
Research review paper
Biotransformation of terpenes
Carla C.C.R. de Carvalho *, M. Manuela R. da Fonseca
Centro de Engenharia Biolo´gica e Quı´mica, Instituto Superior Te´cnico, Av. Rovisco Pais, 1049-001 Lisboa, Portugal
Received 17 July 2005; accepted 13 August 2005
Available online 5 October 2005
Abstract
The main application of terpenes as fragrances and flavors depends on the absolute configuration of the compounds because
enantiomers present different organoleptic properties. Biotransformations allow the production of regio- and stereoselective
compounds under mild conditions. These products may be labeled as bnaturalQ. Commercially useful chemical building-blocks
and pharmaceutical stereo isomers can also be produced by bioconversion of terpenes. Enzymes and extracts from bacteria,
cyanobacteria, yeasts, microalgae, fungi, plants, and animal cells have been used for the production and/or bioconversion of
terpenes. In addition, whole cell catalysis has also been used. A variety of media and reactors have been assessed for these
biotransformations and have produced encouraging results, as discussed in this review.
D 2005 Elsevier Inc. All rights reserved.
Keywords: Monoterpenes; Terpenoids; Biotransformations; Enantiomeric resolution
Contents
1.
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
134
2.
Biocatalysts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
135
3.
Reaction media . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
137
4.
Reactor type. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
138
5.
Bio-kinetic resolutions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
139
6.
Concluding remarks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
140
Acknowledgements. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
140
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
140
1. Introduction
Terpenes occur widely in nature. Terpenes such as lim-
onene and a-pinene are inexpensively available in large
quantities. Monoterpenes in plants are known to have
mainly ecological roles in acting as deterrents against
feeding by herbivores, as antifungal defenses and attrac-
tants for pollinators (
). In mammals
terpenes are involved in stabilizing cell membranes,
metabolic pathways and as regulators of enzymatic re-
actions. For example, cholesterol and related steroids
are triterpenes that are derived from 6 isoprene units.
Herbs and higher plants containing terpenoids and
their oxygenated derivatives have been used as fra-
0734-9750/$ - see front matter
D 2005 Elsevier Inc. All rights reserved.
doi:10.1016/j.biotechadv.2005.08.004
* Corresponding author. Tel.: +351 21 8417681; fax: +351 21
8419062.
E-mail address: ccarvalho@ist.utl.pt (C.C.C.R. de Carvalho).
Biotechnology Advances 24 (2006) 134 – 142
www.elsevier.com/locate/biotechadv
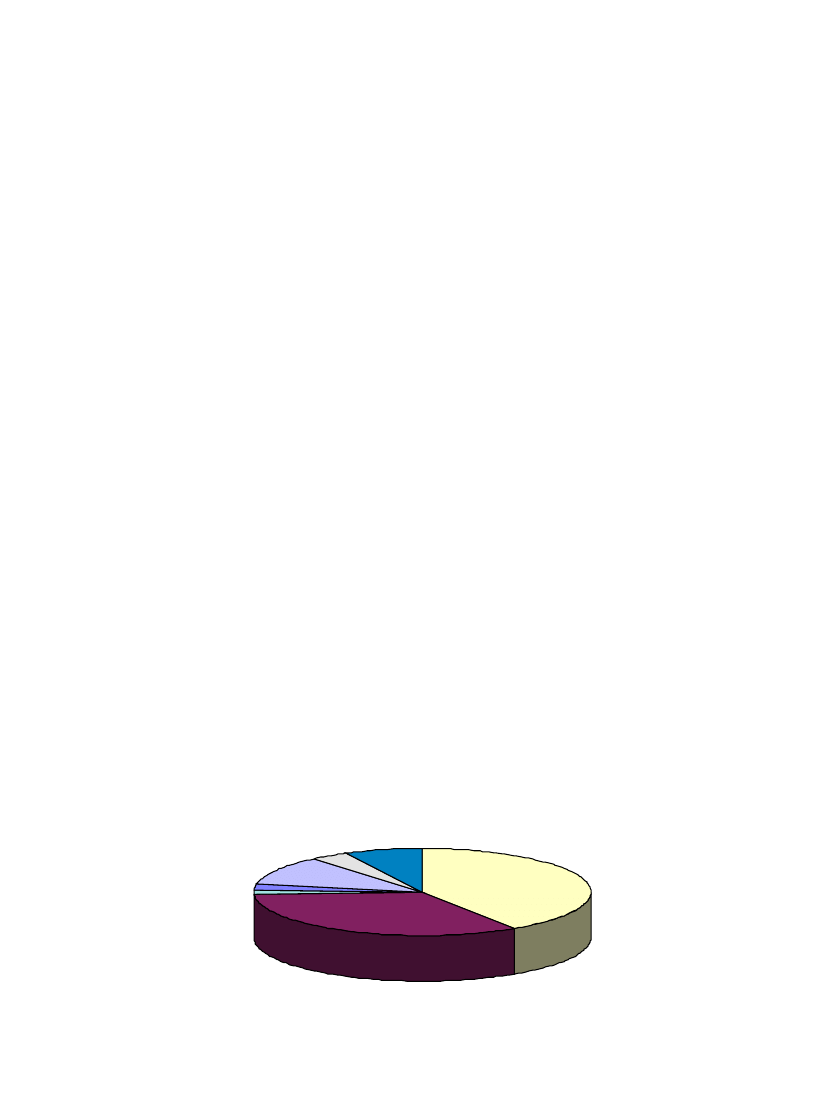
grances and flavors for centuries. More than 22,000
individual terpenoids are known at present, making
terpenoids the largest group of natural products. Ter-
penes have drawn increasing commercial attention be-
cause of increasing understanding of their roles in
prevention and therapy of several diseases, including
cancer; their activity as natural insecticides and antimi-
crobial agents; properties that can be useful in storing
agricultural produce (e.g., sprouting inhibitor in pota-
toes); and as building blocks for the synthesis of many
highly value compounds.
