Journal of Ethnopharmacology 103 (2006) 455–460
Effect of Cistus laurifolius L. leaf extracts and flavonoids on
acetaminophen-induced hepatotoxicity in mice
Esra K¨upeli
, Didem Deliorman Orhan, Erdem Yesilada
Gazi University, Faculty of Pharmacy, Department of Pharmacognosy, Hipodrom 06330, Ankara, Turkey
Received 26 April 2005; received in revised form 15 August 2005; accepted 19 August 2005
Available online 10 October 2005
Abstract
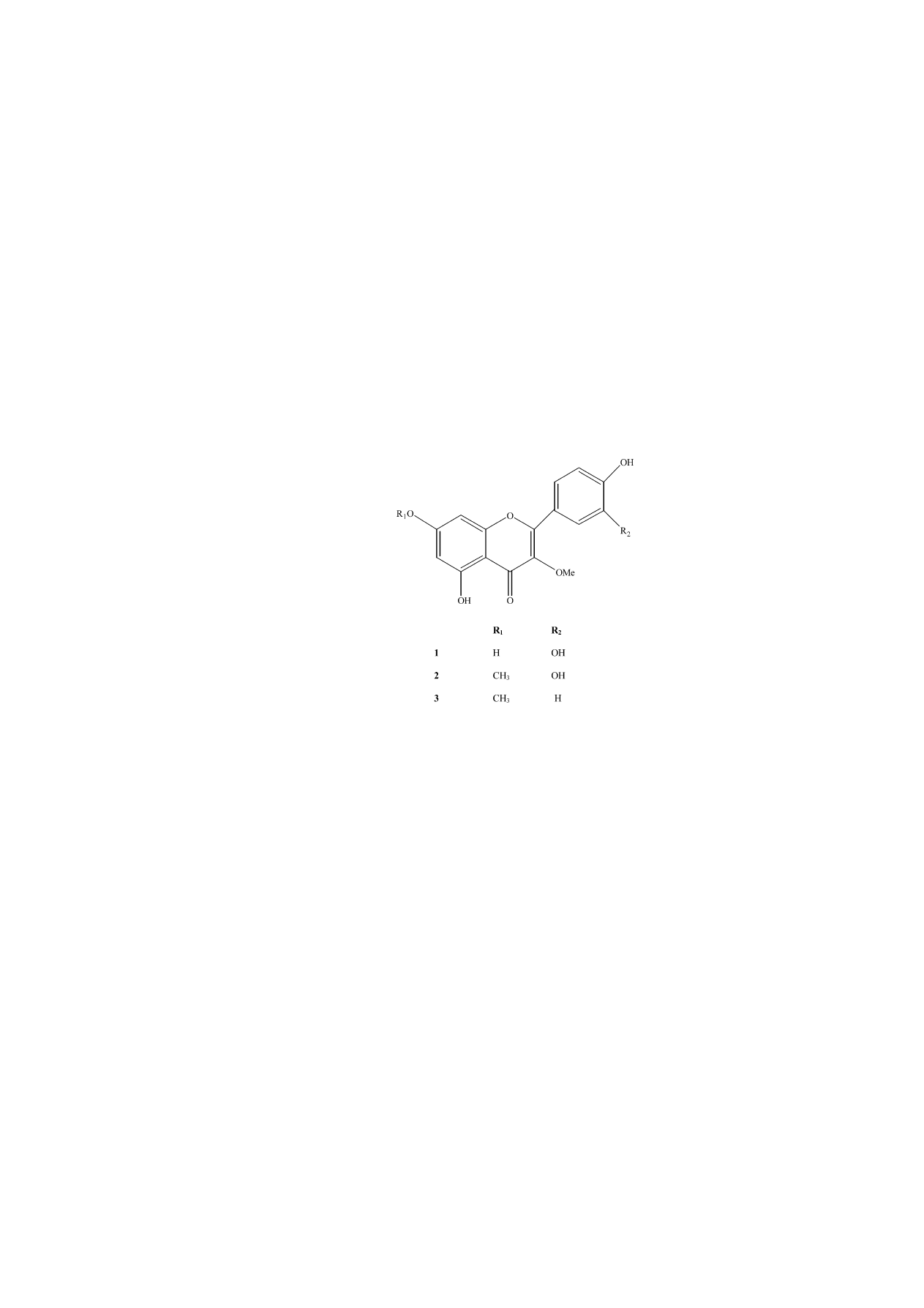
In this study, the effect of the flavonoids quercetin-3-methyl-ether (isorhamnetin) (1), quercetin-3,7-dimethyl-ether (2) and kaempferol-3,7-
dimethyl-ether (3) isolated from Cistus laurifolius L. (cistaceae) leaves was assessed on lipid peroxidation (liver and plasma), cellular glutathione
(GSH) level and plasma AST (aspartate aminotransferase), ALT (alanine aminotransferase) enzyme activities in acetaminophen-induced liver
damage in mice. At 114 mg/kg oral dose quercetin-3,7-dimethyl-ether was shown to possess potent antioxidative activity.
© 2005 Elsevier Ireland Ltd. All rights reserved.
