
International Journal of Inorganic Materials 2 (2000) 451–454
Change in antibacterial characteristics with doping amount of ZnO in
MgO–ZnO solid solution
*
Osamu Yamamoto , Jun Sawai, Tadashi Sasamoto
Department of Applied Chemistry
, Kanagawa Institute of Technology, 1030 Shimo-ogino, Atsugi-shi, 243-0292 Japan
Accepted 11 May 2000
Abstract
Antibacterial activity for MgO–ZnO solid solution was studied by measuring the change in electrical conductivity with bacterial
growth. MgO–ZnO solid solution powders were prepared by heating at 14008C for 3 h in air. A single phase with cubic type structure was
obtained in the weight ratio range (MgO / ZnO) of 4.0 and 1.5, but the ratio of 0.67 resulted in a ZnO phase in addition to solid solution.
After milling the solid solution powders by planetary ball mill, the average particle size and the specific surface area of these powders
2
became 0.1 mm and 26 m / g, respectively, which were used in the test of antibacterial activity. From the results of antibacterial tests, the
activity increased with increasing the powder concentration in the medium. With increasing the doping amount of ZnO in MgO–ZnO
solid solution, it was found to show a decrease in the antibacterial activity against Escherichia coli and Staphylococcus aureus. The pH
value in physiological saline at the powder concentration of 2.5 mg / ml showed the alkali region above 10.0, and decreased with the
increase of ZnO amount in solid solution. The decrease in antibacterial activity, therefore, was associated with the decrease of pH value in
medium
2000 Elsevier Science Ltd. All rights reserved.
Keywords
: MgO; ZnO; Solid solution; Antibacterial activity
1. Introduction
cancellation in activity, because these three ceramics are
known to form solid solutions easily.
Microbial pollution and contamination that take place by
In the present work, MgO–ZnO solid solution powders
microorganisms have produced various problems in indus-
were prepared with the various weight ratios (MgO / ZnO)
try and other vital fields, including medicine, such as
at 14008C for 3 h in air. After preparing the slurries of the
degradation and infection, etc. In order to solve these
powders obtained, the change in antibacterial activity as a
problems, therefore, new pasteurization and antibacterial
function of MgO–ZnO composition in the solid solution
techniques have been demanded and studied [1–3].
was studied by measuring the change in electrical con-
Recently, the occurrence of antibacterial activity by
ductivity with bacterial growth.
using ceramic powders has been pointed out with much
attention as a new technique that can substitute for
conventional ones using organic agents. Ceramic powders
2. Experimental
of zinc oxide (ZnO), calcium oxide (CaO) and magnesium
oxide (MgO) were found to show a marked antibacterial
2.1. Preparation of samples and test bacteria
activity without the presence of light [4–7]. The use of
these ceramics has the following advantages; mineral
MgO and ZnO powders of reagent grade were used as
elements essential to the human body and strong anti-
starting materials. These powders were mixed with differ-
bacterial activity in small amount without the irradiation of
ent weight ratio (MgO / ZnO) and then heated at 14008C
light [8–10]. However, it is not yet clear what change in
for 3 h in air. The as-prepared powder samples of MgO–
antibacterial activity is expected by the formation of solid
ZnO solid solution were milled by planetary ball mill. The
solution among these powders, either reinforcement or
sample code and the chemical composition of solid
solution are listed in Table 1. In order to examine the
formation of solid solution, X-ray diffraction measurement
*Corresponding author. Tel.: 181-46-291-3148; fax: 181-46-242-
8760.
