
Biomaterials 23 (2002) 517–526
Inflammatory responses to orthopaedic biomaterials
in the murine air pouch
Paul H. Wooley
a,b,
*, Robert Morren
a
, John Andary
a
, Sudha Sud
a
, Shang-You Yang
a
, Lois
Mayton
a
, David Markel
a
, Allison Sieving
a
, Sam Nasser
a,b
a
Department of Orthopaedic Surgery, Wayne State University School of Medicine, Hutzel Hospital, One South, 4707 St. Antoine Boulevard,
Detroit, MI 48201, USA
b
The Veterans Administration Medical Center, Detroit, MI 48201, USA
Received 4 December 2000; accepted 3 April 2001
Abstract
An in vivo model of the inflammatory response to orthopaedic biomaterials was used to examine cellular and cytokine responses
to polymer particles of ultra high molecular weight polyethylene (UHMWPE) and polymethylmethacrylate (PMMA), and metal
particles of cobalt–chrome (Co–Cr) and titanium alloy (Ti–6Al–4V). Responses were determined separately and in combinations, to
examine interactions between different forms of biomaterials. Murine air pouches were injected with particle suspensions, and
reactions evaluated using histological, immunological, and molecular techniques. All particulate biomaterials caused significant
increases in membrane thickness compared with control (saline) air pouches, with the highest reaction seen in response to Ti–6Al–
4V particles. A synergistic increase in membrane thickness was observed when PMMA was combined with UHMWPE, suggesting
that multiple biomaterial stimuli markedly increase the inflammatory reaction. Cellular analysis indicated that all particles
increased the absolute number and the percentage of macrophages in the membrane over the control level, with the most
pronounced increase due to individual biomaterial occurring with UHMWPE particles. Cytokine analysis revealed that biomaterials
provoked a strong IL-1 response. Ti–6Al–4V stimulated the highest IL-6 gene transcription and the lowest IL-1 gene transcription.
The data suggest that synergism in the inflammatory response to biomaterials may be important in adverse responses to orthopaedic
wear debris. r 2001 Elsevier Science Ltd. All rights reserved.
Keywords:
Orthopaedic biomaterials; Particulates; Inflammation; Polymers; Alloys
1. Introduction
A critical aspect of adverse responses to the bioma-
terials used in the construction of orthopaedic prosthetic
components is the stimulation of cells in the peri-
prosthetic tissue by particles resulting from wear of the
prosthesis. The peri-prosthetic tissue serves as an inter-
face between the prosthesis and the bone, and contains
cells of the macrophage/monocyte lineage. Thus small
particles are readily phagocytosed by the resident cells of
the peri-prosthetic membrane. Plastics and metal are
impervious to enzymatic destruction, and wear particles
disturb the biological degradative function of phago-
cytes that ingest the debris [1]. Repeated phagocytosis of
the particles results in activated cells that secrete both
proteolytic enzymes and high levels of the inflammatory
cytokines IL-1 and TNFa, which may in turn contribute
to the osteolytic process by providing activation signals
to osteoclasts [2]. Biomaterial debris also appears to
provoke other biological effects, including granuloma
formation and inflammatory cell influx, which may also
contribute to bone resorption, osteolysis and the
eventual loss of the prosthesis support. The tissue from
the areas of osteolysis shows a synovial-like layer at the
cement surface, and the presence of macrophages and
foreign-body giant cells invading the femoral cortices.
This appearance shares some histological characteristics
of both rheumatoid arthritis (RA) and a foreign body
reaction. Microscopic examination of specimens ob-
tained at revision surgery of failed hip replacements has
*Corresponding author. Tel.: +1-313-745-6828; fax: +1-313-993-
0857.
E-mail address:
ad8754@wayne.edu (P.H. Wooley).
0142-9612/02/$ - see front matter r 2001 Elsevier Science Ltd. All rights reserved.
PII: S 0 1 4 2 - 9 6 1 2 ( 0 1 ) 0 0 1 3 4 - X

revealed a varied cellular composition of the pseudosy-
novium, with histiocytes, giant cells, lymphocytes,
plasma cells and neutrophils all present, with the areas
around the loosened prostheses characterized as aggres-
sive granulomatous lesions consisting of well organized
tissue containing histiocytic–monocytic and fibroblastic
reactive zones [3]. Furthermore, immunohistological
evaluation has revealed the presence of multinucleated
giant cells and C3bi-receptor bearing monocyte–macro-
phages [4]. Particles of acrylic cement and shards
of polyethylene appear incorporated into the histio-
cyte/macrophage or giant cell population, resulting in
‘‘foci’’ of cellular activity within the synovial-like
membrane [5].
It is recognized that any type of particulate debris,
including that generated by poor surgical technique, loss
of mechanical fixation of the polymethylmethacrylate
(PMMA) bone cement, or wear at the ultra-high
molecular weight polyethylene (UHMWPE)–metal in-
terface, may lead to the aseptic loosening process.
However, the critical aspects of biomaterial debris that
provoke the most severe cellular responses remain
unclear, and quantitative analysis of cell types present
in this periprosthetic tissue revealed considerable
heterogeneity between tissues from different individuals.
Hence a predictable model of the inflammatory response
to biomaterials in particulate form would be useful to
examine the molecular pathogenesis of the steps leading
to the osteolytic process. We have adapted a murine
model of inflammation that closely resembles the peri-
prosthetic tissue encountered in aseptically loosened
prosthetic components to evaluate the cellular response
to biomaterials in vivo. The rodent air pouch has been
identified as a useful model for the evaluation of the
response to orthopaedic biomaterials [6,7], providing
cellular infiltration and mediators of inflammation that
appear to closely resemble the pseudosynovium asso-
ciated with aseptic loosening. In this study we have
examined the cellular and cytokine reactions to both
metal and polymeric biomaterials, and demonstrate that
the model is sensitive differences in the material
composition of the particles under investigation.