Keywords: Cistus laurifolius; Cistaceae; Flavonoid; Hepatotoxicity; Lipid peroxidation; Serum transaminase enzymes; Cellular GSH
1. Introduction
The genus Cistus is one of the characteristic genera of the
Mediterranean region, colonizing degraded areas (
). Cistus laurifolius L. (cistaceae) is a common plant
in Anatolia and is used against various ailments in traditional
medicine. The plant leaves are used to treat rheumatic and related
inflammatory diseases, externally as a bath or poultice to reduce
pain in rheumatism, against fever in common cold or applied
externally as a plaster on the dorsal part of the body in a line of
the kidneys for urinary inflammations (
A tea prepared from the leaves is used as hypoglycaemic and the
flowers and buds decoction is used for the treatment of peptic
ulcers in Turkish folk medicine (
Sezik et al., 1991; Yesilada et
). In previous phytochemical studies, Cistus laurifolius
have been reported to contain flavonoids including quercetin,
kaempferol, apigenin, luteolin and theirs methyl–ethers, the
coumarine scopoletin, diterpenoids, sesquiterpenoids, and sug-
ars (
Demetzos et al., 1989; Vogt et al., 1987; Wollenweber and
Due to the widespread utilization of the plant as a traditional
remedy, it is essential to investigate the potential effects of the
crude drug on hepatic tissue for the evaluation of potential health
∗
Corresponding author. Fax: +90 312 223 50 18.
E-mail address: esrak@gazi.edu.tr (E. K¨upeli).
risks to users as well as to disclose the possible antioxidant
effect in the folkloric use. In the present study, the antioxi-
dant effect of Cistus laurifolius ethanol extract and fractions
were investigated against acetaminophen-induced liver dam-
age on subacute administration in mice. In order to assess the
activity, some biochemical plasma and hepatic tissue parame-
ters including malondialdehyde formation (MDA), transaminase
levels in plasma; aspartate transferase (AST) and alanine trans-
ferase (ALT), and cellular glutathione (GSH) level in hepatic
tissue were measured. Through bioassay-guided fractionation,
the active antioxidant constituent(s) were isolated and identified
by spectroscopy.
2. Material and methods
2.1. General experimental procedures
NMR spectra were acquired on a JEOL instrument.
1
H NMR:
500 MHz,
13
C NMR: 125 MHz, using TMS as internal standard.
FAB-MS were obtained on a JEOL HX-110A instrument.
The active compounds were isolated after extensive
chromatography using Sephadex LH-20 (25–100
m, Lot
124H0053, Sigma Chem. Co., column size 30 mm
× 500 mm).
Silica gel (Kieselgel 230–400 mesh, Merck Art. No. 1.09385,
column size 35 mm
× 750 mm) and reversed phase 18 (LiChro-
prep RP-18, Merck, column size 18.5 mm
× 352 mm) columns.
0378-8741/$ – see front matter © 2005 Elsevier Ireland Ltd. All rights reserved.
doi:10.1016/j.jep.2005.08.038
456
E. K¨upeli et al. / Journal of Ethnopharmacology 103 (2006) 455–460
2.2. Animals
Male Swiss albino mice (20–25 g) were purchased from the
animal breeding laboratories of Refik Saydam Central Institute
of Health (Ankara, Turkey). The animals left for 2 days for
acclimatization to animal room conditions were maintained on
standard pellet diet and water ad libitum. The food was with-
drawn on the day before the experiment, but allowed free access
of water. A minimum of six animals was used in each group.
Throughout the experiments, animals were processed according
to the suggested international ethical guidelines for the care of
laboratory animals.
2.3. Plant material
Cistus laurifolius L. leaves were collected from Bolu,
D¨ortdivan in May 2002 and was identified by Prof. Dr. M. Vural
from the Department of Botany, Faculty of Science, Gazi Uni-
versity. A voucher specimen is deposited in the Herbarium of
Faculty of Pharmacy, Gazi University (GUE-2300).
2.4. Extraction and fractionation
2.4.1. Preparation of extract and fractions
Some 2 kg of powdered material was extracted three times
with EtOH (10 L) by stirring in a 60
◦
C water bath for 8 days
each. The combined ethanol extract was evaporated to dry-
ness under reduced pressure to yield ‘EtOH extract’ (315 g).
The EtOH extract was then resuspanded in 1500 ml of MeOH
and extracted with n-hexane (7
× 500 ml). Combined hex-
ane extract was evaporated under reduced pressure to yield
‘Hexane fraction’ (64.8 g). MeOH was removed from the
remaining solution and diluted with distilled H
2
O to 2000 ml
and further fractionated by successive extractions with chlo-
roform (7
× 500 ml), ethyl acetate (5 × 250 ml) and water-
saturated n-butanol (3
× 200 ml). The extracts as well as the
remaining aqueous phase were evaporated to dryness under
reduced pressure to yield the “CHCl
3
Fr.” (98.4 g), “EtOAc
Fr.” (28.3 g), “BuOH Fr.” (29.7 g) and “R–H
2
O Fr.” (84.5 g),
respectively.