(XRD) was carried out and the specific surface area of
1466-6049 / 00 / $ – see front matter
2000 Elsevier Science Ltd. All rights reserved.
P I I : S 1 4 6 6 - 6 0 4 9 ( 0 0 ) 0 0 0 4 5 - 3
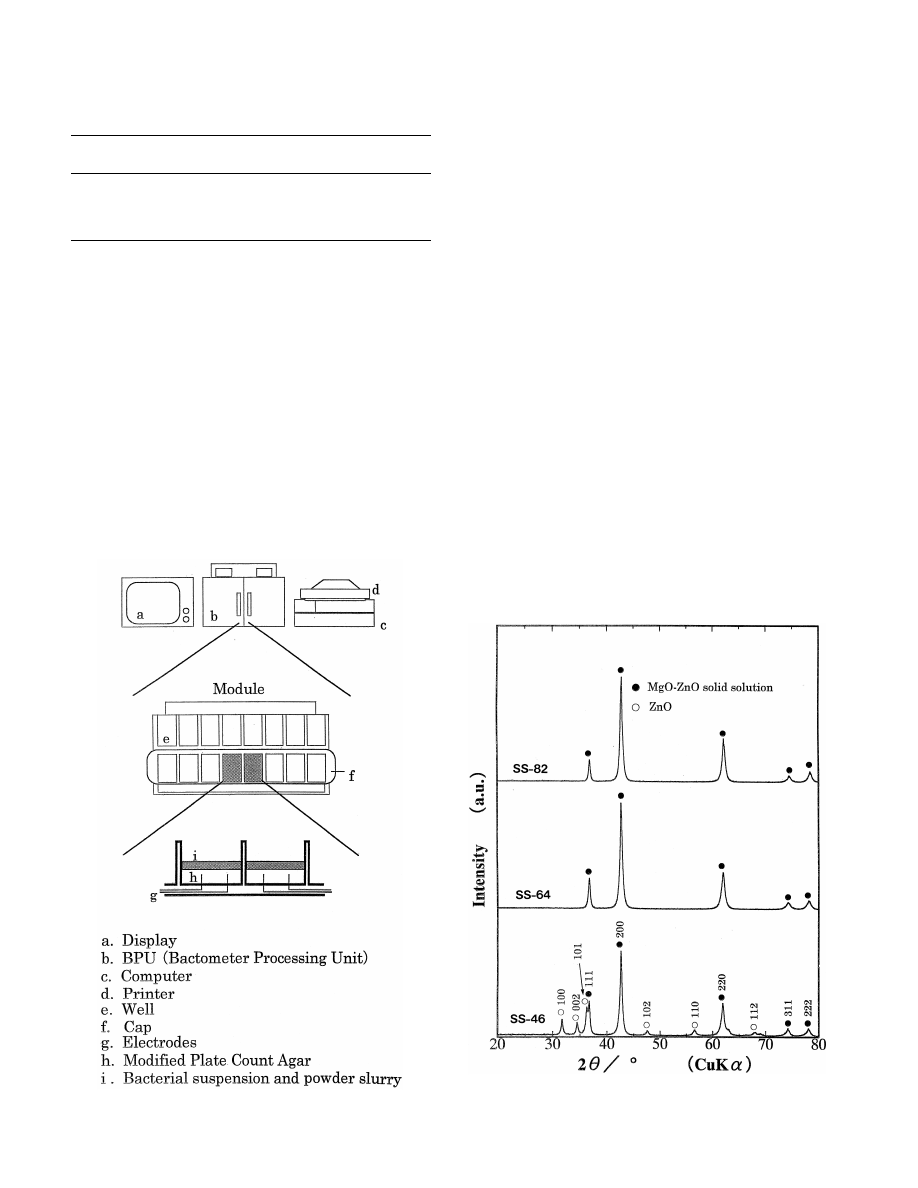
452
O
. Yamamoto et al. / International Journal of Inorganic Materials 2 (2000) 451 –454
Table 1
measuring the change in electrical conductivity with
Sample code and chemical composition of solid solution powders used in
bacterial growth. The apparatus for measuring the con-
this study
ductivity was Bactometer Microbial Monitoring System
Sample code
Mass ratio
Model 64 (company: bioMerieux) as shown in Fig. 1.
MgO:ZnO
Preparation of bacteria into the wells of a module for the
SS-10
1:0
Bactometer was carried out as follows; adding the powder
SS-82
8:2
samples into the well containing Modified Plate Count
SS-64
6:4
Agar (MPCA) and then dispensing the bacterium suspen-
SS-46
4:6
sion into the well. After setting the module in the
Bactometer, the change in electrical conductivity was
powder samples was measured. The powder samples
monitored during the incubation at 378C for 25 h in a dark
obtained were suspended with physiological saline in the
place. The details of the procedures were reported in a
concentration range from 1.6 to 100 mg / ml and then the
previous paper [4].
slurries prepared were used in antibacterial tests.
In order to examine indirectly the pH values when the
Staphylococcus aureus 9779 (S
. aureus) and Escherichia
powder samples were added into the well, the samples
coli 745 (E
. coli) were used as test bacteria and stored at
were dispersed into physiological saline at a powder
Tokyo Metropolitan Research Laboratory of Public Health.
concentration of 2.5 mg / ml. After keeping the dispersed
These bacteria were cultured in Brain Heat Infusion (BHI)
solutions for 24 h, the pH values of physiological saline
at 37C for 24 h on a reciprocal shaker. The bacterial
were measured.
culture was suspended in a sterile physiological saline with
3
a final concentration of approximately 10 CFU / ml (CFU:
Colony Forming Unit).
