebsiella pneumoniae and
Citrobacter freundii
[30] was studied by HS-
SPME. Collection of volatiles was performed
with 5 min to 24 h headspace extractions
using 100 mm PDMS fibers; identification and
quantification of extracted materials was made
by GC-MS, GC-FID and GC-FTD. Com-
pared with volatile trapping by ORBO silica-
gel cartridges, a larger number of already
proven fly attractants were identified in the
HS-SPME extracts (ammonia, aromatic and
aliphatic
nitrogenated
compounds,
thio-
compounds, etc.).
Faldt et al [31] also employed HS-SPME and
GC-MS to monitor emission profiles of two
insect-attractor
fungi–Fomitopsis
pinicola
and
Fomes fomentarius
. In the sporulating phase of the
fungi, they observed a significant increase on
the emission rate of insect attractants, such
as (R)- and (S)-oct-1-en-3-ol and octan-3-one
for F. pinicola and octan-3-one, linalool oxide,
b-phellandrene and b-myrcene for F. fomentarius.
Additionally, the sesquiterperne b-barbatene
was identified for the first time as a fungal
metabolite. In a similar work [32], HS-SPME
was applied for analysis of attractor chemicals
(for example, 3-octen-1-ol) released by the
Ganoderma applanatum
; SPME was found to be
more sensitive for these samples than other
methodologies, such as adsorptive purge and
trap or absorption in cold methanol.
Finally, SPME in-vivo bioanalysis has also
been used in studies related to environmental
problems. The production of unsaturated ali-
phatic aldehydes by chrysophyte algae (Uroglena
americana
, Synedra delicatissima and others), which
are in part responsible for the disagreeable odor
of polluted surface waters, has already been
evaluated by SPME [19]. Concentrations up to
several mg L
-1
of malodorous compounds, such
as 2,4-heptadienal, were found after direct
extraction of aqueous samples with PDMS/
DVB fibers and GC-MS analysis; terpenoid
compounds, such as limonene, geosmin and
methylisoborneol, were also detected and iden-
tified as algal metabolites. The sensitivity of
this SPME method (detection limits up to 2 mg
L
-1
) is reported as comparable to, or better
than, that of the conventional liquid-liquid
extraction.
Eriksson et al [33] applied HS-SPME to
assess bacterial degradation of diesel fuel in soil
and water: they determined that the commercial
bacterial inoculum, LRC-1, was able to decom-
pose completely the diesel fuel compounds in 5
weeks (water) and 3 weeks (soil). Results for
very fast (10 s) headspace extraction of test
samples with 100 mm PDMS fibers were similar
to those obtained with the standard–and much
more
time-consuming–extraction
procedure
with pentane.
trends in analytical chemistry, vol. 21, no. 6+7, 2002
433

4.2. Plant BVOC monitoring
The study of plant BVOC emission is sig-
nificant for several reasons. Isoprene and other
volatile compounds released in large amounts
by plants are important in global atmospheric
chemistry [4]. Also, apart from the obvious
interest for the cosmetics industry, knowledge
about the composition and emission dynamics
of floral scents is interesting because of their
numerous roles in plant reproductive processes,
defense against predators and in intra-species
communication [34].
Vereen et al [35] applied SPME and GC-MS
to study BVOCs released by leaves of Fraser
firs (Abies fraseri). Live branches of fir were
enclosed inside 100 mL Tedlar bags and the air
inside was extracted with 100 mm PDMS fibers
for periods from 5 min to 4 h. Experiments
using intact and mechanically damaged leaves
were performed. For 5 min extractions, mono-
terpernes such as 3-carene are predominant; for
3 h extractions, the major component in the
chromatograms is bornyl acetate, and minor
amounts of heavier compounds (for example,
camphor
and
borneol),
not
detected
in
faster extractions, are observed. Two analytes,
b-phellandrene and g-terpinene, detected only
on the emission mix from damaged leaves, were
assigned as possible wound-response com-
pounds released from the plant. However, it
was pointed out that the inadequate precision
(RSD > 20%) of the assays performed and
slow equilibration times for heavier compounds
prevented quantitative application of SPME to
these samples.