2.4.2. Isolation of the active constituents
Four grams of the CHCl
3
fraction was permeated on a
Sephadex LH-20 column using MeOH as eluent. Some 43
fractions of 15 ml each were collected. Fractions were com-
pared by TLC on silica gel using CHCl
3
/MeOH (8:2) and
toluene/ether (1:1) as mobile systems and combined as follows:
Fr.1–13 (1.23 g), Fr.14–19 (0.72 g), Fr.20–30 (0.91 g), Fr.31–43
(1.12 g). Some additional 5.36 g of the CHCl
3
fraction were
worked-up under identical conditions to obtain more material for
further purification. Vacuum-chromatography of the flavonoid-
enriched fractions (silica gel; petroleum ether/EtOAc (1:1), (1:2)
and EtOAc/MeOH (8:2) as solvent system) and reverse-phase
(RP-18) chromatography using MeOH/H
2
O yielded three pure
compounds: (1) (1.23 g), (2) (0.95 g), (3) (0.82 g). The struc-
ture of compounds was elucidated by spectroscopic methods as
quercetin-3-methyl-ether (1), quercetin-3,7-dimethyl-ether (2)
Scheme 1.
and kaempferol-3,7-dimethyl-ether (3) (
). The spec-
tral data are in agreement with the reported values (
al., 1986; Guerrero et al., 2002; Smolarz et al., 2003; Stevens et
al., 1995
2.5. Pharmacological procedures
2.5.1. Preparation of test samples for bioassay
The extract, fractions and pure compounds were suspended
in 0.5% CMC in distilled water prior to oral administration
to experimental animals. Test groups of mice were orally
treated with EtOH extract (500 mg/kg body weight), hexane
(206 mg/kg), CHCl
3
(312 mg/kg), EtOAc (90 mg/kg), n-BuOH
(94 mg/kg) or R-H
2
O fractions (268 mg/kg) for 7 follow-
ing days, once a day by gastric gavage. The control group
(untreated) and acetaminophen group (positive control) were
administered in 0.5% CMC suspension for the same period.
The reference drug, ascorbic acid (Vitamin C) suspended in
0.5% CMC was directly administered to animals at 100 mg/kg
dose.
2.5.2. Experimental procedure
Some 60 min after the administration of the last dose on
seventh day, except the control group mice, each of the
acetaminophen group and test group animals was challenged
with the suspension of acetaminophen (800 mg/kg body weight)
in 0.5% CMC to induce hepatic injury (
Four hours after acetaminophen administration, blood samples
were withdrawn by cardiac puncture and then the mice were sac-
rificed by overdose of diethylether. Blood samples collected in
heparinized tubes were centrifuged at 3000
× g (4
◦
C) for 10 min
to obtain plasma. Plasma samples were used to determine the
lipid peroxide levels and the enzyme (AST, ALT) activity. Liver
of each mouse was promptly removed and used to determine
the tissue levels of malondialdehyde (MDA) and cellular glu-
tathione (GSH).

E. K¨upeli et al. / Journal of Ethnopharmacology 103 (2006) 455–460
457
2.5.3. Determination of plasma lipid peroxidation level
The methodology described by
was
used. Briefly, 1 ml of plasma was mixed with 2.0 ml of
trichloroacetic acid (TCA; 15%, w/v)–thiobarbituric acid (TBA;
0.375%)–0.25 N HCl and mixed throughly and centrifuged at
10,000
× g for 5 min. The supernatant was mixed with 20 l
of butyl hydroxy toluene (BHT; 0.02% in 95% EtOH, w/v) to
prevent further oxidation and heated for 15 min in a boiling
water bath. After cooling under running water, the flocculent
precipitate was removed by centrifugation at 10,000
× g for
5 min. The absorbance of the sample was measured at 532 nm
against blank that contained all the reagents except plasma.
1,1,3,3-Tetraethoxypropan was used as standard for the curve
calibration.
2.5.4. Determination of lipid peroxidation in liver tissue
The method of
as modified by
was used to determine lipid peroxidation in tis-
sue samples. Mice were sacrified by an overdose of diethylether.
The liver of each mouse was immediately excised and chilled in
ice-cold 0.9% NaCl and then perfused via the portal vein with
ice-cold 0.9% NaCl. After washing with 0.9% NaCl, 1.0 g of wet
tissue was weighted exactly and homogenized in 9 ml of 0.25 M
sucrose using a teflon homogenizer to obtain a 10% suspension.
The cytosolic fraction was obtained by a two-step centrifuga-
tion first at 1000
× g for 10 min and then at 2000 × g for 30 min
at 4
◦
C. A volume of the homogenate (0.20 ml) was transferred
to a vial and was mixed with 0.2 ml of a 8.1% (w/v) sodium
dodecyl sulphate solution, 1.50 ml of a 20% acetic acid solution
(adjusted to pH 3.5 with NaOH) and 1.50 ml of a 0.8% (w/v)
solution of TBA and the final volume was adjusted to 4.0 ml
with distilled water. Each vial was tightly capped and heated in
boiling water bath for 60 min. The vials were then cooled under
running water.
Equal volumes of tissue blank or test sample and 10%
TCA were transferred into a centrifuge tube and centrifuged at
1000
× g for 10 min. The absorbance of the supernatant fraction
was measured at 532 nm in a Beckman DU 650 spectrometer.