2. Methods
2.1. Murine air pouches
Air
pouches
were
generated
according
to
the
methods of Sedgewick et al. [8] in groups of 10–15
female BALB/c mice. An area of the dorsal skin (2 cm
2
)
was cleaned with alcohol and shaved to provide the
pouch site. A subcutaneous injection of 3 ml of air was
injected at a single site with 25-gauge needle and 3 ml
syringe. The air pouches were injected with 1 ml of air
on alternate days for 5 days to establish a definitive fluid
filled pouch. On day 6, the pouches were injected with
500 ml of suspension containing 5% weight/volume
biomaterial particles. Control pouches received 500 ml
of sterile PBS alone. The mice were sacrificed 48 h after
the introduction of particles into the pouch. The
pouches were lavaged with 1 ml of saline, and the lavage
fluid rapidly frozen at
@801C for evaluation of
cytokines. The pouches were then dissected free from
the surrounding tissue. The pouch was divided, with
one-half fixed in formalin for histological evaluation,
and the remainder snap-frozen in liquid nitrogen for
RNA extraction.
2.2. Biomaterial particles
Four orthopaedic biomaterials in particulate form
were evaluated for reactions in the murine air pouch,
alone and in combinations (Table 1). The size and
distribution of the particles was evaluated with a
Coulter particle counter equipped with interchangeable
(100, 30, and 15 mm pore) attachments, and by scanning
electron microscopy (SEM). Particles for SEM analysis
were dispersed on a 0.1 mm Isopore membrane filter, and
dried for 24 h. PMMA and UHMWPE samples were
gold coated using a Fullam sputter coater prior to SEM
imaging using an S-2400 Hitachi scanning electron
microscope. Samples were imaged at 800 magnifica-
tion to visual particle size characteristics and particle
concentration distribution, which was analyzed using
the Image Pro software package (Media Cybernetics,
Table 1
Experimental study groups. Mice were injected with 500 ml of suspension containing 5% weight/volume particles. Groups with particle combinations
were injected with 500 ml comprised of 250 ml from each individual biomaterial suspension
Group
Injection suspension
Mean diameter (mm)
Size range (mm)
1
Vehicle control (sterile PBS)
na
na
2
UHMWPE
3.6
2.0–23
3
PMMA
4
0.1–10
4
Co–Cr
5.7
1.0–20
5
Ti–6Al–4V
2.3
0.1–68
6
UHMWPE+PMMA
3.7
0.1–23
7
UHMWPE+Co–Cr
4.2
1.0–23
8
UHMWPE+Ti–6Al–4V
3.1
0.1–68
P.H. Wooley et al. / Biomaterials 23 (2002) 517–526
518
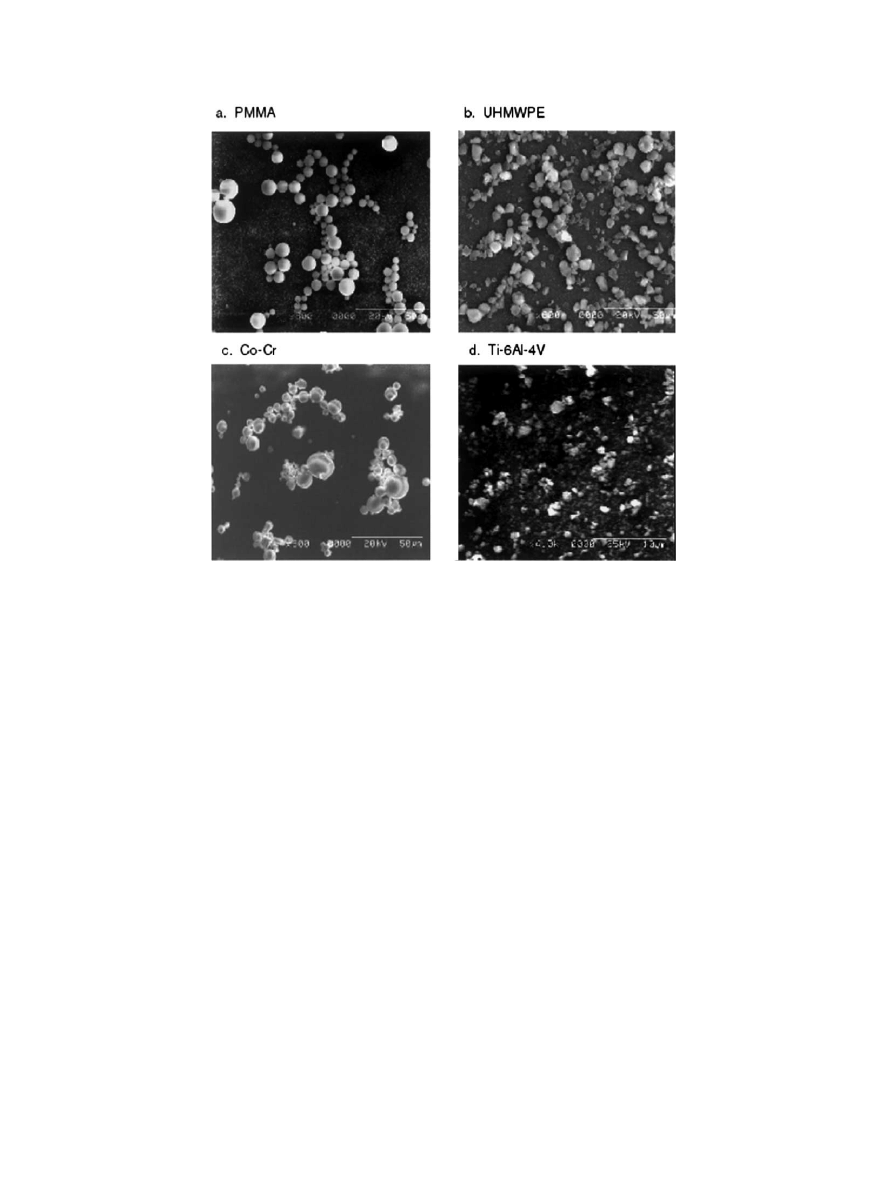
Maryland). SEM imaging (Fig. 1), image analysis, and
particle analysis revealed all materials to be predomi-
nantly spherical in shape. Ultra-high molecular weight
polyethylene (UHMWPE) particles (the generous gift of
Dr. John Cuckler, University of Alabama, Birmingham,
AL) had a mean particle diameter of 3.6 mm, with
diameters ranging from 2.0 to 23 mm. Cobalt–chromium
alloy particles (mean particle size 5.7 mm, range 1–
20 mm) were the generous gift of Dr. Jack Parr (Wright
Medical Inc., Memphis, TN). Titanium alloy particles
(Ti–6Al–4V) (mean particle size 2.3 mm, size range 0.1–
68 mm) were the generous gift of the Zimmer Corpora-
tion (Warsaw, Indiana). PMMA particles with (mean
diameter 4.0 mm, range 0.1–10 mm) were obtained from a
commercial source (Polyscience, Warrington, PA).