Zini et al [15] employed HS-SPME and GC-
ITMS (ion trap mass spectrometry) to assess the
BVOC-emission profiles from both intact and
mechanically damaged live leaves of Eucalyptus
citriodora
. Leaves from live trees were enclosed in
the device shown in Fig. 4, and 1 min extrac-
tions with PDMS/DVB fibers were performed
every 30 min for continuous periods between 8
h and 10 h. The main BVOCs identified in the
samples were isoprene, citronellal, citronellol
and b-caryophyllene. Different patterns of
dependence between analyte peak areas and
leaf-enclosure times were observed; for exam-
ple, for rose oxide (cis-4-Methyl-2-(2-methyl-1-
propenyl)-tetrahydropyran), a maximum in the
area versus time curves appears after 300 min to
400 min of leaf enclosure; and, for citronellal,
the peak areas decay exponentially immediately
after the start of the experiments.
A similar study was conducted on live flow-
ering Boronia megastigma plants by MacTavish et
al [36]. Volatiles from specimens in vases and
placed inside glass or steel vessels were extrac-
ted for 30 min with 100 mm PDMS fibers; the
collected material was analysed by GC-MS and
GC-FID. The principal BVOCs identified were
a-pinene, 5-acetoxylinalool, dodecyl acetate, Z-
n-heptadec-8-ene and several b-ionone isomers.
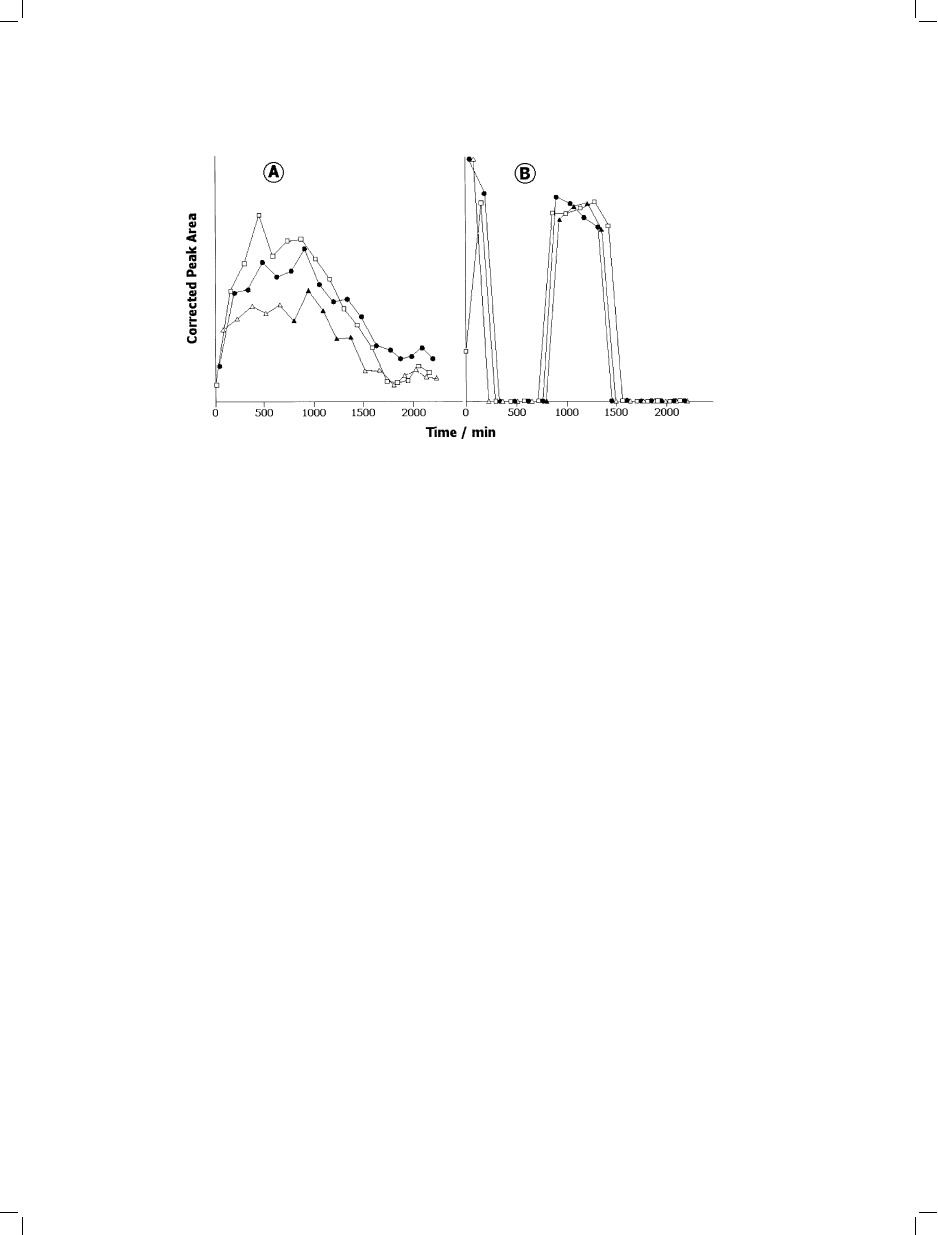
From the examination of emission profiles
under varied illumination conditions monitored
during 26 h periods of plant enclosure (Fig. 7),
it was ascertained that the emission pattern for
this
plant
is
both
endogenously
and
environmentally controlled.