3. Results
2.2. Test of antibacterial activity
3.1. Powder samples
Antibacterial activity of powder samples was judged by
In Fig. 2, XRD patterns of the powder samples are
shown. A single phase of solid solution with NaCl type
structure was formed in SS-82 and SS-64 samples. For the
SS-46 sample, however, ZnO with the hexagonal wurtzite
Fig. 2. XRD patterns of MgO–ZnO solid solution heated at 14008C for 3
Fig. 1. Schematic illustration of the apparatus used in antibacterial test.
h in air.
O
. Yamamoto et al. / International Journal of Inorganic Materials 2 (2000) 451 –454
453
7
type structure coexisted with the solid solution of the cubic
change occurs at the bacterial concentration of about 10
phase with the NaCl type structure. This result agreed with
CFU / ml in the medium.
the phase diagram [11]. A diffraction line of solid solution
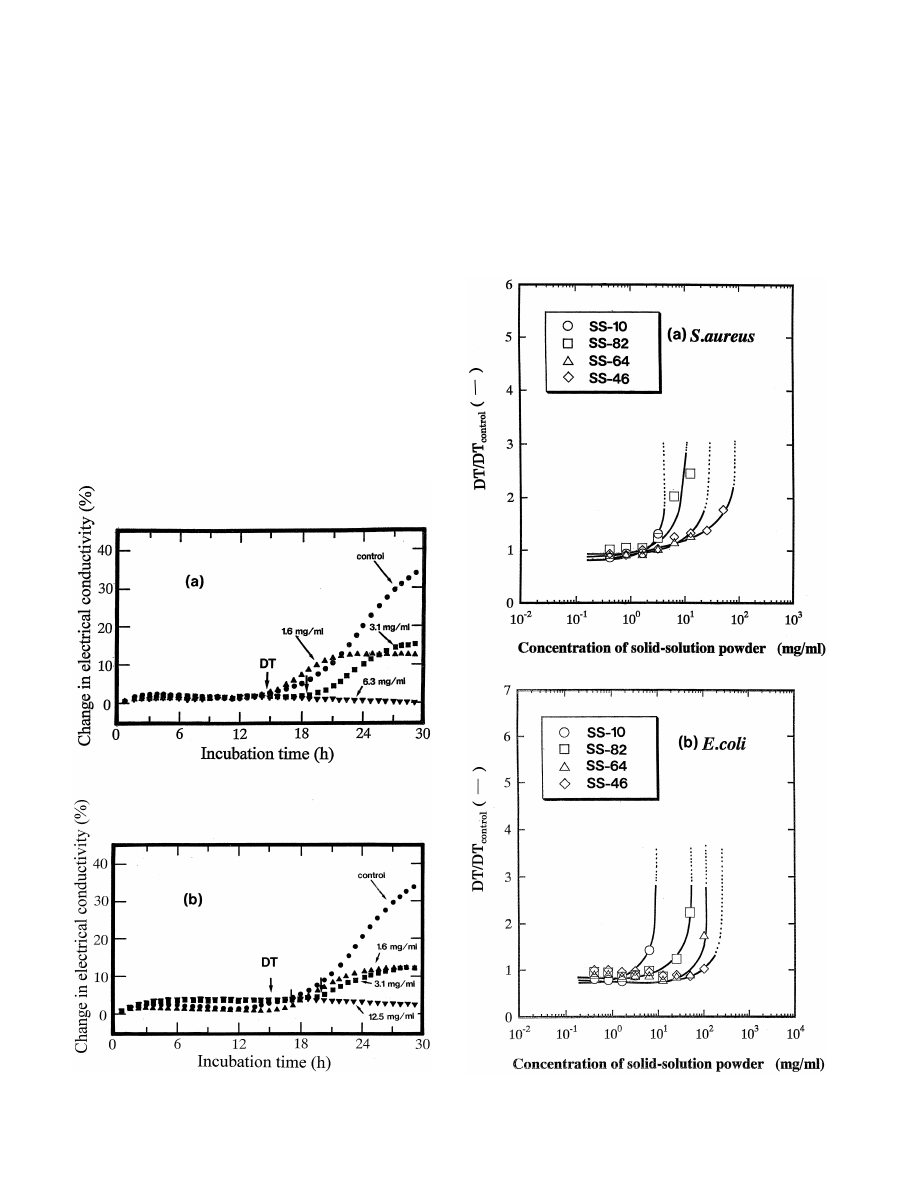
Fig. 3(a) and (b) show the changes in electrical con-
with the index of 200 shifted to high-angle side with
ductivity of SS-10 and SS-64, respectively, with the
increasing the amount of ZnO and the lattice constant of
incubation time of S
. aureus. In the figures, DT (Detection
the cubic phase changed from 0.211 to 0.204 nm. This
Time) shows the incubation time at which an electrical
21
shift is considered to be due to the replacement of Mg
change can be detected. Hence, if the value of DT is
21
ions (ion radius: 0.065 nm) with larger Zn
ions (ion
delayed by adding the powder samples, it can be judged
radius: 0.074 m). ZnO detected in SS-46 sample seems to
that the samples have the effect of an inhibition of the
be due to excess ZnO in the formation of solid solution.