Control experiment was processed using the same experimental
procedure except the TBA solution was replaced with distilled
water due to the peroxidative effect of acetaminophen on tissue.
Livers of acetaminophen-treated mice were used as positive con-
trol and 1,1,3,3-tetraethoxypropan was used as standard for the
curve calibration.
2.5.5. Nonprotein sulfhydryl groups (cellular GSH) in liver
tissue (
Some 200 mg of liver was homogenized in 8.0 ml of 0.02 M
EDTA in an ice bath. The homogenates were kept in the ice bath
until used. Aliquots of 5.0 ml of the homogenates were mixed
in 15.0 ml test tubes with 4.0 ml distilled water and 1.0 ml of
50% trichloroacetic acid (TCA). The tubes were centrifuged for
15 min at approximately 3000
× g. Some 2.0 ml of supernatant
was mixed with 4.0 ml of 0.4 M Tris–buffer, pH 8.9, 0.1 ml Ell-
man’s reagent [5,5
-dithiobis-(2-nitro-benzoic acid)] (DTNB)
added, and the sample shaken. The absorbance was read within
5 min of the addition of DTNB at 412 nm against a reagent blank
with no homogenate. Results were expressed as
mol GSH/g
tissue.
2.5.6. Aspartate transferase (AST) and alanine transferase
(ALT) in plasma
Biocon standard kits and DAX-48 autoanalyzer were used to
measure AST and ALT activities in plasma samples according
to the method of
2.5.7. Acute toxicity
Animals employed in the experiments were observed during
48 h and morbidity or mortality was recorded, if happens, for
each group at the end of observation period.
2.6. Statistical analysis
The data obtained were analyzed by one-way of variance
(ANOVA) and Student–Newman–Keuls post hoc tests for the
significant interrelation between the various groups using Instat
computer software. P < 0.05 was considered to be significant
different from the control.
3. Results and discussion
In living systems, dietary antioxidants such as
␣-tocopherol,
ascorbic acid, carotenoids, as well as flavonoids and related
phenolic compounds are suggested in protection from oxida-
tive damage of tissues in the body and eventually for a healthy
life (
). Especially flavonoids have been shown
to scavenge various reactive oxygen species and implicated as
inhibitors of lipid peroxidation (
Acetaminophen (paracetamol), a frequently used analgesic
and antipyretic drug, is known to be hepatotoxic in high doses,
which is primarily metabolized by sulfation and glucuronidation
to unreactive metabolites, and then activated by the cytochrome
P-450 system to produce liver injury. It is established that
acetaminophen is bioactivated to a toxic electrophile, N-acetyl-
p-benzoquinone imine (NAPQI), which binds covalently to tis-
sue macromolecules, and probably also oxidizes lipids, or the
critical sulphydryl groups (protein thiols) and alters the home-
ostasis of calcium (
). Post-mitochondrial super-
natants isolated from livers of rats given a single large oral dose
of acetaminophen (800 mg/kg) showed rapid rates of lipid per-
oxidation when incubated in vitro. Lipid peroxidation probably
occurs simultaneously with the proposed covalent binding of
the active metabolite of acetaminophen. Since the former pro-
cess is known to cause severe and extensive membrane damage,
it may be a very important factor in acetaminophen-induced liver
necrosis (
As shown in
, in the liver and plasma of
acetaminophen-treated group, tissue and plasma lipid peroxida-
tion levels (219.2 and 1172%) as evidenced by MDA determina-
tion increased significantly as compared to control group, how-
ever, the content of GSH in liver decreased (8.7%). Additionally,
acetaminophen was found to cause several folds increases in
plasma AST and ALT levels (106 and 71.6%). Ethanol (EtOH)
extract of Cistus laurifolius leaves administered in 500 mg/kg

458
E. K¨upeli et al. / Journal of Ethnopharmacology 103 (2006) 455–460
Table 1
Effect of Cistus laurifolius EtOH extract, subfractions and the isolated flavonoids on MDA and GSH levels against acetaminophen-induced liver damage in mice
Materials
Dose (mg/kg)
Plasma MDA level
Liver homogenate MDA level
Tissue GSH
Plasma (nmol/ml)
% Change
Liver (nmol/g)
% Change
mol/g
% Change
Control (0.5% CMC)
1.8
± 0.1
118.2
± 15.4
106.1
± 8.1
Acetaminophen
800
11.4
± 2.1
+1172
377.8
± 29.6
+219.2
96.9
± 10.6
−8.7
Ascorbic acid
100
4.6
± 0.2
−59.5
183.7
± 17.9
−51.4
126.3
± 11.5
+30.3
EtOH extract
500
5.8
± 0.9
−49.3
194.7
± 19.3
−48.5
135.6
± 7.9
+39.9
Hexane Fr.
206
7.9
± 1.5
−31.1
296.1
± 24.1
−21.6
117.3
± 8.6
+21
CHCl
3
Fr.
312
6.7
± 0.8
−41.3
260.8
± 16.7
−31.0
153.4
± 6.1
+58.3
EtOAc Fr.
90
7.0
± 1.0
−38.4
278.8
± 14.4
−26.2
117.8
± 7.2
+21.6
n-BuOH Fr.