The biotransformation of terpenes is of interest
because it allows the production of enentiomerically
pure flavors and fragrances under mild reaction condi-
tions. Products produced by biotransformation process-
es may be considered as bnaturalQ. Industrial use of
monoterpenes as substitutes of ozone-depleting chlor-
ofluorocarbons is also flourishing (
Terpenes may be used as substitutes for chlorinated
solvents in applications such as cleaning of electronic
components and cables, degreasing of metal and clean-
ing of aircraft parts (
). This review
discusses recent developments in biotransformation
catalysts, reaction media, reactor types and biokinetic
resolution of terpenes.
2. Biocatalysts
Studies describing the biotransformation of terpenes
using enzymes, cell extracts and whole cells of bacteria,
cyanobacteria, yeasts, microalgae, fungi and plants
have been published. Both soluble and immobilized
enzymes have been used in biotransformations of ter-
penes. Isolation and purification of the relevant
enzymes can be expensive and difficult. Whole cell
biocatalysts may be cheaper and simpler to obtain
than isolated enzymes, but can add contaminants to
the reaction mixture. In whole cells, membranes and
walls protect the enzymes from shear forces and other
factors while cofactors can be regenerated within the
cell under certain conditions. Cascade of reactions, such
as those needed for steroid production, can be carried
out by a single whole cell biocatalyst. Nevertheless,
control and reproducibility of the bioconversions with
whole cells are more difficult to accomplish than in
enzymatic processes and side reactions may occur.
Cells can be used as freely suspended or immobilized.
The cells used as biocatalysts may be in various phys-
iological states: viable and growing; viable, but non
growing; and non viable. In the latter case, in situ
regeneration of cofactors will not occur. A good com-
parison of the various forms of biocatalyst was pre-
sented by
Straathof and Adlercreutz (2000)
.
Werf et al. (1997)
discussed opportunities in microbial
biotransformation of monoterpenes and the difficulties
associated with the conduct of these biotransformations
on industrial scale.
In nearly two-thirds of the manuscripts published on
production and/or biotransformation of terpenes in the
last decade, the biocatalysts used were either bacteria or
fungi (
). Only 7% of the studies used isolated
enzymes. Simple furan compounds from molecules
containing an a-isopropylidene ketone unit were enzy-
matically synthesized by
(2002)
. The role of cytochrome P450 in this transfor-
mation was described. Synthesis of terpenes esters
using a Candida rugosa lipase encapsulated in a dioctyl
sulfosuccinate-reversed-micellar solution showed a rel-
atively high activity for the transesterification reaction
of geraniol with tributyrin (
), but this
was not regarded as a feasible commercial process for
producing terpene esters.
The first sesquiterpene cyclase obtained and purified
from a whole plant/pathogen system catalyzed the con-
version of (E,E)-farnesyl diphosphate to (+)-y-cadinene
(
showed that,
Bacteria
41%
Fungi
33%
Plants
11%
Microalgae
4%
Enzymes
7%
Yeasts
2%
Cyanobacteria
2%
Fig. 1. Percentage of papers published on various biocatalyst types in the last ten years.
C.C.C.R. de Carvalho, M.M.R. da Fonseca / Biotechnology Advances 24 (2006) 134–142
135

although ( )-(4S)-limonene synthase and ( )-(4S)-lim-
onene/( )-(1S,5S)-a-pinene synthase from grand fir
(Abies grandis) has around 91% amino acid sequence
homology, they produced significantly different mix-
tures of monoterpenes olefins starting from the same
substrate. The results indicated that fewer than 10% of
the amino acid residues of the two enzymes determined
the reaction velocity and the product distribution
achieved.
Among enzymes, epoxide hydrolases are probably
one of the most versatile biocatalysts. These enzymes
belong to the a/h-hydrolase-fold family. They are able
to carry out the asymmetric hydrolysis of racemic
epoxides, producing the corresponding vicinal diols
and the biokinetically resolved non-hydrolyzed epox-
ides. The processes they catalyze can be enantiocon-
vergent, with the production of a single enantiomeric
diol from a racemic oxirane. Use of microbial epoxide
hydrolases for preparative biotransformations has been
reviewed by
.
Werf et al. (1998)
characterized a novel hydrolase
(i.e., limonene-1,2-epoxide hydrolase) from Rhodococ-
cus erythropolis DCL14 that did not belong to the a/h-
hydrolase superfamily, but to a separate class of epox-
ide hydrolases. This enzyme catalyzed the hydrolysis of
limonene-1,2-epoxide to limonene-1,2-diol and its ac-
tivity in cell extracts was 795 nmol/min mg protein
when the cells were grown in (+)-limonene (
Werf et al., 1998
). An organic:aqueous phase reaction
system that used whole cells of R. erythropolis strain
DCL14 was reported by
. A
production level of 72.4 g diol/g protein was obtained
in a magnetically stirred fed-batch reactor. This corre-
sponded to yields of 98.5% and 94.1% of trans-limo-
nene-1,2-epoxide and limonene-1,2-diol, respectively
(
). In situ separation of the
product was achieved in an external loop by recircula-
tion of the aqueous phase through a column filled with
an adsorbent. Production could be further improved by
using mechanical stirring. In this case, 197.2 g of diol
per g of protein were produced and the trans-epoxide
and diol yields were 98.2% and 67.9%, respectively.
The reactor operated for 22 days.