After milling the powder samples by planetary ball mill,
it was found that the specific surface area and particle size
2
was about 26 m / g and 0.1 mm, respectively.
3.2. Antibacterial activity of powder samples
With the growth of bacteria, it is known that electrolytes
such as organic and amino acids are produced with the
digestion of proteins in the medium [12]. The electrical
conductivity in such a growth medium, therefore, increases
with an increase of the electrolytes produced, of which the
Fig. 3. The change in electrical conductivity with incubation time on S
.
Fig. 4. Comparison of antibacterial activity against (a) S
. aureus and (b)
aureus. (a) SS-10 and (b) SS-64 sample.
E
. coli by adding powder samples.

454
O
. Yamamoto et al. / International Journal of Inorganic Materials 2 (2000) 451 –454
bacterial growth. In the case of no addition of SS-10
ZnO in MgO–ZnO solid solution. From this result, it is
(control), the DT value was approximately 15 h. By adding
found that bacteria growth is inhibited with increasing the
SS-10, however, the DT value increased with the increase
pH value in the medium.
of powder concentration and no DT value was detected in
Sawai et al. [16] have even found the generation of
2
the powder concentration of 6.3 mg / ml (see Fig. 3(a)). The
super-oxide, O , from the surface of MgO and considered
2
change of DT value of SS-64 was similar to that of SS-10
to have an effective activity for the inhibition of bacterial
and no DT was observed in powder concentration of 12.5
growth. And also, it has been known that the super-oxide
mg / ml (see Fig. 3(b)). The results indicate an increase in
is stable under alkali region and then the diffuse distance
the antibacterial activity against S
. aureus by increasing
of super-oxide increases with increasing pH value [17]. In
the powder concentration in medium.
the present work, therefore, super-oxide is expected to
Based on the change in electrical conductivity described
generate from the surface of MgO–ZnO solid solution.
above, the antibacterial activity of all powder samples
Based on the above discussion, the decrease in the
prepared was examined on two bacteria, S
. aureus and E.
antibacterial activity with increasing ZnO content in solid
coli.
solution is anticipated to be due to the decrease of stability
2
Fig. 4(a) and (b) show the comparison of antibacterial
of O
generated from the surface of the solid-solution and
2
activity of four samples on S
. aureus and E. coli, respec-
the decrease of pH value in the medium.
tively. The vertical axis, ‘DT / DT
’ represents the ratio
In conclusion, by measuring the change in electrical
control
of the DT value at specified concentration of powder
conductivity with bacterial growth, it was found that the
samples to that at no addition of powder samples (control).
antibacterial activity against Escherichia coli and Staphy-
If the values of DT / DT
are changed with a steep rise
lococcus aureus decreased by increasing the doping
control
at the lower powder concentration, it can be judged to
amount of ZnO in MgO–ZnO solid solution.
show the stronger antibacterial activity. As shown in Fig.
4(a), with the increase of ZnO amount in solid solution, the
pronounced change of the value is observed at high
Acknowledgements
powder concentration, that is, the decrease of ZnO in solid
solution results in an effective antibacterial activity on S
.
The authors thank Professor Michio Inagaki of Aichi
aureus. In E
. coli (see Fig. 4(b)), the change of DT /
Institute of Technology for his discussion and encourage-
DT
value occurs at a little higher powder concen-
control
ment.
tration with the increase of ZnO amount in solid solution
than in S
. aureus. The change of antibacterial activity of
powder samples on E
. coli was similar to those on S.
aureus.
References
The pH value in physiological saline dispersing powder
samples was examined, in order to know the pH value in
[1] Kusaka T, Takagi Y. J Antibact Antifung Agents 1992;20:451.
medium. The value in saline at the powder concentration
[2] Saito M. J Antibact Antifung Agents 1993;21:17.