The metal and PMMA particles were washed in
70% ethanol solution to remove bound endotoxin [9],
and the absence of endotoxin was confirmed using the
Limulus assay (Endosafe; Charles Rivers, Charlestown,
SC). Particles were suspended in sterile PBS for
injection.
2.3. Histological evaluation and image analysis
Tissue samples were fixed, dehydrated, and embedded
in paraffin blocks with particular care to preserve the
original shape of the pouch tissue. Sections were cut
along a longitudinal axis at approximately the pouch
midline, mounted and stained with hematoxylin and
eosin (HaE) and Esterase stain. After staining, the
slides were permanently bonded with coverslips. Mini-
mums of three separate sections per specimen were
evaluated in a blinded fashion using the Image Pro
image analysis software package. Pouch thickness was
determined at six points on each section, with an even
distribution of measurement on the proximal side, distal
side, and transition curve of the pouch. Total number of
cells (based upon nucleus count) was determined as cells
per mm
2
, and cells differentiated into fibroblastic and
histiocytic type based upon nuclear morphology. All
data was exported to an Excel spreadsheet, and mean
cellular tissue densities calculated.
2.4. Cytokine gene activation
Cytokine RNA signals were determined in pouch
membrane tissues using reverse transcription–polymer-
ase chain reactions (RT–PCR) techniques described
previously [10,11]. Tissues were homogenized with a
Polytron RT 2000 in 7.5 m guanidium–HCl for 3 min,
and the sodium lauryl sarcosinate added to a final
concentration of 0.5%. After centrifugation to remove
Fig. 1. Scanning electron microscopy (SEM) appearance of the particle preparations. 800 .
P.H. Wooley et al. / Biomaterials 23 (2002) 517–526
519
debris, and the addition of 2 m potassium acetate and
1 m acetic acid, RNA was precipitated by the addition of
cold absolute ethanol. Quantity and purity of RNA was
determined by absorbance on a spectrophotometer
(Beckman Instruments, Fullerton, CA.) at 260 and
280 nm. Samples with ratios >1.7 were accepted for
analysis. To analyze the inflammatory cytokine gene
expression by the RT–PCR method, RNA was first
reverse transcribed into complementary DNA (cDNA).
PCR amplification was performed with primers specific
for murine IL-1, IL-6 and TNFa (Clontech Inc.).
Reactions for an individual sample were performed
simultaneously to ensure consistency in the analysis.
One hundred nanograms of each cDNA was combined
in a reaction mixture with each primer pair, PCR
buffer, MgCl
2
, dNTPs, and AmpliTaq DNA polymerase
was
added
to
a
final
concentration
of
5 U/ml.
The reaction was started by heating at 941C for
1 min to denature the RNA–cDNA hybrid, then
annealing the primers at 551C for 1 min, and extending
the primer sequence at 721C for 1 min. This cycle
was repeated 35 times using the DNA thermal
cycler (Perkin Elmer, Norwalk, CT). Following ampli-
fication, 10 ml of each reaction solution was mixed with
1 ml of loading buffer (Perkin Elmer), loaded on a 1.8%
agarose gel containing 0.5 mg/ml ethidium bromide
(Sigma, St. Louis, MO) and run at 50 V for 30 min.
All PCR assays were repeated to confirm the presence or
absence of the bands. The amplified PCR products were
visualized by ultraviolet light. The densities of all the
visualized bands of cytokine gene products were
measured with an ISO 2000 Digital Imaging system
(Alpha Innotech, San Leandro, CA). Each cytokine
signal was normalized relative to the PCR product from
a housekeeping gene (GADPH) using cDNA from the
same sample.
2.5. Cytokine levels
Levels of murine IL-1 and TNFa in pouch lavage fluid
were determined by ELISA as described previously [12].
Commercial kits (Genzyme, Cambridge, MA) were used
for these experiments, in accordance with the manufac-
turers instructions. The levels of each cytokine in the
sample were calculated by regression analysis from a
standard curve provided with the kit, assayed on the
same plate concomitantly.