R. erythropolis strain DCL14 is known to have
several carveol dehydrogenase activities that allow the
cells to carry out the cofactor dependent stereoselective
oxidation of carveol (
). The
catalytic efficiency is much higher for the (6S)-stereo-
isomers of carveol than for the (6R)-stereoisomers.
der Werf et al. (1999)
achieved a specific activity of 115
nmol/min mg
prot
for the DCPIP-dependent enzyme in
cell extracts of R. erythropolis DCL14 grown on lim-
onene. In 1 : 5 dodecane:aqueous phase systems that
used whole cells of R. erythropolis DCL14, a maximum
production rate of 124.1 nmol/min mg
prot
was attained
de Carvalho and da Fonseca, 2002a
). In a mechanical-
ly stirred reactor, a maximum production rate of 188
nmol/min mg
prot
was maintained for nearly 23 h (
Carvalho and da Fonseca, 2002b
). Several additions of
substrate were possible during reactor operation, result-
ing in a productivity of 0.12 mg/hd mL. When the cells
were allowed to adapt/grow in the presence of both
carveol and carvone in dodecane, they were able to
overcome carvone toxicity. In a column reactor, after
an adaptation period of 268 h, the freely suspended
cells produced carvone at 0.19 mg/hd mL, yielding
0.96 g
carvone
/g
carveol
).
The cell population was able to endure a final concen-
tration of 1.03 M of carvone. Whole cells thus allowed
in situ cofactor regeneration under conditions in which
cell viability remained high. In the air-driven column
reactor, maximum production rates could be main-
tained by whole cells for remarkably longer periods
than those that would be possible with cell extracts.
The problems encountered during lipase-or esterase-
catalyzed esterification and transesterification (e.g., in-
hibitory effects of acyl donors, alcohols and esters;
inactivation of enzymes by added organic solvents)
were overcome by
by coupling
acetyl coenzyme A formation and microbial esterifica-
tion. Pichia quercuum IFO 0949 and Pichia heedii IFO
10019 showed a strong double coupling activity of
acetyl-CoA formation and microbial esterification
with alcohol acetyltransferase in an interface bioreactor
and a triple coupling activity of acetyl-CoA formation,
microbial reduction of citronellal to citronellol and
microbial esterification of the latter with acetyl-CoA.
Fungal cells have also been used in biotransforma-
tion of terpenes and terpenoids (
). Aspergillus
niger was used in hydroxylation reactions with terpenes
(
) and
showed the resemblance between bioconversion of ter-
penoid pharmaceuticals by A. niger and mammalian
systems. A. niger was further used by
Reese (2002)
to bioconvert terpenes from Stemodia
maritima. Fungal spores of Penicillium digitatum
were able to biotransform geraniol, nerol, citrol (mix-
ture of the alcohols nerol and geraniol) and citral (mix-
ture of the aldehydes neral and geranial) to 6-methyl-5-
hepten-2-one (
Demyttenaere and De Kimpe, 2001
).
Aleu and Collado (2001)
reviewed biotransformations
carried out by Botrytis sp. Some of these transforma-
tions of terpenoids to volatile substances in grapes are
important in producing a distinctive aroma in wine.
C.C.C.R. de Carvalho, M.M.R. da Fonseca / Biotechnology Advances 24 (2006) 134–142
136

Prior to the development of high-pressure homoge-
nization and other methods of large scale cell disruption
(
Hetherington et al., 1971; Chisti and Moo-Young,
), only extracellular enzymes or those extractable
by chemical cell lysis were available to industry. Note-
worthy growth of biotransformation as an alternative to
chemical synthesis was made possible by the ability to
produce large quantities of intracellular enzymes by cell
disruption and development of immobilization techni-
ques that allowed the recovery and reuse of enzymes
(
). Furthermore, enzyme immobilization
allowed modification of enzyme properties and en-
hancement of stability of enzymes. Similarly, immobi-
lization of cells provided a method of regulating
metabolism and hence product formation (
1994
). Freely suspended cells tend to readily metabo-
lize exogenous terpenes (
). Acceptable product
yields are generally only attained if the desired product
can be extracted to a non-polar organic phase or
adsorbed on a resin (
Do¨rnenburg and Knorr, 1995
).
3. Reaction media
Biotransformations have been traditionally carried
out in aqueous systems. This is because aqueous
media are generally compatible with enzymes and
growing whole cells. Unfortunately, terpenes are poorly
soluble in water. Water solubilities at 25 8C of some of
the common terpenes are the following (in mmol/L):
(R)-(+)-limonene, 0.15; ( )-a-pinene, 0.037; ( )-a-pi-
nene oxide, 2.55; ( )-carveol, 19; and (+)-carvone, 8.8
(
). Use of an organic phase in the
aqueous reaction system improves enzymatic and mi-
crobial biotransformations of terpenes compared with
the use of pure aqueous media.
By allowing continuous removal of the product
from the aqueous phase, the presence of a water im-
miscible organic phase decreases product inhibition of
the biocatalyst. Furthermore, removal of product shifts
the thermodynamic equilibrium of kinetically unfavor-
able reactions so that more product can be produced
(
). In addition, the re-
covery of both product and biocatalyst becomes easier
compared to when only the aqueous phase is used.
Enzyme catalysis in non-aqueous media have been
reviewed recently (
Halling, 2000; Klibanov, 2001;
Lee and Dordick, 2002
). Similarly, whole cell biocata-
lysis in organic media has been reviewed (
1998
). While multiphasic production systems have
advantages, organic-solvents can inactivate enzymes
and cause loss of cell viability by interfering with the
cell membrane.
In a biotransformation system, the organic phase
may be the only liquid phase present with minute
quantities of water dissolved within it (
al., 1998; de Carvalho et al., 2004; Angelova et al., in
press
), or a distinct aqueous phase and an organic-
phase may coexist (
van Keulen et al., 1998; de Car-
valho et al., 2000b; Tecela˜o et al., 2001; de Carvalho
and da Fonseca, 2002a
). In the latter case, the organic
phase may be the substrate itself or an organic solvent
that acts as a reservoir for the substrate/product. Most
terpenes have antimicrobial properties and dilution with
the organic solvent likely helps in reducing their tox-
icity towards the microorganisms being used for the
biotransformation. The overall log P of the organic
phase also appears to have an impact on the biotrans-
formation capability and viability of the microbial cells
used in the biotransformation (
Fonseca, 2002a
).