[3] Tsunoda Y, Egawa H, Yuge O. J Antibact Antifung Agents
of 2.5 mg / ml showed pH510.7 in SS-10 sample, 10.5 in
1992;20:571.
SS-82 sample, 10.4 in SS-64 sample and 10.1 in SS-46
[4] Yamamoto O, Hotta M, Sawai J, Sasamoto T, Kojima H. J Ceram
sample.
Soc Jpn 1998;106:1007.
[5] Sawai J, Igarashi H, Hashimoto A, Kokugan T, Shimizu M. J Chem
Eng Jpn 1995;28:288.
4. Discussion
[6] Sawai J, Kawada E, Kanou F, Igarashi H, Hashimo A, Kokugan T,
Shimizu M. J Chem Eng Jpn 1996;29:251.
[7] Yamamoto T, Uchida M, Kurihara Y. J Antibact Antifung Agents
The following four factors may affect the antibacterial
1991;19:425.
activity of ceramic powders: (1) the cations eluted from
[8] Kurihara Y. New Ceramics 1996;1996:39.
powder, (2) active oxygen generated from powder, (3) the
[9] Yamamoto O, Sawai J, Hotta M, Kojima H, Sasamoto T. J Mater
pH value, and (4) the mechanical destruction of cell
Sci Soc Jpn 1998;35:258.
[10] Sawai J, Yamamoto O, Hotta M, Kojima H, Sasamoto T. J Chem
membrane [4,6,9,13,14]. However, Yamamoto et al. [4,13]
Soc Jpn 1998;1998:633.
and Sawai et al. [14] reported that factors (1) and (4) had
[11] Segnit ER, Holland AE. J Am Ceram Soc 1965;43:412.
no effect on the activity. In the case of MgO powder, it has
[12] Oya A, Banse T, Ohashi F, Otani S. Appl Clay Sic 1991;6:135.
been reported that the pH value in physiological saline
[13] Yamamoto O, Sawai J, Ishimura N, Kojima H, Sasamoto T. J Ceram
increases with the increase of powder concentration and
Soc Jpn 1999;107:853.
[14] Sawai J, Kojima H, Igarashi H, Hashimoto A, Shoji S, Kokugan T,
antibacterial activity appears with increasing value of pH,
Shimizu M. J Ferment Bioeng 1998;86:521.
i.e., under alkali above pH510 [15,16]. Therefore, it is
[15] Ohkouchi S, Murata R, Ishihara Y, Maeda N, Moriyoshi Y. Inorg
essential to examine the pH value in physiological saline
Mater 1996;3:111.
dispersed powder samples. In the results of the examina-
[16] Sawai J, Kawada E, Kanou F, Igarashi H, Hashimoto A, Kokugan T,
tion, the pH value in saline at the powder concentration of
Shimizu M. J Chem Eng Jpn 1996;29:627.
2.5 mg / ml decreases with an increase in doping amount of
[17] Kobayashi K. Protein, Nucleic Acid and Enzyme 1988;33:2678.
Wyszukiwarka
Podobne podstrony:
Lord of the Flies Character Changes in the Story
Changes in Negative Affect Following Pain (vs Nonpainful) Stimulation in Individuals With and Withou
Changes in Brain Function of Depressed Subjects During
Changes in the quality of bank credit in Poland 2010
Changes in Levels of Phosphorus Metabolites in Temporal Lobes of Drug Naive Schizophrenic Patients
16 Changes in sea surface temperature of the South Baltic Sea (1854 2005)
Hypothesized Mechanisms of Change in Cognitive Therapy for Borderline Personality Disorder
#0804 – Dealing with Time Changes in Scheduling
Woziwoda, Beata; Kopeć, Dominik Changes in the silver fir forest vegetation 50 years after cessatio
Jażdżewska, Iwona The Warsaw – Lodz Duopolis in the light of the changes in the urban population de
Delay in diphtheria, pertussis, tetanus vaccination is associated with a reduced risk of childhood a
Bondeson; Aristotle on Responsibility for Ones Character and the Possibility of Character Change
Biomechanic Changes in Passive Properties of Hemiplegic
19 Mechanisms of Change in Grammaticization The Role of Frequency
Stages of change in dialectical behaviour therapy for BPD
Changes in human gut flora with age
Communist Propaganda Charging United States with the Use of BW in Korea, 20 August 1951 (biological
więcej podobnych podstron