2.6. Data analysis
Statistical comparisons of the experimental para-
meters between groups were made using the ANOVA
test, with the Scheffe formula for post hoc multiple
comparisons, using the SPSS software package (SPSS,
Chicago, IL).
3. Results
3.1. Air pouch membrane
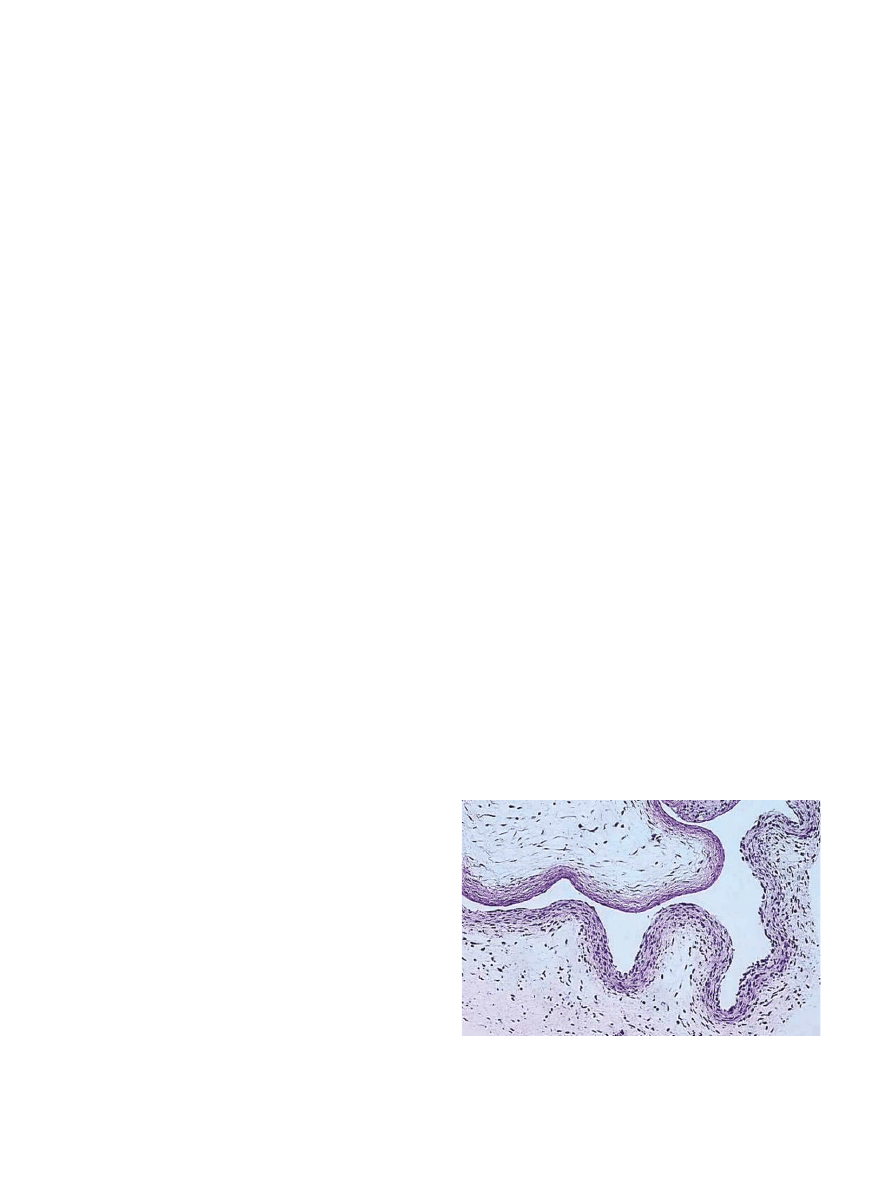
The histological appearance of the control (saline) air
pouch membrane is illustrated by Fig. 2. The control
membrane was characterized by an outer fibrous layer
populated mainly by fibroblastic cells and an inner
inflammatory layer predominantly comprised of macro-
phages. The mean thickness of control pouch mem-
branes was 62.0 mm (S.D. 11.6 mm) while the number of
nucleated cells was 960/mm
2
with a standard deviation
of 54 cells. The differential count revealed the control
membrane was comprised of 65% cells with fibroblastic
morphology, with the remaining cells (320 cells/mm
2
)
classified as macrophage/monocytes based upon cellular
morphology and esterase histochemical staining. Giant
cells were not observed in the saline-injected air pouch
membrane.
3.2. Tissue response to individual biomaterials
The introduction of any biomaterial particles into the
air pouch caused dramatic increases in histological
parameters of membrane thickness and cellularity,
compared with control pouch membranes. These
changes are illustrated by Fig. 3A–D. The objective
measurements of changes in response to the different
biomaterials (membrane thickness and cell count),
determined by image analysis of the histological
sections, are summarized in Figs. 4 and 5. Membrane
thickness in pouches stimulated by PMMA injection
(Fig. 3A) was significantly increased (225.6 mm, S.D.
22.7) compared to saline injected controls (p
o0.001),
and the number of cells per mm
2
were also elevated
(1180 cells/mm
2
), which was also statistically significant
Fig. 2. The histological appearance of the control (saline) air pouch
membrane. The membrane is characterized by an outer fibrous layer
populated mainly by fibroblastic cells and an inner inflammatory layer
predominantly comprised of histiocytic cells.