94
8.7
± 2.2
−23.4
307.8
± 21.6
−18.5
128
± 8.4
+32.1
R-H
2
O Fr.
268
8.9
± 1.9
−21.3
303.2
± 18.8
−19.8
106
± 4.4
+9.4
147
6.8
± 0.6
−40.3
316.6
± 12.3
−16.2
109.7
± 13.1
+13.2
114
6.1
± 0.4
−46.8
300.8
± 14.8
−20.5
115.5
± 6.3
+19.2
Kaempferol-3,7-dimethyl-ether
98
7.1
± 0.5
−37.5
355.15
± 14.3
−6.0
104.5
± 9.5
+7.8
Results are presented as mean
± S.E.M.
a
(+) Represents percentage of increase and (
−) decrease when compared to either vehicle or acetaminophen.
b
Compared to vehicle control (0.5% CMC).
c
Compared to acetaminophen as hepatotoxin.
*
Change to significance from control or acetaminophen; p < 0.05.
**
Change to significance from control or acetaminophen; p < 0.01.
***
Change to significance from control or acetaminophen; p < 0.001.
dose as well as ascorbic acid, as reference compound, showed
a significant effect at plasma AST, ALT, MDA and liver tissue
MDA, GSH levels (
). Besides, we observed that
EtOH extract was more effective than ascorbic acid in plasma
AST levels.
The active EtOH extract was fractionated through successive
solvent-solvent extractions and five fractions namely Hexane
Fr., CHCl
3
Fr., EtOAc Fr., n-BuOH Fr. and R-H
2
O Fr. were
obtained.
As shown in
3
Fr. (41.3% for plasma and 31%
for tissue) and, in a lesser degree, EtOAc Fr. (38.4% for plasma
and 26.2% for tissue) possessed a potent activity against both
plasma and tissue MDA levels. Of the five fractions tested, the
CHCl
3
Fr. (58.3%) appeared to be the most effective in increas-
ing the hepatic GSH levels, as compared to the acetaminophen
group. Additionally, only CHCl
3
(21.5%), EtOAc (10.1%) and
n-BuOH (19.6%) fractions produced a decrease in plasma ALT
levels while all of the fractions caused reduction in plasma AST
Table 2
Effect of Cistus laurifolius EtOH extract, subfractions and the isolated flavonoids on plasma transaminase enzyme levels against acetaminophen-induced liver damage
in mice
Materials
Dose (mg/kg)
ALT
AST
IU (L)
% Change
IU (L)
% Change
Control (0.5% CMC)
3474
± 16
1683
± 57
Acetaminophen
800
5960
± 251
+71.6
3468
± 219
+106.0
Ascorbic acid
100
5864
± 188
−1.6
2108
± 115
−39.2
EtOH extract
500
4692
± 113
−21.3
2319
± 119
−33.1
Hexane Fr.
206
6452
± 114
+8.3
2660
± 167
−23.3
CHCl
3
Fr.
312
4680
± 115
−21.5
2576
± 235
−25.7
EtOAc Fr.
90
5360
± 240
−10.1
2194.4
± 161
−36.7
n-BuOH Fr.
94
4792
± 138
−19.6
2796
± 258
−19.4
R-H
2
O Fr.
268
6180
± 139
+3.7
2656
± 300
−23.4
147
4704
± 124
−21.1
2289.6
± 121
−34.0
114
4932
± 147
−17.3
3120
± 167
−10.0
Kaempferol-3,7-dimethyl-ether
98
4904
± 124
−17.7
2288
± 150
−34.0
Results are presented as mean
± S.E.M.
a
(+) Represents percentage of increase and (
−) decrease when compared to either vehicle or acetaminophen.
b
Compared to vehicle control (0.5% CMC).
c
Compared to acetaminophen as hepatotoxin.
*
Change to significance from control or acetaminophen; p < 0.05.
**
Change to significance from control or acetaminophen; p < 0.01
***
Change to significance from control or acetaminophen; p < 0.001

E. K¨upeli et al. / Journal of Ethnopharmacology 103 (2006) 455–460
459
levels (19.4–36.7%). The results showed that pretreatment with
CHCl
3
Fr. (in ALT) and EtOAc Fr. (in AST) represented the most
significant alleviation of the plasma enzyme activity induced by
acetaminophen. AST can be generally found in the liver, car-
diac muscle, skeletal muscle, kidneys, brain, pancreas, lungs,
leukocytes, and erythrocytes, whereas, ALT is present in high-
est concentration in liver (
). Therefore, the CHCl
3
Fr. was considered to be more effective than the EtOAc Fr. on
acetaminophen-induced liver damage.
According to the biochemical test results, the following
experiments were directed to CHCl
3
Fr. On TLC analysis,
CHCl
3
Fr. was found to be rich in flavonoids. Through suc-
cessive column chromatographies three main flavonoids were
isolated, and their structures were elucidated as quercetin-3-
methyl-ether (isorhamnetin) (1), quercetin-3,7-dimethyl-ether
(2), kaempferol-3,7-dimethyl-ether (3) by spectral techniques.