Vercammen et al [37] evaluated two sorptive
extraction techniques—SPME with 100 mm
PDMS and 85 mm PA fibers and dynamic
trapping & thermal desorption (T&D) with
Tenax and PDMS packed sorption tubes—for
collection and GC-MS analysis of BVOCs
released by live rose and jasmine (Jasminum poly-
anthum
). For the SPME experiments, fibers were
exposed for 30 min to the air inside glass bell
jars containing the live plants; for dynamic
trapping, air from these jars (100 mL min
1
flow for 10 min) was passed through the sorp-
tive tubes and the collected materials were des-
orbed
in
sequence
using
a
commercial
apparatus. PDMS (either using SPME or T&D)
was assigned as the best extracting phase for the
evaluated samples, because of limited produc-
tion of artefacts and good analyte recovery.
However, the authors pointed out that T&D
was superior to SPME for these analyses: the
larger mass of sorbent on the trapping tubes
(300 mg PDMS less than 1 mg coating on a
100 mm PDMS SPME fiber) allowed recovery
of greater amounts of analytes; also, broadening
of chromatographic peaks was smaller in T&D
because of cryofocusing and fast desorption of
434
trends in analytical chemistry, vol. 21, no. 6+7, 2002
extracts provided by the commercial thermal
desorption device.
4.3. Isolation of insect semiochemicals
The
substances
used
for
long-range
communication between organisms are known
as semiochemicals, which are classified in two
groups - pheromones, which act in intra-species
communications, and allelochemicals, respon-
sible for inter-species signaling [38]. SPME has
been successfully applied to in-vivo studies of
such substances as a replacement for, or com-
plementary to, traditional sample preparation
techniques.
Sex pheromones from Phyllonorycter sylvella
moths were collected and identified by HS-
SPME using 100 mm PDMS fibers and GC-MS
[39]. For the extractions, the SPME fiber was
exposed for up to 3 h to the air close to the
abdominal secretory glands of single, live,
female moths. Comparing the profiles obtained
for signaling and non-signaling individuals, three
compounds were identified as the sex pher-
omones: Z-8- and E-10-tetradecenyl acetates
(for the first time assigned as attractants in the
genus Phyllonorycter), as well as Z-10-tetradecenyl
acetate, which was already a known attractor for
this genus. The results obtained are comparable
with the method previously used - extraction
with ultra-pure pentane of the excised glands
from 100 sacrificed moths. The same procedure
was further applied to study the periodicity of
the pheromone-release cycles of Ph. sylvella, Ph.
heegerella
and Ph. ulmifoliella moths [40].
Rochat et al [41] also employed HS-SPME to
quantify volatile sex pheromones released by
live, individual, Strategus aloeus beetles (Fig. 8).
Extractions were performed exposing 75 mm
CAR / PDMS fibers for 5 min to the air in the
entrance of the galleries dug into soil by these
beetles and containing one specimen inside.
Each extraction was able to collect (0.34 0.06)
mg of a pheromone mixture containing 95.5%
2-butanone, 4.0% 3-pentanone and 0.5% sec-
butyl acetate. Another insect, the pheromone
blend of which was studied by HS-SPME, was
the phytophagous Nezara viridula, an important
pest that attacks soybean and other crops [14].