In studies comparing responses of the bacteria R.
erythropolis, Xanthobacter Py2, Arthrobacter simplex
and Mycobacterium sp. to toxic solvents and substrates,
the major factor that influenced the behavior of the cells
in organic-aqueous multiphase systems was found to be
the toxicity of the solvent (
2004a,b
). More than 33% of the variability of the data
could be explained by solvent toxicity; the remaining
variability was ascribed to factors related with substrate
concentration, cells’ ability to adapt and the composi-
tion of the cell membrane. The latter may be signifi-
cantly influenced by the carbon source used for
growing the cells. Growth history of cells influences
their ability to carry out biotransformations in the rest-
ing state. In one study (
), all
alkanes, long-chain alkanols and terpenes tested as
carbon sources caused a dose-dependent increase in
the degree of saturation of the fatty acids of the cell
membrane. Growing the cells with short-chain alcohols
caused a dose-dependent decrease in the degree of
saturation of the fatty acids of the membrane. The
resulting differences in membrane composition led to
cells with different membrane hydrophobicities and
therefore differences in the cells’ ability to uptake
hydrophobic/hydrophilic compounds.
Cell growth has traditionally been considered det-
rimental in biotransformations with bresting cellsQ if
the cells use the substrate, product or both as carbon
source. However, cell growth during the incubation
phase and/or during the biotransformation of carveol
into carvone in a n-dodecane:mineral medium system
resulted in the emergence of better adapted cells and
consequent high production of carvone (
et al., in press
). The adapted cells retained viability in
C.C.C.R. de Carvalho, M.M.R. da Fonseca / Biotechnology Advances 24 (2006) 134–142
137

the presence of 1.03 M carvone. In contrast, cells that
were not adapted died at a carvone concentration of
50 mM.
Other non conventional media that may be used in
biotransformation studies include ionic liquids and su-
percritical fluids. Ionic liquids are low melting point
salts. They are non-aqueous polar solvents and can
dissolve many compounds. Several enzymes have
been successfully used in ionic liquids (for a review
see e.g.,
). These liquid should prove
useful in production/transformation of terpenes.
Pfruen-
der et al. (2004)
showed that ionic liquids can act as a
substrate reservoir and sink in a biotransformation per-
formed with viable whole cells.
(S)-( )-terpene esters were stereoselectively synthe-
sized by Candida cylindracea lipase in supercritical
carbon dioxide in the near-critical region (
1997
). The supercritical carbon dioxide triggered the
activation of the enzyme by causing movement of the
surface groups and creating active sites. However, most
studies with supercritical media have focused on their
use in extraction of terpenes from essential oils and not
their use as reaction media.
4. Reactor type
Approximately 48% of the papers describing bio-
transformation of terpenes concern reactions carried out
in shake flasks (
). Shake flasks are simple and
efficient for screening microorganisms, substrates and
reaction conditions rapidly and inexpensively. Cultures
of bacteria (e.g.,
Carter et al., 2003; Carballeira et al.,
2004
), fungi (e.g.,
Demyttenaere and De Kimpe, 2001;
Chen and Reese, 2002
), plant cells (e.g.,
Henriksson et al., 2004
) and microalgae (e.g.,
et al., 2002; Hook et al., 2003
) have been investigated
for terpene biotransformations in shake flasks.
Vials, reaction wells and other microscale devices
have been used to obtain reliable biotransformation data
at extremely low costs. For example,
carried out sesquiterpene cyclase activity assays using
ca. 1 Ag of total protein and 5 AL of substrate stock
solution in a total reaction volume of 245 AL. The
bioconversion pathway for limonene in Xanthobacter
sp. C20 was established by
reaction mixtures having a total volume of 1.5 mL in 15
mL vials fitted with Teflon Mininert valves to prevent
Table 1
Examples of terpene biotransformations using whole cells
Publication
Microorganism
Result
Aspergillus niger
3h-Hydroxy derivatives of confertifolin
Gongronella butleri, Schizosaccharomyces
octosporus and Diplogelasinospora grovesii
Stereoselective reduction of ketones
Escherichia coli
( )-Carvone, ( )-limonene
Rhodococcus erythropolis
Trans-limonene-1,2-epoxide and limonene-1,2-diol
de Carvalho and da Fonseca, 2002a,b
Rhodococcus erythropolis
( )-Carvone from ( )-carveol
de Carvalho and da Fonseca, 2003
Rhodococcus opacus
Trans-carveol and carvone from limonene
Aspergillus niger
Several metabolites from stemodin, stemodinone and
stemarin
Botryococcus braunii
Novel terpene epoxides
Rhodococcus erythropolis
Trans-carveol from limonene
13 Airborne fungi
Volatile organic compounds
Pseudomonas rhodesiae
Isonovalal from a-pinene oxide
Mucor plumbeus
Several products from ribenone
Fulzele et al., 1995
Artemisia annua
Several terpenoids
Alcaligenes defragans
Isoterpinolene from isolimonene
Microalgae
Aliphatic and aromatic ketones
Penicillium caseifulvum
Limonene, h-caryophyllene and other terpenoids
Lindmark-Henriksson et al., 2004
Picea abies cell cultures
Trans-pinocarveol and other minor products from
h
-pinene
Glomerella cingulata
Transformation of (+)-cis-nerolidol and nerylacetone
Pichia quercuum and P. heedii
Citronellol
Onken and Berger, 1999
Pleurotus sapidus
Carveol and carvone from limonene
Bovista sp.
Bovistol and new sesquiterpenes
Xanthobacter sp. C20
Limonene-8,9-epoxide from limonene
Pseudomonas putida
a-Pinene oxide from a-pinene
Verstegen-Haaksma et al., 1995
Several bacterial and fungal strains
Biologically active compounds from (S)-(+)-carvone
Pseudomonas sp. PIN
Conversion of a-and h-pinene and related compounds
Peganum harmala
Several terpenes and non-terpenes
C.C.C.R. de Carvalho, M.M.R. da Fonseca / Biotechnology Advances 24 (2006) 134–142
138

evaporation of limonene.
studied the metabolism of terpenes by denitrifying
Alcaligenes defragrans strains in systems with 30 AL
monoterpene, 150 AL nitrate and 15 mL of aqueous
medium.
used 15 mL test tubes to
study terpene ester production in a solvent phase con-
taining a reverse micelle-encapsulated lipase.