Effect of the isolated flavonoid aglycones on acetaminophen-
induced liver damage was also studied using the same biochem-
ical methods on plasma and liver tissue.
Mice treated with flavonoid 2 significantly reduced
acetaminophen-induced increases in plasma (46.8%) and liver
(20.5%) MDA production compared with acetaminophen-
treated mice. On the other hand, flavonoid 1 also caused reduce
of acetaminophen-induced increase in plasma (40.3%) and liver
(16.2%) MDA production. According to these results, flavonoid
2 is considered to have more inhibitory effect than that of
flavonoid 1 on acetaminophen-induced lipid peroxidation.
Glutathione (GSH) plays an essential role in the detoxifica-
tion of acetaminophen and protects hepatocytes by uniting with
reactive metabolites of acetaminophen. Thus, it prevents them
from binding covalently to liver proteins. Intracellular decrease
of the reducted GSH exposes the cell to the destructive effects of
the oxidative stress (
). In other word,
the hepatic GSH is non-protein reserve of the liver and responsi-
ble in reducing the NAPQI-induced liver damage (
). The GSH activities showed alleviation of 13.2, 19.2
and 7.8%, respectively, in liver of mice, received flavonoids 1,
2 and 3, compared with the acetaminophen group.
The rise in serum AST and ALT levels has been attributed
to the damaged structural integrity of the liver (
), because these are cytoplasmic in location
and are released into circulation after cellular damage (
). Pretreatment of mice with flavonoid 1, 2 and 3
noticeably decreased the ALT activities (21.1, 17.3 and 17.7%)
compared with the acetaminophen group. Flavonoids 1 (34%)
and 3 (34%) provided a significant reduction in AST activities,
whereas flavonoid 2 (10%) exhibited an insignificant reduc-
tion. The reversal of alleviation of plasma enzyme activity in
acetaminophen-induced hepatic damage by these flavonoids
could explain the prevention of leakage of the intracellular
enzymes by its membrane stabilizing activity (
While quercetin-3,7-dimethyl-ether (2) displayed identical
activity than the CHCl
3
fraction in MDA levels, it did not show
any comparable effect with CHCl
3
fraction in tissue GSH and
MDA levels. As to ALT levels, all three flavonoids had equal
activity than the CHCl
3
fraction, whereas quercetin-3-methyl-
ether (1) and kaempferol-3,7-dimethyl-ether (3) were less active
than the CHCl
3
fraction.The isolated flavonoids did not induce
any apparent acute toxicity during the 48 h observation period.
As shown in
, flavonoid 2 was the most active
compound, as can be expected due to the presence of ortho-
catechol group (3
,4
-OH) in the B-ring, which is known to be
an important substituent pattern in the antioxidant activity of
flavonoids. Although the same functional group also exists in
flavonoid 1, the effect was somewhat lesser, probably due to the
weaker lipophilic nature of the compound.
Previously,
studied the neuroprotec-
tive effects of three antioxidant flavonoids, including quercetin,
quercetin 3-methyl-ether (1), and 1-dihydroquercetin isolated
from Opuntia ficus-indica var. saboten and the results indicated
that quercetin 3-methyl-ether was the most potent and suggested
as a promising neuroprotectant. It is evident that due to the neu-
roprotective activity of this flavonoid, it would be beneficial for
the prevention and treatment of oxidative stress-induced neuro-
logical disorders (
On the other hand, several studies have reported that quercetin
3-methyl-ether (1) displayed a remarkable activity against a
wide range of human picornaviruses, platelet aggregation and
histamine-induced tracheal relaxation in vitro (
Lin et al., 1995; Vanden Berghe et al., 1986
). Moreover,
quercetin 3-methyl-ether isolated from Cistus laurifolius has
been reported to be effective for inhibiting the aldose reduc-
tase activity. Therefore, this compound could be used to treat
the complications associated with diabetes (
reported that the methanolic Cis-
tus laurifolius extract and liposoluble fractions (hexane and
chloroform) were active on IL-1
␣ when assayed high con-
centrations. In a previous study, the polysaccharide mixture
obtained from the flowers and flower buds of Cistus lauri-
folius was found to be active against pylorus ligation-, absolute
ethanol-, indomethacin-, indomethacin plus HCl/EtOH-induced
gastric and cysteamine-induced duodenal lesions (
). The CHCl
3
extract and the precipitate obtained
from Cistus laurifolius leaves showed significant analgesic
activity on the tail flick assay (
The in vivo antioxidant effect of the flavonoid aglycones iso-
lated from Cistus laurifolius, quercetin-3,7-dimethyl-ether (2)
and kaempferol-3,7-dimethyl-ether (3) is reported for the first
time.
According to the results of the present study, the extracts
and isolated flavonoids from Cistus laurifolius possess a potent
antioxidant activity. Since oxidation is known to be involved in
the pathogenesis of many diseases in which treatment with Cis-
tus laurifolius is claimed to be effective, further studies should
be carried out on the active compound(s) using other in vivo
and in vitro antioxidant models in order to assess the role and
elucidate the action mechanism.
Acknowledgement
The authors are extremely thankful to Dr. Emi Okuyama of
Graduate School of Pharmacy Science, Chiba University, Chiba,
Japan, for NMR and MS measurements.