Some of the most innovative applications of
SPME to in-vivo analysis of insect semi-
ochemicals involve extraction of analytes with
varied volatilities by direct contact of the fiber
with the live specimen. The small dimensions of
the SPME fibers (the length of commercial
fibers is 10 mm and their diameters range from
124 mm to 300 mm, depending on the coating
thickness [42]) allow them to be rubbed in the
Fig. 7. Dependence of corrected peak areas (peak area per plant) on plant-enclosure time for Boronia megastigma flowered plants
(modified from [36]). A=5-acetoxy-linalool; B=a-pinene. *=continuous darkness; &=continuous illumination; and, ~,
~=alternating darkness and illumination.
trends in analytical chemistry, vol. 21, no. 6+7, 2002
435
insect cuticle or directly inserted inside secretion
glands through external openings, with reduced
damage and stress to the specimen. This strat-
egy was applied by Fre´rot et al [43] to investi-
gate the sex-pheromone mix produced by single
individuals of Sesamia nonagriodes, an important
pest for Mediterranean maize crops. The secre-
tion gland of live, calling females was extruded
by gentle pressure on the abdomen of the
insects, a 7 mm PDMS fiber was carefully rub-
bed for 5 min in the tegument of the glandular
area and the extracts analysed by GC-MS. The
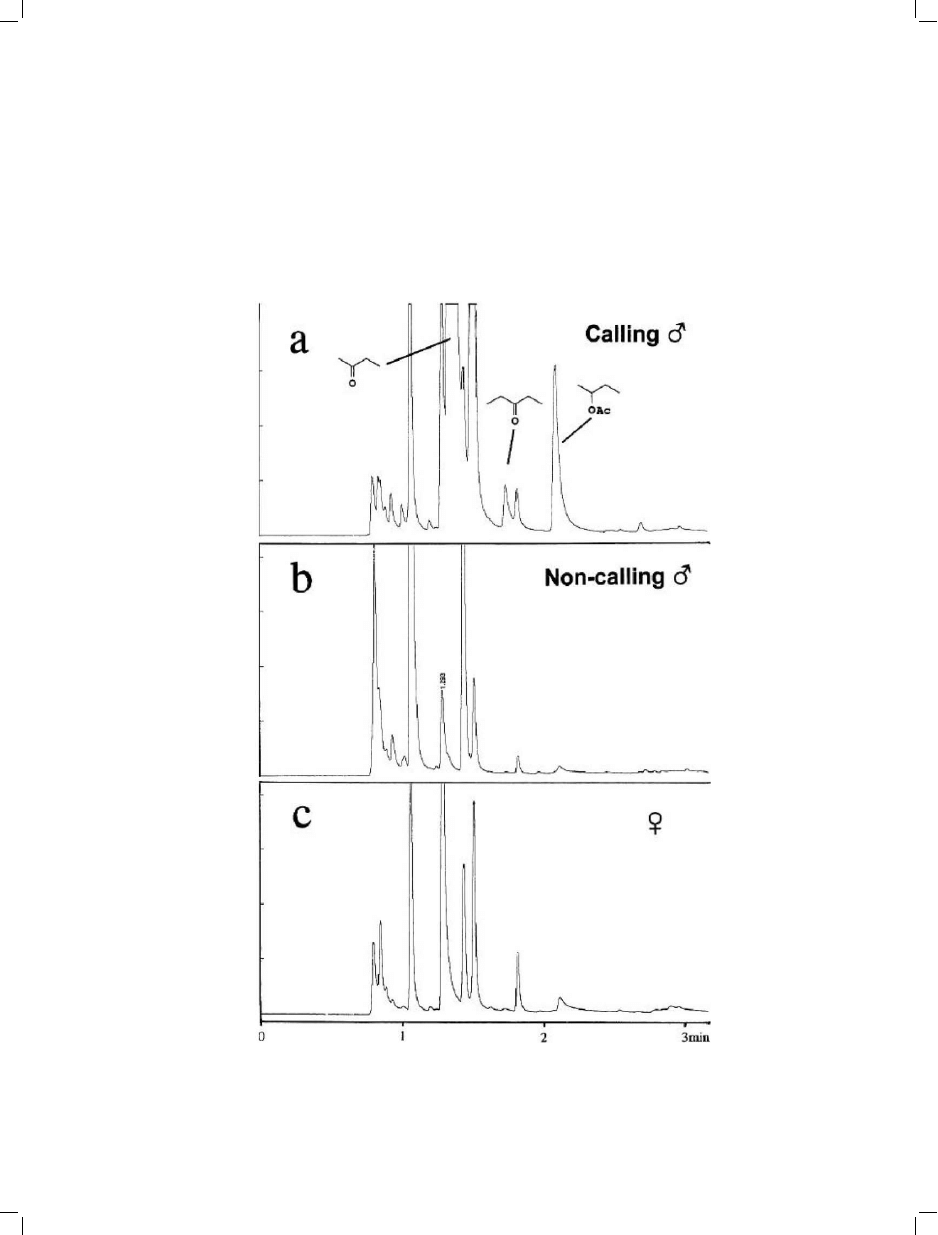
Fig. 8. Gas chromatography-mass spectrometry chromatograms for volatiles extracted with PDMS/CAR fibers from single
Strategus aloeus beetles: a=calling male; b=non-calling male; and, c=female. (Reprinted from [41]).