Petri dishes with agar media are good reactors for
terpene producing/transforming fungi (
1997; Larsen, 1998; Fischer et al., 1999
). Although such
solid-state fermentations are not easily scaled up, they
are used in several commercial large volume processes
(
). Solid-state fermentation has certain im-
portant advantages compared to submerged culture
(
The application of terpene biotransformations in
bioreactors at the liter-scale suggests that these process-
es are technically feasible at larger scales.
Metzger (1997)
used an airlift reactor to produce ter-
pene epoxides with two strains of the green microalga
Botryococcus braunii. Further work on the occurrence
and biotransformation of various terpenes in B. braunii
was reviewed by
. For biotrans-
formations involving fungi, processing conditions ap-
parently need to be selected to avoid swelling of cell
membrane that is in contact with the solvent and ter-
penes (
We have tested magnetically and mechanically
stirred tank reactors for the transformation of terpenes
with freely suspended whole cells (
2000b; de Carvalho and da Fonseca, 2002b
); mem-
brane reactors (
de Carvalho and da Fonseca, 2002b
);
and air-driven column reactors (
Fonseca, 2002b; de Carvalho et al., in press
). The
stirred reactors with suspended cells were particularly
efficient for the diastereomeric resolved conversion of
limonene-1,2-epoxide in limonene-1,2-diol, because
the epoxide partitioned preferentially to the organic
phase while the diol remained in the aqueous phase. In
situ removal of the product could be achieved by
recirculating the aqueous phase through a column
filled with an adsorbent. The membrane reactor was
able to maintain the aqueous and organic phases sep-
arate, thus improving downstream processing, but the
production rate became mass transfer controlled due to
biofilm adhesion to the membrane. The air-driven
column reactor performed best in terms of productiv-
ity. Cell viability and activity could be maintained for
several weeks after the cells were adapted to the
substrate, product and solvent. Also, the relatively
gentle mixing in the air-driven reactor contributed to
its long term stability.
5. Bio-kinetic resolutions
Compared to conventional chemical catalysts, bio-
catalyts function under mild conditions to perform reac-
tions that are regio-, stereo- and enantiospecific. Chiral
building blocks, pharmaceutical and agrochemical com-
pounds and food additives are commercially considered
as pure enantiomers only when one enantiomer is pres-
ent in excess of ca. 98%. Although the majority of flavor
and pharmaceutical compounds are racemic, usually
only one of the enantiomers has the desired activity.
The other enantiomer may be inactive or it may have an
unwanted activity. The US Food and Drug Administra-
tion has declared that if a drug is chiral, the biological
effects of both enantiomers must be studied because the
non-therapeutic enantiomer may have unwanted side
effects (
). As with pharmaceu-
tical products, chirality is important in fragrances and
flavors because perception of odor and flavor depends
on the absolute conformation of the isomers. Different
isomers of the same compound can have quite different
Shake flasks
48%
Membrane reactor
3%
Agar plates
8%
Small scale
15%
Column reactor
5%
Stirred tank reactor
18%
Airlift reactor
3%
Fig. 2. Percentage of papers published on various reactor types in the last ten years.
C.C.C.R. de Carvalho, M.M.R. da Fonseca / Biotechnology Advances 24 (2006) 134–142
139

odors (
). This subject has been reviewed in depth
and
.
Compared to biotransformations with isolated en-
zymes, microorganisms generally produce compounds
with lower enantioselectivity because a microbial cell
may have multiple enzymes that are capable of trans-
forming a substrate (
). Enantioselectiv-
ity of microorganisms can be altered by adding organic
solvents and inhibitors of undesired enzyme activities.
An optically active epoxide was prepared using
whole cells of Bacillus megaterium (
2005
). Resolution of racemic glycidyl phenyl ether
was carried out in an iso-octane:aqueous phase system
in a mechanically stirred reactor to obtain a 44.5% yield
of the (S)-isomer with a 100% enantiomeric purity. R.
erythropolis DCL14 cells were able to diastereomeri-
cally resolve a mixture of (+)-limonene-1,2-epoxide
because only the cis-limonene-1,2-epoxide was con-
verted to limonene-1,2-diol and the trans-epoxide
remained unchanged (
2002
). Reactions were carried out in two-phase sys-
tems. When the degree of conversion of limonene-1,2-
epoxide reached 43%, the diastereomeric excess of the
trans-limonene-1,2-epoxide was greater than 99%. The
same cells also stereoselectively carried out the oxida-
tion of ( )-trans-carveol to ( )-carvone (
and da Fonseca, 2002a; de Carvalho et al., 2002
). The
unreacted ( )-cis-isomer was recovered at the end of
the reaction. A diastereomeric excess higher than 98%
was attained when the conversion of ( )-carveol was
59% in n-dodecane:aqueous phase systems. As in these
examples, some substrate mixtures are non-racemic,
i.e., they initially contain unequal amounts of the two
enantiomers. A model relating the initial enantiomeric
ratio to the extent of substrate conversion and enantio-
meric excess was reported by
6. Concluding remarks
The low water solubility, high volatility and cyto-
toxicity of both terpenes and terpenoids make their
production at industrial scale a difficult task. Although
processes have been published for producing a single
target compound, microbial metabolism usually results
in the production of multiple products which compli-
cates downstream processing. In recent years, the yields
obtained in biotransformations and improved technolo-
gy of production suggest that economically viable pro-
duction of many terpene compounds will become
possible in the future.
Acknowledgements
This work was supported by a post-doctoral grant
(SFRH/BPD/14426/2003) awarded to Carla da C. C. R.
de Carvalho by Fundac¸a˜o para a Cieˆncia e a Tecnolo-
gia, Portugal.