460
E. K¨upeli et al. / Journal of Ethnopharmacology 103 (2006) 455–460
References
Ark, M., ¨
Ust¨un, O., Yes¸ilada, E., 2004. Analgesic activity of Cistus laurifolius
in mice. Pharmaceutical Biology 42, 176–178.
Attaguile, G., Russo, A., Campisi, A., Savoca, F., Acquaviva, R., Ragusa,
N., Vanella, A., 2000. Antioxidant activity and protective effect on DNA
cleavage of extracts from Cistus incanus L. and Cistus monspeliensis L.
Cell Biology and Toxicology 16, 83–90.
Barbera, O., Marco, J.A., Sanz, J.F., Parareda, J.S., 1986. 3-Methoxyflavones
and coumarins from Artemisia incanescens. Phytochemistry 25, 2357–
2360.
Chenoweth, M., Hake, C., 1962. The smaller halogenated aliphatic hydrocar-
bons. Annual Reviews of Pharmacology 2, 363–398.
Demetzos, C., Homatidou, V., Loukis, A.E., Philianos, S.M., 1989. The essen-
tial oil of Cistus creticus: comparison with five other species of the genus
Cistus. Planta Medica 55, 633–634.
Dok-Go, H., Lee, K.H., Kim, H.J., Lee, E.H., Lee, J., Song, Y.S., Lee, Y.H.,
Jin, C., Lee, Y.S., Cho, J., 2003. Neuroprotective effects of antioxidative
flavonoids, quercetin, (1)-dihydroquercetin and quercetin 3-methyl-ether,
isolated from Opuntia ficus-indica var. saboten. Brain Research 965,
130–136.
Enomoto, S., Okada, Y., Guvenc, A., Erdurak, C.S., Coskun, M., Okuyama,
T., 2004. Inhibitory effect of traditional Turkish folk medicines on aldose
reductase (AR) and haematological activity, and on AR inhibitory activity
of quercetin-3-O-methyl-ether isolated from Cistus laurifolius L. Biolog-
ical and Pharmaceutical Bulletin 27, 1140–1143.
Fairhurst, S., Barber, D.J., Clark, B., Horton, A.A., 1982. Studies on
paracetamol-induced lipid peroxidation. Toxicology 23, 249–259.
Guerrero, M.F., Puebla, P., Carro, R., Martin, M.L., Roman, L.S., 2002.
Quercetin 3,7-dimethyl-ether: a vasorelaxant flavonoid isolated from Cro-
ton schiedeanus Schlecht. Journal of Pharmacy and Pharmacology 54,
1373–1378.
Haraguchi, H., 2001. Antioxidative plant constituents. In: Tringali, C. (Ed.),
Bioactive Compounds from Natural Sources. Taylor and Francis Group,
London (Chapter 9).
Ischiropolus, H., Zhu, L., Chen, J., Tsai, M., Martin, J.C., Smith, C.D.,
Beckman, J.S., 1992. Peroxynitrite-mediated tyrosine nitration catalyzed
by superoxide dismutase. Archives of Biochemistry and Biophysics 298,
431–437.
Jamall, I.S., Smith, J.C., 1985. Effects of cadmium on glutathione perox-
idase, superoxide dismutase, and lipid peroxidation in the rat heart: a
possible mechanism of cadmium cardiotoxicity. Toxicology and Applied
Pharmacology 80, 33–42.
Ko, W.C., Kuo, S.W., Sheu, J.R., Lin, C.H., Tzeng, S.H., Chen, C.M., 1999.
Relaxant effects of quercetin-methyl-ether derivatives in isolated guinea
pig trachea and their structure-activity relationships. Planta Medica 5,
273–275.
Kurtel, H., Granger, D.N., Tso, P., Grisham, M.B., 1992. Vulnerability of
intestinal interstitial fluid oxidant stress. American Journal of Physiology
268, 573–575.
Lauterburg, B.H., Velez, M.E., 1988. Glutathione deficiency in alcoholics:
risk factor for acetaminophen hepatotoxicity. Gut 29, 1153–1157.
Lin, C.N., Lu, C.M., Lin, H., Ko, F.N., Teng, C.M., 1995. Novel antiplatelet
naphthalene from Rhamnus nakaharai. Journal of Natural Product 58,
1934–1940.
Lin, C.C., Shieh, D.E., Yen, M.H., 1997. Hepatoprotective effect of the frac-
tions of Ban-zhi-lian on experimental liver injuries in rats. Journal of
Ethnopharmacology 56, 193–200.
Mora, A., Paya, M., Rios, J.L., Alcaraz, M.J., 1990. Structure-activity rela-
tionships of polymethoxyflavones and other flavonoids as inhibitors of
non-enzymic lipid peroxidation. Biochemistry and Pharmacology 40,
793–797.
Ohkawa, H., Ohishi, N., Yagi, K., 1979. Assay for lipid peroxides in ani-
mal tissues by thiobarbituric acid reaction. Analytical Biochemistry 95,
351–358.
Rej, R., 1978. Aspartate aminotransferase activity and isoenzyme proportions
in human liver tissues. Clinical Chemistry 24, 1971.