P.H. Wooley et al. / Biomaterials 23 (2002) 517–526
520
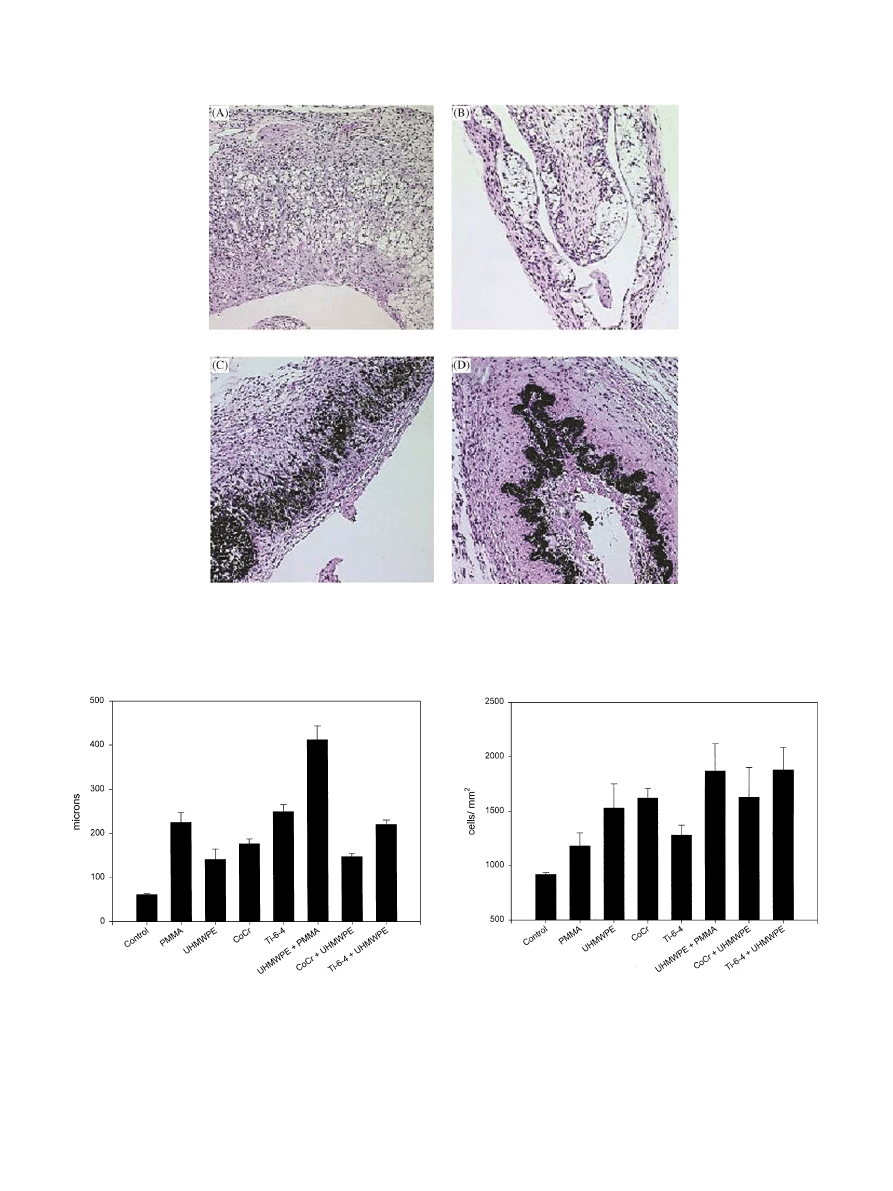
(p
o0.05). Fig. 3B illustrates the membrane changes that
occur when the air pouch was injected with UHMWPE.
Membrane thickness was increased (141 mm, S.D. 23.2)
compared with control, and UHMWPE caused the
highest increase in the density of cells infiltrating the
pouch membrane (1550 cells/mm
2
). Both of these
changes were significant (p
o0.01) compared with
control values. Co–Cr also increased both membrane
Fig. 3. The histological appearance of particle-stimulated air pouch membranes. Five day established air pouches were injected with (A) PMMA, (B)
UHMWPE, (C) Co–Cr, or (D) Ti–6Al–4V particles.
Fig. 4. Membrane thickness of control and particle-stimulated air
pouch membranes.
Fig. 5. Membrane cellularity of control and particle-stimulated air
pouch membranes.
P.H. Wooley et al. / Biomaterials 23 (2002) 517–526
521
thickness (177 mm, S.D. 11.1) and membrane cellularity
(1460 cells/mm
2
) to a highly significant degree over
saline control (p
o0.001 and o0.01, respectively), as
shown in Fig. 3C. The highest increase in membrane
thickness was observed using Ti–6Al–4V particles
(249 mm, S.D. 16.1) as illustrated in Fig. 3D. Ti–6Al–
4V also provoked a significant increase (p
o0.05) in the
cell count within the membrane (1280 cells/mm
2
). When
individual biomaterials were compared for variations in
the membrane responses, there were no substantial
differences among the different particles with respect to
the total cell count per unit area within the membrane.
This may suggest that an equivalent degree of cell
stimulation occurred using the different particle sources.
However, it was observed that Ti–6Al–4V particles
caused a significant increase (p
o0.05) membrane thick-
ness compared with UHMWPE particles, suggesting
that this metallic alloy provokes a stronger membrane
response compared to UHMWPE.
3.3. Tissue response to biomaterials in combination
The objective measurements of changes (membrane
thickness and cell count) in response to the combination
of different biomaterials with UHMWPE are shown in
Figs. 4 and 5. While combinations of metallic particles
with UHMWPE did not provoke increased membrane
thickness over either particle alone, the combination of
UHMWPE with PMMA proved synergistic, with the
resulting membrane thickness markedly elevated over
findings with either individual biomaterial. This in-
creased thickness with the combination of UHMWPE
and PMMA was significantly different from either
PMMA or UHMWPE alone (p
o0.001).