References
Aleu J, Collado IG. Biotransformations by Botrytis species. J Mol
Catal, B Enzyme 2001;13:77 – 93.
Angelova B, Fernandes P, Cruz A, Pinheiro HM, Mutafov S, Cabral
JMS. Hydroxylation of androstenedione by resting Rhodococcus
sp. cells in organic media. Enzyme Microb Techno in press.
Aranda G, Moreno L, Corte´s M, Prange´ T, Maurs M, Azerard R. A
new example of 1a-hydroxylation of drimanic terpenes through
combined
microbial
and
chemical
processes.
Tetrahedron
2001;57:6051 – 6.
Banerjee A, Sharma R, Chisti Y, Banerjee UC. Botryococcus braunii:
a renewable source of hydrocarbons and other chemicals. Crit Rev
Biotechnol 2002;22:245279.
Brenna E, Fuganti C, Serra S. Enantioselective perception of chiral
odorants. Tetrahedron: Asymmetry 2003;14:1 – 42.
Brown LM, Springer J, Bower M. Chemical substitution for 1,1,1-
trichloroethane and methanol in an industrial cleaning operation.
J Hazard Mater 1992;29:179 – 88.
Carballeira JD, Valmaseda M, Alvarez E, Gago JVS. Gongronella
butleri, Schizosaccharomyces octosporus and Diplogelasinospora
grovesii: novel microorganisms useful for the stereoselective
reduction of ketones. Enzyme Microb Technol 2004;34:611 – 23.
Carter OA, Peters RJ, Croteau R. Monoterpene biosynthesis path-
way construction in Escherichia coli. Phytochemistry 2003;64:
425 – 33.
Chen ARM, Reese PB. Biotransformation of terpenes from Stemodia
maritima by Aspergillus niger ATCC 9142. Phytochemistry
2002;59:57 – 62.
Chisti Y. Solid substrate fermentations, enzyme production, food
enrichment. In: Flickinger MC, Drew SW, editors. Encyclopedia
of bioprocess technology: fermentation, biocatalysis, and biose-
paration, vol. 5. New York7 Wiley; 1999. p. 2446 – 62.
Chisti Y, Moo-Young M. Disruption of microbial cells for intracellular
products. Enzyme Microb Technol 1986;8:194 – 204.
Clark DS. Can immobilisation be exploited to modify enzyme
activity? Trends Biotechnol 1994;12:439 – 43.
Davis EM, Tsuji J, Davis GD, Pierce ML, Essenberg M. Purification
of (+)-y-cadinene synthase, a sesquiterpene cyclase from bacteria-
inoculated cotton foliar tissue. Phytochemistry 1996;41:1047 – 55.
de Carvalho CCCR, da Fonseca MMR. Maintenance of cell
viability in the biotransformation of ( )-carveol with whole
Table 2
Odor properties of enantiomers of some terpene compounds
Monoterpene
Enantiomer
Fragrance
Carvone
(R)-( )
Spearmint
(S)-(+)
Caraway
Limonene
(R)-(+)
Orange
(S)-( )
Turpentine
a-Pinene
(1R,5R)-(+)
Slightly minty
(1S,5S)-( )
Pine tree
Menthol
(1R,3R,4S)-( )
Minty
(1S,3S,4R)-(+)
Phenolic
C.C.C.R. de Carvalho, M.M.R. da Fonseca / Biotechnology Advances 24 (2006) 134–142
140

cells of Rhodococcus erythropolis. J Mol Catal, B Enzyme
2002a;19–20C:389 – 98.
de Carvalho CCCR, da Fonseca MMR. Influence of reactor config-
uration on the production of carvone from carveol by whole cells
of Rhodococcus erythropolis DCL14. J Mol Catal, B Enzyme
2002b;19–20C:377 – 87.
de Carvalho CCCR, da Fonseca MMR. Towards the bio-production
of trans-carveol and carvone from limonene: induction after
cell growth on limonene and toluene. Tetrahedron: Asymmetry
2003;14:3925 – 31.
de Carvalho CCCR, da Fonseca MMR. Principal component analysis
applied to bacterial cell behaviour in the presence of organic
solvents. Biocatal Biotransform 2004a;22:203 – 14.
de Carvalho CCCR, da Fonseca MMR. Solvent toxicity in organic-
aqueous systems analysed by multivariate analysis. Bioprocess
Biosyst Eng 2004b;26:361 – 75.
de Carvalho CCCR, van Keulen F, da Fonseca MMR. Biotransfor-
mation of limonene-1,2-epoxide to limonene-1,2-diol by Rhodo-
coccus erythropolis cells An Introductory approach to selective
hydrolysis and product separation. Food Technol Biotechnol J
2000a;38:181 – 5.
de Carvalho CCCR, van Keulen F, da Fonseca MMR. Production and
recovery of limonene-1,2-diol and simultaneous resolution of a
diastereomeric mixture of limonene-1,2-epoxide with whole cells
of Rhodococcus erythropolis DCL14. Biocatal Biotransform
2000b;18:223 – 35.
de Carvalho CCCR, van Keulen F, da Fonseca MMR. Modelling the
biokinetic resolution of diastereomers present in unequal initial
amounts. Tetrahedron: Asymmetry 2002;13:1637 – 43.
de Carvalho CCCR, Cruz A, Angelova B, Fernandes P, Pons MN,
Pinheiro HM, et al. Behaviour of Mycobacterium sp NRRL B-
3805 whole cells in aqueous, organic-aqueous and organic media
studied by fluorescence microscopy. Appl Microbiol Biotechnol
2004;64:695 – 701.
de Carvalho CCCR, Parren˜o-Marchante B, Neumann G, da Fonseca
MMR, Heipieper HJ. Adaptation of Rhodococcus erythropolis
DCL14 to growth on n-alkanes, alcohols and terpenes. Appl
Microbiol Biotechnol 2005;67:383 – 8.
de Carvalho CCCR, Poretti A, da Fonseca MMR. Cell adaptation to
substrate, solvent and product: a successful strategy to overcome
product inhibition in a bioconversion system. Appl Microbiol
Biotechnol. In press.