Sallie, R., Tredger, J., William, R., 1991. Drugs and the liver. Biopharma-
ceuticals Drug Disposition 12, 251–259.
Sedlak, J., Lindsay, R.H., 1968. Estimation of total protein-band and non-
protein sulfhydryl group in tissue with Ellman’s reagent. Analytical Bio-
chemistry 25, 192–205.
Sezik, E., Tabata, M., Yesilada, E., Honda, G., Goto, K., Ikeshiro, Y., 1991.
Traditional medicine in Turkey I. Folk medicine in Northeast Anatolia.
Journal of Ethnopharmacology 35, 191–196.
Smolarz, H.D., Surdacka, A., Rolinski, J., 2003. Influence of ethyl acetate
extract and quercetin-3-methyl-ether from Polygonum amphibium on acti-
vation lymphocytes from peripheral blood of healthy in vitro. Phytother-
apy Research 17, 744–747.
Stevens, J.F., Hart, H.T., Wollenweber, E., 1995. The systematic and evo-
lutionary significance of exudate flavonoids in Aeonium. Phytochemistry
39, 805–813.
Thabrew, M., Joice, P., Rajatissa, W., 1987. A comparative study of the
efficacy of Pavetta indica and Osbeckia octandra in the treatment of
liver dysfunction. Planta Medica 53, 239–241.
Vanden Berghe, D.A., Vlietinck, A.J., Van Hoof, L., 1986. Plant products as
potential antiviral agents. Bulletin De L’Institut Pasteur 84, 101–147.
Vogt, T., Prosch, P., G¨ulz, P.G., 1987. Epicuticular flavonoid aglycones in the
genus Cistus, Cistaceae. Journal of Plant Physiology 131, 25–36.
Wilkinson, J.H., Baron, D.N., Moss, D.W., Wolter, P.G., 1972. Standardiza-
tion of clinical enzyme assays: reference method for aspartate and alanine
transaminases. Journal of Clinical Pathology 25, 940.
Wollenweber, E., Mann, K., 1984. Flavonoid aglycones in the leaf resin of
some Cistus species. Zeitschrift f¨ur Naturforschung 39c, 303–306.
Yesilada, E., Honda, G., Sezik, E., Tabata, M., Fujita, T., Tanaka, T., Takeda,
Y., Takaishi, Y., 1995. Traditional medicine in Turkey V. Folk medicine
in inner Taurus mountains. Journal of Ethnopharmacology 46, 133–152.
Yes¸ilada, E., Ustun, O., Sezik, E., Takaishi, Y., Ono, Y., Honda, G., 1997a.
Inhibitory effects of Turkish folk remedies on inflammatory cytokines:
interleukin-1
␣, interleukin-1 and tumor necrosis factor α. Journal of
Ethnopharmacology 58, 59–73.
Yes¸ilada, E., G¨urb¨uz, ˙I., Ergun, E., 1997b. Effects of Cistus laurifolius L.
flowers on gastric and duodenal lesion. Journal of Ethnopharmacology
55, 201–211.
Document Outline
- Effect of Cistus laurifolius L. leaf extracts and flavonoids on acetaminophen-induced hepatotoxicity in mice
- Introduction
- Material and methods
- General experimental procedures
- Animals
- Plant material
- Extraction and fractionation
- Pharmacological procedures
- Preparation of test samples for bioassay
- Experimental procedure
- Determination of plasma lipid peroxidation level
- Determination of lipid peroxidation in liver tissue
- Nonprotein sulfhydryl groups (cellular GSH) in liver tissue (Sedlak and Lindsay, 1968)
- Aspartate transferase (AST) and alanine transferase (ALT) in plasma
- Acute toxicity
- Statistical analysis
- Results and discussion
- Acknowledgement
- References
Wyszukiwarka
Podobne podstrony:
Nickel and Dimed On (Not) Getting By in Barbara Ehrenreich
The effects of plant flavonoids on mammalian cells implication for inflammation, heart disease, and
Assessment of cytotoxicity exerted by leaf extracts
53 755 765 Effect of Microstructural Homogenity on Mechanical and Thermal Fatique
31 411 423 Effect of EAF and ESR Technologies on the Yield of Alloying Elements
21 269 287 Effect of Niobium and Vanadium as an Alloying Elements in Tool Steels
Effects of the Great?pression on the U S and the World
Effect of aqueous extract
Possible Effects of Strategy Instruction on L1 and L2 Reading
extraction and analysis of indole derivatives from fungal biomass Journal of Basic Microbiology 34 (
Effect of caffeine on fecundity egg laying capacity development time and longevity in Drosophila
Effects Of 20 H Rule And Shield Nieznany
Effect of Drugs and Alcohol on Teenagers
A systematic review and meta analysis of the effect of an ankle foot orthosis on gait biomechanics a
Effect of heat treatment on microstructure and mechanical properties of cold rolled C Mn Si TRIP
71 1021 1029 Effect of Electron Beam Treatment on the Structure and the Properties of Hard
Assessment of cytotoxicity exerted by leaf extracts
53 755 765 Effect of Microstructural Homogenity on Mechanical and Thermal Fatique
więcej podobnych podstron