Cellular infiltration in membranes with combined
particles was elevated compared with individual parti-
cles, but this increase was not significant compared with
UHMWPE particles alone. However, the increases in
membrane cellularity were significant (p
o0.02) when
the respective combinations were compared to PMMA
alone or Ti–6Al–4V alone.
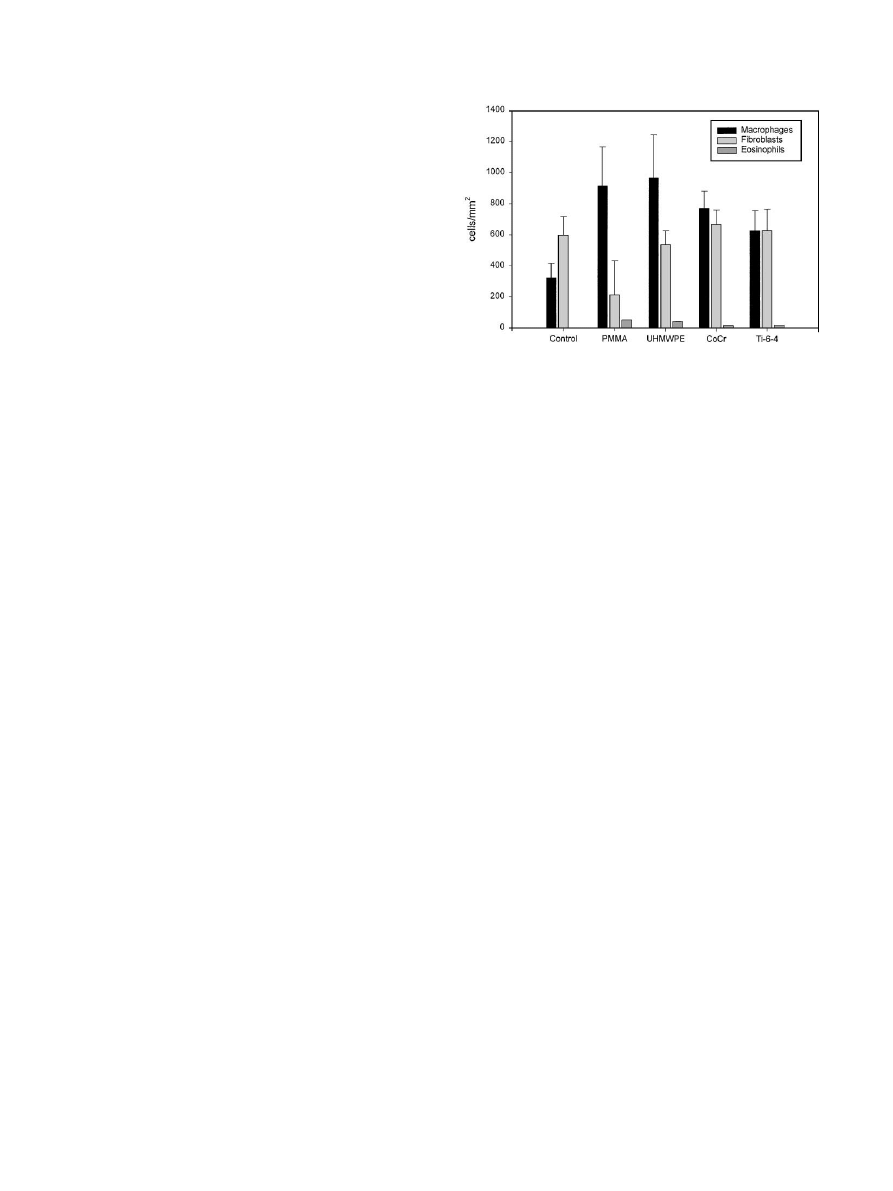
3.4. Differentiation cellular response to biomaterials
A differential count of the cellular infiltrate was
determined using histochemical staining and image
analysis of nuclear morphology. In contrast to the
predominantly fibroblastic composition of the control
(saline) membrane, the introduction of biomaterials into
the pouch resulted in a major accumulation of macro-
phages (Fig. 6) and the appearance of eosinophils and
occasional giant cells (
o1% of total cells). The
differential count revealed that the significant increase
in cellularity of the membrane in response to biomater-
ials was due to the macrophage component; there were
no significant changes in the fibroblast component of the
membrane when biomaterial stimulated pouches were
compared with either the saline control or one another.
The level of significance of the increase in macrophages
in biomaterial-stimulated membranes was increased
compared with the statistic for the total cellular counts,
with p values ranging from p
o0.01 to o0.001. How-
ever, there were no significant variations in membrane
macrophage count when the different biomaterials were
compared with each other. This finding was also true for
biomaterial combinations (data not shown). Eosinophils
were invariably absent from the saline control mem-
brane, and were present at consistent low levels in
particle stimulated membranes. The highest level of
eosinophil accumulation was seen in PMMA stimulated
membrane (4.4% of total cells), although this level was
not significantly elevated compared with other bioma-
terials.
3.5. Cytokine gene activation in the air pouch membrane
The result of a typical RT–PCR using membrane
extracted mRNA is shown in Fig. 7. Positive bands
were generated using primers specific for murine IL-1
and IL-6, but weak or negative results were observed
using primers specific for TNFa. This surprising
difference between the two predominant inflammatory
cytokines did appear to be specific for particle stimula-
tion; the introduction of 2 mg of lippopolysachaccaride
into the air pouch resulted in positive TNFa gene
activation within 24 h (not shown). In all instances, the
strength of the PCR signal for IL-1b appeared to be
higher than the strength of the PCR signal for IL-6,
suggesting that the major inflammatory cytokine re-
sponse was mediated via IL-1. The addition of
biomaterial particles to the air pouch resulted in an
increase in strength of the cytokine gene PCR signals
over the control air pouch membrane. No marked
Fig. 6. Differential count of the cellular infiltrate of control and
particle-stimulated air pouch membranes.