Delahais V, Metzger P. Four polymethylsqualene epoxides and one
acyclic tetraterpene epoxide from Botryococcus braunii. Phyto-
chemistry 1997;44:671 – 8.
Demyttenaere J, De Kimpe N. Biotransformation of terpenes by fungi
Study of the pathways involved. J Mol Catal, B Enzyme
2001;11:265 – 70.
de Oliveira BH, dos Santos MC, Leal PC. Biotransformation of the
diterpenoid isosteviol by Aspergillus niger, Penicillium chryso-
genum and Rhizopus arrhizus. Phytochemistry 1999;51:737 – 41.
Do¨rnenburg H, Knorr D. Strategies secondary for the improvement of
metabolite production in plant cell cultures. Enzyme Microb
Technol 1995;17:674 – 84.
Duetz WA, Fjallman AHM, Ren SY, Jourdat C, Witholt B. Biotrans-
formation of D-limonene to (+)-trans-carveol by toluene-grown
Rhodococcus opacus PWD4 cells. Appl Environ Microbiol
2001;67:2829 – 32.
Fernandes P, Cabral JMS, Pinheiro HM. Influence of some opera-
tional parameters on the bioconversion of sitosterol with immo-
bilized whole cells in organic medium. J Mol Catal, B Enzyme
1998;5:307 – 10.
Fichan I, Larroche C, Gros JB. Water solubility, vapor pressure, and
activity coefficients of terpenes and terpenoids. J Chem Eng Data
1999;44:56 – 62.
Fischer G, Schwalbe R, Mo¨ller M, Ostrowski R, Dott W. Species-
specific production of microbial volatile organic compounds
(MVOC) by airborne fungi from a compost facility. Chemosphere
1999;39:795 – 810.
Fontanille P, Le Fle`che A, Larroche C. Pseudomonas rhodesiae PF1:
a new and efficient biocatalyst for production of isonovalal from
a-pinene oxide. Biocatal Biotransform 2002;20:413 – 21.
Fraga BM, Herna´ndez MG, Gonza´lez P, Lo´pez M, Sua´rez S. Bio-
transformation of the diterpene ribenone by Mucor plumbeus.
Tetrahedron 2001;57:761 – 70.
Fritter G, Bajgrowicz JA, Kraft P. Fragrance chemistry. Tetrahedron
1998;54:7633 – 703.
Fulzele DP, Heble MR, Rao PS. Production of terpenoid from Arte-
misia annua L. plantlet cultures in bioreactor. J Biotechnol
1995;40:139 – 43.
Gaikwad NW, Madyastha KM. Biosynthesis of b-substituted furan
skeleton in the lower furanoterpenoids: a model study. Biochem
Biophys Res Commun 2002;290:589 – 94.
Gong PF, Xu JH. Bio-resolution of a chiral epoxide using whole cells
of Bacillus megaterium ECU1001 in a biphasic system. Enzyme
Microb Technol 2005;36:252 – 7.
Halling PJ. Thermodynamic predictions for biocatalysis in nonconven-
tional media: theory, tests and recommendations for experimental
design and analysis. Enzyme Microb Technol 1994;16:178 – 206.
Halling PJ. Biocatalysis in low-water media: understanding effects of
reaction conditions. Curr Opin Chem Biol 2000;4:74 – 80.
Hashimoto T, Noma Y, Asakawa Y. Biotransformation of terpenoids
from the crude drugs and animal origin by microorganisms.
Heterocycles 2001;54:529 – 59.
Hetherington PJ, Follows M, Dunnill P, Lilly MD. Release of protein
from baker’s yeast (Saccharomyces cerevisiae) by disruption in an
industrial homogeniser. Trans Inst Chem Eng 1971;49:142 – 8.
Heyen U, Harder J. Cometabolic isoterpinolene formation from iso-
limonene by denitrifying Alcaligenes defragrans. FEMS Micro-
biol Lett 1998;169:67 – 71.
Ho¨lker U, Ho¨fer M, Lenz J. Biotechnological advantages of labora-
tory-scale solid-state fermentation with fungi. Appl Microbiol
Biotechnol 2004;64:175 – 86.
Hook IL, Ryan S, Sheridan H. Biotransformation of aliphatic and
aromatic ketones, including several monoterpenoid ketones and
their derivatives by five species of marine microalgae. Phyto-
chemistry 2003;63:31 – 6.
Ikushima Y. Supercritical fluids: an interesting medium for chemical
and biochemical processes. Adv Colloid Interface Sci 1997;
71 72:259 – 80.
Jirage KB, Martin CR. New developments in membrane-based
separations. Trends Biotechnol 1999;17:197 – 200.
Katoh S, Hyatt D, Croteau R. Altering product outcome in Abies
grandis ( )-limonene synthase and ( )-limonene/( )-a-pinene
synthase by domain swapping and directed mutagenesis. Arch
Biochem Biophys 2004;425:65 – 76.
Kirchner EM. Environment, health concerns force shift in use of
organic solvents. Chem Eng News 1994;72:13 – 20.
Klibanov AM. Enzymes that work in organic solvents. Chemtech
1986;16:354 – 9.
Klibanov AM. Improving enzymes by using them in organic solvents.
Nature 2001;409:241 – 6.
Kragl U, Eckstein M, Kaftzik N. Enzyme catalysis in ionic liquids.
Curr Opin Biotechnol 2002;13:565 – 71.
C.C.C.R. de Carvalho, M.M.R. da Fonseca / Biotechnology Advances 24 (2006) 134–142
141

Langenheim JH. Higher plant terpenoids: a phytocentric overview of
their ecological roles. J Chem Ecol 1994;20:1223 – 80.