436
trends in analytical chemistry, vol. 21, no. 6+7, 2002

average recovery of pheromone mix (Z-11-hex-
adecen-1-ol, Z11-hexadecenyl acetate and hex-
adecanyl acetate) was 120 ng/individual by
SPME and 60 ng/individual for the standard
method previously employed (extraction of two
excised glands with 20 mL of diethylether).
Direct extraction with SPME was also
employed to study pheromones produced by
Brazilian ‘‘dinosaur’’ ants, Dinoponera quadriceps
[44,45]. Communication between individuals in
‘‘dinosaur’’ ant colonies is accomplished when
they scratch their antennae in the tegument of
other ants; the status of a specimen is assigned
by the composition of the mixture of cuticular
hydrocarbons sensed by the antennae. For
SPME extraction of these hydrocarbons, live
ants were immobilized, gently forced to bend
and 7 mm PDMS fiber was rubbed for 2 min in
the exposed intertergital membrane. Statistical
analysis of GC-MS data from SPME extracts
proved that the hydrocarbon 9-hentriacontane
was, for the most part, responsible by the dif-
ferentiation between the several categories of
ants (mated dominant males, virgin dominant
males, young sterile workers and old sterile
workers).
A similar study on the social wasp Polistes
dominulus
was conducted by Sledge et al [46].
Pheromones from these wasps (heavy alcohols,
hydrocarbons and acids) were extracted by
introducing a 7 mm PDMS fiber directly inside
the secreting gland of live specimens. The
results were comparable to those obtained with
HS-SPME
and solvent-washing extractions
from excised glands, which demand the sacrifice
of sampled individuals.
5. Final remarks and conclusions
SPME has been applied successfully to a wide
range of analytical applications involving sam-
pling of living organisms. For the majority of
the examples mentioned above, the sensitivity
and the precision provided by SPME were
comparable to, or better than, those of the
techniques traditionally employed for the same
samples. Moreover, some of these applications
(such as direct extraction of pheromones with
high molecular weight from live insects) would
not be feasible using other sample-preparation
techniques, since they would cause severe
damage to the live organisms or would demand
their sacrifice. Also, the minimal equipment
necessary for SPME and the basic training
needed for its use are reduced—characteristics
that can be especially suitable for biological
research, since the typical personnel involved
frequently have a limited background in analy-
tical chemistry. Finally, in contrast with alter-
native techniques that are sometimes applied to
in-vivo
biochemical analysis (such as dynamic
headspace trapping and thermal desorption),
SPME does not demand dedicated analytical
instruments or expensive commercial sample-
preparation devices, which may not be acces-
sible to most laboratories around the world.
SPME is a young technique, and some fea-
tures, still not fully investigated, could have a
remarkable impact on its future application for
in-vivo
bioanalysis. Coupling of SPME to
separation and detection techniques other than
GC and HPLC is a topic under study, and
combinations, such as SPME/capillary electro-
phoresis [47], could be powerful tools for spe-
cies of biological interest, such as proteins and
enzymes. Also, development of new dedicated
SPME devices for field analysis [48] could be
important for some in-vivo applications in future.