P.H. Wooley et al. / Biomaterials 23 (2002) 517–526
522
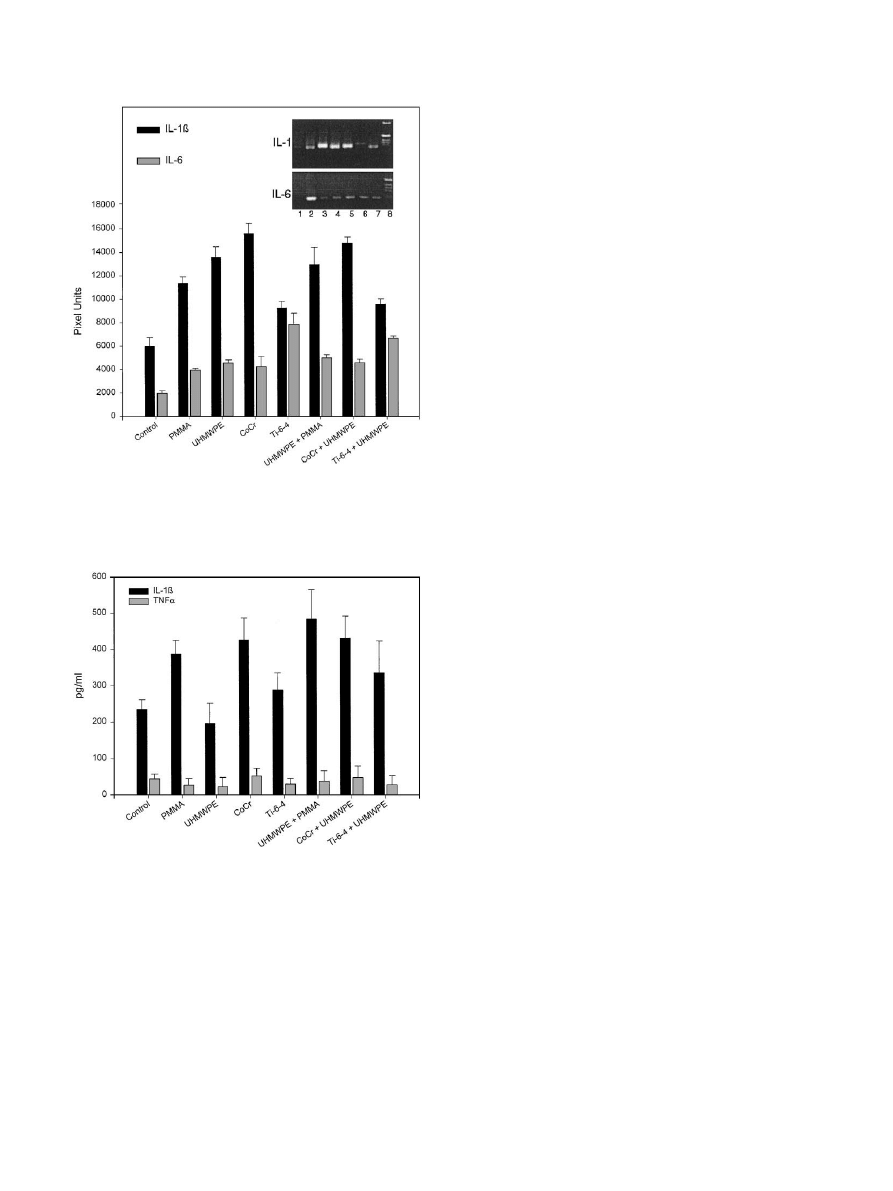
variations in cytokine production were observed be-
tween the different biomaterials, with the exception of
the results with Ti–6Al–4V particles. Membranes
stimulated with Ti–6Al–4V appeared to be skewed
towards an IL-6 response; Ti–6Al–4V provoked the
lowest IL-1b band density, and the highest IL-6 band
density.
The
combination
of
Ti–6Al–4V
with
UHMWPE also generated a cytokine gene activation
that was weighted towards an IL-6 response.
3.6. Cytokine levels in the air pouch fluid
The levels of IL-1b and TNFa in pouch fluid
determined by ELISA assay are shown in Fig. 8. Not
all biomaterials provoked increased IL-1b levels above
the results seen in the saline control pouch (230 pg/ml),
although stimulation with PMMA and Co–Cr, and
particles in combination did result in significant
increases in the fluid cytokine levels (p
o0.05–o0.01).
There was reasonable agreement between the band
strength of IL-lb gene activation in the pouch membrane
and the level of cytokine protein detected in the pouch
fluid, with the exception of the findings for UHMWPE
alone, where protein levels of IL-Ib were unexpectedly
low. Levels of TNFa in pouch fluid were low, as
predicted by the gene activation with the membrane. No
significant increases in the level of TNFa in pouch fluid
due to particle stimulation were observed.
4. Discussion
In order to evaluate the mechanisms of the biological
response to orthopaedic biomaterials, it is important to
establish accurate models of the disease process. We
have utilized the murine air pouch model, initially
developed to investigate the response to orthopaedic
materials by Schumacher et al. [6,7], for the investiga-
tion of the inflammatory response to particulate debris.