Larsen TO. Volatile flavour production by Penicillium caseifulvum.
Int Dairy J 1998;8:883 – 7.
Lee MY, Dordick JS. Enzyme activation for nonaqueous media. Curr
Opin Biotechnol 2002;13:376 – 84.
Lee KKB, Poppenborg LH, Stuckey DC. Terpene ester production in
a solvent phase using a reverse micelle-encapsulated lipase. En-
zyme Microb Technol 1998;23:253 – 60.
Leo´n R, Fernandes P, Pinheiro HM, Cabral JMS. Whole-cell bio-
catalysis in organic media. Enzyme Microb Technol 1998;23:
483 – 500.
Lilly MD. Advances in biotransformation processes. Chem Eng Sci
1994;49:151 – 9.
Lindmark-Henriksson M, Isaksson D, Vanek T, Valterova´ I, Ho¨gberg
HE, Sjo¨din K. Transformation of terpenes using a Picea abies
suspension culture. J Biotechnol 2004;107:173 – 84.
Miyazawa M, Nankai H, Kameoka H. Biotransformations of acyclic
terpenoids, (+)-cis-nerolidol and nerylacetone, by plant pathogenic
fungus, Glomerella cingulata. Phytochemistry 1995;40:1133 – 7.
Nakamura K. Highly stereoselective reduction of ketones by Geotri-
chum candidum. J Mol Catal, B Enzyme 1998;5:129 – 32.
Oda S, Ohta H. Double coupling of acetyl coenzyme A production
and microbial esterification with alcohol acetyltransferase in an
interface bioreactor. J Ferment Bioeng 1997;83:423 – 8.
Onken J, Berger RG. Effects of R-(+)-limonene on submerged cul-
tures of the terpene transforming basidiomycete Pleurotus sapi-
dus. J Biotechnol 1999;69:163 – 8.
Pfruender H, Amidjojo M, Kragl U, Weuster-Botz D. Efficient whole-
cell biotransformation in a biphasic ionic liquid/water system.
Angew Chem Int Ed 2004;43:4529 – 31.
Rasser F, Ankea T, Sternerb O. Terpenoids from Bovista sp 96042.
Tetrahedron 2002;58:7785 – 9.
Scha¨fer S, Schrader J, Sell D. Oxygen uptake rate measurements to
monitor the activity of terpene transforming fungi. Process Bio-
chem 2004;39:2221 – 8.
Steinreiber A, Faber K. Microbial epoxide hydrolases for preparative
biotransformations. Curr Opin Biotechnol 2001;12:552 – 8.
Straathof AJJ, Adlercreutz P. Applied biocatalysis. Reading7 Harwood
Academic Publishers; 2000.
Tecela˜o CSR, van Keulen F, da Fonseca MMR. Development of a
reaction system for the selective conversion of ( )-trans-carveol
to ( )-carvone with whole cells of Rhodococcus erythropolis
DCL14. J Mol Catal, B Enzyme 2001;11:719 – 24.
van der Werf MJ, de Bont JAM, Leak DJ. Opportunities in microbial
biotransformation of monoterpenes. Adv Biochem Eng Biotech-
nol 1997;55:147 – 77.
van der Werf MJ, Overkamp KM, de Bont JAM. Limonene-1,2-
epoxide hydrolase from Rhodococcus erythropolis DCL14
belongs to a novel class of epoxide hydrolases. J Bacteriol
1998;180:5052 – 7.
van der Werf MJ, van der Ven C, Barbirato F, Eppink MHM, de Bont
JAM, van Berkel JH. J Biol Chem 1999;274:26296 – 304.
van der Werf MJ, Keijzer PM, van der Schaft PH. Xanthobacter
sp. C20 contains a novel bioconversion pathway for limonene.
J Biotechnol 2000;84:33 – 143.
van Keulen F, Correia CN, da Fonseca MMR. Solvent selection for
the biotransformation of terpenes by Pseudomonas putida. J Mol
Catal, B Enzyme 1998;5:295 – 9.
Verstegen-Haaksma AA, Swarts HJ, Jansen BJM, de Groot A, Bot-
tema-MacGillavry N, Witholt B. Application of S-(+)-carvone in
the synthesis of biologically active natural products using chem-
ical transformations and bioconversions. Ind Crops Prod 1995;
4:15 – 21.
Yoo SK, Day DF. Bacterial metabolism of a-and h-pinene and related
monoterpenes by Pseudomonas sp. strain PIN. Process Biochem
2002;37:739 – 45.
Zhu W, Asghari G, Lockwood GB. Factors affecting volatile terpene
and non-terpene biotransformation products in plant cell cultures.
Fitoterapia 2000;71:501 – 6.
C.C.C.R. de Carvalho, M.M.R. da Fonseca / Biotechnology Advances 24 (2006) 134–142
142
Document Outline
Wyszukiwarka
Podobne podstrony:
Biotransformation of Tryptamine in Fruiting Mycelia of Psilocybe cubensis Planta Med (1989) 55 (3) 2
Biotransformation of Tryptamine in Fruiting Mycelia of Psilocybe cubensis Planta Med (1989) 55 (3) 2
Biotransformation of menthol and geraniol by hairy root cultures of Anethum graveolens
biotransformation of tryptamine derivatives in mycelial cultures of Psilocybe J Basic Microbiol 29 (
biotransformation of some monoterpenoid ketones by chlorella vulgaris
Factors affecting volatile terpene and non terpene biotransformation products in plant cell cultures
~$Production Of Speech Part 2
World of knowledge
The American Society for the Prevention of Cruelty
The law of the European Union
05 DFC 4 1 Sequence and Interation of Key QMS Processes Rev 3 1 03
terms of trade
Izoprenoidy, terpeny
Historia gry Heroes of Might and Magic
24 G23 H19 QUALITY ASSURANCE OF BLOOD COMPONENTS popr
7 Ceny międzynarodowe trems of trade Międzynarodowe rynki walutowe
więcej podobnych podstron