Acknowledgements
The authors would like to thank Carol H.
Collins for reviewing this manuscript.
References
[1] L. Tollsten, G. Bergstrom, Phytochemistry 27 (1988) 4013.
[2] J. Takabayashi, S. Takahashi, M. Dicke, M.A. Posthumus,
J. Chem. Ecol. 21 (1995) 273.
[3] J.B.F. Gervlieet, M.A. Posthumus, L.E.M. Vet, M. Dicke,
J. Chem. Ecol. 23 (1997) 2395.
[4] J. Kesselmeier, M. Staudt, J. Atmos. Chem. 33 (1999) 23.
[5] J.P.F.G. Helsper, J.A. Davies, H.J. Boumeester, A.F. Krol,
M.H. van Kampen, Planta 207 (1998) 88.
trends in analytical chemistry, vol. 21, no. 6+7, 2002
437

[6] J. Pawliszyn (Editor), Applications of Solid Phase Micro-
extraction, RSC, Cambridge, UK, 1999.
[7] J. Pawliszyn (Editor), Solid Phase Microextraction: Theory
and Practice, Wiley-VCH, New York, USA, 1997, p. 43.
[8] Supelco Inc., Solid Phase Microextraction: Theory and
Optimization of Conditions (Bulletin 923), Supelco, Belle-
fonte, PA, USA, 1998, p. 1.
[9] Z.Y. Zhang, M.J. Yang, J. Pawliszyn, Anal. Chem. 66
(1994) 844A.
[10] J. Chen, J. Pawliszyn, Anal. Chem. 67 (1995) 2530.
[11] Supelco Inc., SPME Applications Guide, Supelco, Belle-
fonte, PA, USA, 2000, p. 64.
[12] R. Eisert, J. Pawliszyn, Crit. Rev. Anal. Chem. 27 (1997)
103.
[13] A. Matich in J. Pawliszyn (Editor), Applications of Solid
Phase Microextraction, RSC, Cambridge, UK, 1999, pp.
349–350.
[14] P. Damiani, L. Cossignani, M. Castellini, F. Bin, Ital. J.
Food Sci. 12 (2000) 189.
[15] C.A. Zini, E. Christensen, E.B. Carama˜o, F. Augusto, J.
Pawliszyn, [submitted to Anal. Chem., 2001 – published?].
[16] T. Nilsson, T.O. Larsen, L. Montanarella, J.Ø. Masen, J.
Microbiol. Meth. 25 (1996) 245.
[17] G. Thodoridis, E.H.M. Koster, C.J. de Jong, J. Chroma-
togr. B 745 (2000) 49.
[18] C. Bicchi, S. Drigo, P. Rubiolo, J. Chromatogr. A 892
(2000) 469.
[19] S.B. Watson, B. Brownlee, T. Satchwill, E. McCauley, Wat.
Sci. Tech. 40 (1999) 251.
[20] S. Goupry, N. Rochut, R.J. Robins, E. Gentil, J. Agric.
Food Chem. 48 (2000) 2222.
[21] T. Gorecki, X. Yu, J. Pawliszyn, Analyst 127 (1999) 643.
[22] F. Augusto, J. Koziel, J. Pawliszyn, Anal. Chem. 73 (2001)
481.
[23] K. Sukola, J. Koziel, F. Augusto, J. Pawliszyn, Anal.
Chem. 72 (2001) 13.
[24] S.J. Pirt, Principles of Microbe and Cell Cultivation,
Blackwell Scientific, Oxford, UK, 1975, p. 143.
[25] H. Lord, J. Pawliszyn, J. Chromatogr. A 902 (2000) 17.
[26] L. Vergnais, F. Masson, M.C. Montel, J.L. Berdague´,
R. Talon, J. Agric. Food Chem. 46 (1998) 228.
[27] R. Talon, L. Vergnais, M.C. Montel, J.L. Berdague´, Int. J.
Food Microbiol. 45 (1998) 143.
[28] J.W. Arnold, S.D. Senter, J. Sci. Food Agric. 78 (1998) 343.
[29] D.C. Robacker, R.A. Flath, J. Chem. Ecol. 21 (1995) 1861.
[30] D.C. Robacker, J. Bartelt, J. Chem. Ecol. 23 (1997) 2897.
[31] J. Faldt, J.M. Jonsell, G. Nordlander, A.-K. Borg-Karlson,
J. Chem. Ecol. 25 (1999) 567.
[32] J. Faldt, M. Eriksson, I. Valterova, A.-K. Borg-Karlson, Z.
Naturforsch. C: J. Biosci. 55 (2000) 180.
[33] M. Ericksson, A. Swartling, G. Dalhammar, Appl. Micro-
biol. Biotechnol. 50 (1998) 129.
[34] N. Dudareva, E. Pichersky, Plant Physiol. 122 (2000) 627.
[35] D.A. Vereen, J.P. McCall, D.J. Butcher, Microchem. J. 65
(2000) 269.
[36] H.S. MacTavish, N.W. Davies, R.C. Menary, Ann. Bot. 82
(2000) 347.
[37] J. Vercammen, P. Sandra, E. Balthussen, T. Sandra,
F. David, J. High Resol. Chromatog. 23 (2000) 547.
[38] D.R. Kelly, Chem. Br. 26 (1990) 124.
[39] A.-K. Borg-Karlson, R. Mozuraitis, Z. Naturforsch. C: J.
Biosci. 51 (1996) 599.
[40] R. Mozuraitis, V. Buda, V. Jonusaite, A.-K. Borg-Karlson,
R. Noreika, Entom. Exper. Applic. 94 (2000) 15.
[41] D. Rochat, P. Ramirez-Lucas, C. Malosse, R. Aldana,
T. Kakul, J.P. Morin, J. Chromatogr. A 885 (2000) 433.
[42] V. Mani in J. Pawliszyn (Editor), Applications of Solid
Phase Microextraction, RSC, Cambridge, UK, 1999,
p.60.
[43] B. Fre´rot, C. Malosse, A.-H. Cain, J. High Resolut. Chro-
matogr. 20 (1997) 340.
[44] T. Monnin, C. Malosse, C. Peeters, J. Chem. Ecol. 24
(1998) 473.
[45] C. Peeters, T. Monnin, C. Malosse, Proc. R. Soc. London
266 (1999) 1323.
[46] M. Sledge, G. Moneti, G. Pieraccini, S. Turillazzi, J.
Chromatogr. A 873 (2000) 73.
[47] C.W. Whang, J. Pawliszyn, Anal. Commun. 35 (1998) 353.
[48] L. Mu¨ller in J. Pawliszyn (Editor), Applications of Solid
Phase Microextraction, RSC, Cambridge, UK, 1999, p.
269.
Fabio Augusto and Antonio Luiz Pires Valente received their doc-
toral degrees from the State University of Campinas (Unicamp) in
1997 and 1984, respectively, and presently are part of the faculty
of the Institute of Chemistry of the State University of Campinas,
Sa˜o Paulo, Brazil. Their research interests include sample-pre-
paration techniques (SPME, MESI), atomic emission detection for
GC, and instrumental developments for TLC.
438
trends in analytical chemistry, vol. 21, no. 6+7, 2002
Wyszukiwarka
Podobne podstrony:
Application of solid phase microextraction to the analysis o
Application of Solid Phase Microextraction Gas Chromatograp
application of solid state fermentation to food industry a review
Solid phase microextraction to concentrate volatile products
Optimisation of solid phase microextraction of volatiles
Solid Phase Microextraction Analyses of Flavor Compounds in
Headspace solid phase microextraction profiling of volatile
A Practical Guide to Quantitation with Solid Phase Microextr
Solid phase microextraction for the detection of termite cut
Kinetics of solid phase extraction and solid phase microextr
Comparison of Different Fibers in the Solid Phase Microextra
Solid phase microextraction a powerful sample preparation to
Solid phase microextraction coupled to gas chromatography a
Development of a headspace solid phase microextraction–gas c
Solid phase microextraction for the analysis of biological s
Kinesiotherapy is the application of scientifically?sed exercise principles?apted to enhance the str
Solid phase microextraction as a clean up and preconcentrati
Solid phase microextraction as a tool for trace element spec
więcej podobnych podstron