This model exhibits cellular infiltration and mediators of
inflammation that appear to closely resemble the
pseudosynovium associated with aseptic loosening
[6,13,14], and has been demonstrated to be sensitive to
differences both in the material composition and the
physical form of the particles under investigation [6,15].
The kinetics of our study were based on the reports of
the histological features of this experiment model, and
the 5 day air pouch was selected to provide an organized
mechanical barrier that retained both the particles and
products of the inflammatory response [8]. We observed
marked responses to both polymeric and metallic
particles, and synergistic responses to mixed particle
types. The appearance of the particulate stimulated
membrane, with biomaterials deeply embedded within
the tissue and surrounded by a predominantly macro-
phage infiltrate, is strongly reminiscent of peri-prosthe-
tic
tissue
recovered
during
arthroplasty
revision
procedures [16,17]. The order of the severity of the
tissue reactions to the biomaterials cannot be precisely
ranked in this study, since UHMWPE provoked
a greater cellular infiltrate than PMMA, but resulted
in a less thickened membrane; while Ti–6Al–4V
provoked a thicker membrane than Co–Cr but a lower
intensity of macrophage accumulation. The response to
Ti–6Al–4V in the air pouch appears somewhat different
from the findings observed in subcutaneous implanta-
Fig. 7. RT–PCR using primers specific for murine IL-1b and mRNA
extracted from control and particle-stimulated air pouch membranes.
Fig. 8. The levels of IL-1b and TNFa in pouch fluid from extracted
from control and particle-stimulated air pouch membranes.
P.H. Wooley et al. / Biomaterials 23 (2002) 517–526
523

tion in rodents [18], and in vitro studies with Ti–6Al–4V
particles suggest that they may be less provocative of
inflammatory responses [19]. However, strong macro-
phage reactions to particulate Ti–6Al–4V have been
observed in periprosthetic tissue recovered during
revision arthroplasty [20–23].
Our data suggest that the composition of the
particulate stimulus has a marked effect upon the
inflammatory reaction within the pouch membrane.
This is in agreement with the observations of Gelb et al.
[6], who noted that the introduction of PMMA particles
composed of different sizes and shapes resulted in
variations in the production of cytokines and other
mediators of inflammation. They observed that small,
irregularly shaped PMMA particles (
o20 mm) elicited a
significantly greater inflammatory reaction than large
particles (>50 mm), and the findings were influenced by
the dose of particles introduced within the pouch. They
hypothesize that the total surface area of the PMMA
particles is critical in the inflammatory response, and
propose that a threshold exists at which the inflamma-
tory response increased dramatically. We did not
examine the dose response to particles in this study,
however our mean particle size was fairly consistent
among the different materials studied. There was no
correlation between the density of the biomaterial and
the level of the biological response, suggesting that the
variations between the different materials were not
associated with absolute particle number. In combina-
tion experiments, the total particle load for each
material was one half of that used for the individual
biomaterial experiments, so the increase due to particle
combinations cannot be attributed to increased particle
numbers. In the combination of UHMWPE and
PMMA, synergistic effects were observed with the
membrane reactions significantly greater than either
material alone. These observations suggest that the
composition of the biomaterial may influence the level
and profile of the inflammatory reaction, although the
dose and physical properties of the particles may
significantly alter the degree of biological reactivity,
probably
due
to
alterations
in
the
degree
of
phagocytosis
[24].
However,
there
is
no
readily
accepted method of comparing polymer and metal
debris. If mass is chosen, surface area or particulate
number will vary. If particle number is chosen, surface
area or mass must differ. We have chosen a constant 5%
weight/volume, recognizing these limitations. Even
though the particle numbers are not equal, the response
to combinations of materials clearly suggests synergy.
This result must be extended cautiously to the patient
situation. The range of the particles under investigation
in this study represents a large proportion of the size
range of wear debris extracted from peri-prosthetic
tissue associated with failed joint components. However,
the mean particle size studied was in excess of 1 mm,
which may be larger than the mean particle size in
periprosthetic tissue, and the macrophage response to
the particles under study clearly includes both uptake
(phagocytosis) and extra-cellular adhesion. However,
histological evaluation of peri-prosthetic tissue asso-
ciated with failed implants does reveal a similar
pathology.
Many of the tissue changes associated with particle
stimulation may be associated with the effects of IL-lb
production. Isaji [25] used the rodent air pouch to study
the local tissue response to recombinant human inter-
leukin 1 alpha and beta (rIL-1 beta), and observed a 10-
to 100-fold increase in the cellular accumulation.
Repeated exposure to IL-1b caused the accumulation
of large amounts of fluid within preformed pouches and
an apparent thickening of the connective-tissue lining of
the pouch, with deposition of large quantities of extra-
cellular collagen within the pouch wall. They concluded
that the air pouch changes were consistent with IL-1b
regulating the development and perpetuation of an
inflammatory reaction. In a chronically stimulated air
pouch, Dawson et al. [26] observed large numbers of
macrophages accumulated after 3 days, accompanied by
fibroblast proliferation and new blood vessels. The
reaction was significantly inhibited by dexamethasone
but not by indomethacin, suggesting that chronically
stimulated inflammatory reactions in this model were
correlated with the increase of reactivity at the site of
inflammation and the exudative reaction was not
mediated by cyclo-oxygenase products. Induction of
IL-1 in activated macrophages at the prosthesis interface
in response to the presence of metallic wear debris is a
frequent finding in peri-prosthetic tissue recovered
during revision arthroplastic procedures [27], and IL-1
and IL-6 levels in these cells have been correlated with
markers of the monocyte/macrophage lineage [28].
Jiranek et al. [29] examined patient tissue and demon-
strated IL-1b mRNA production occurred predomi-
nantly in macrophages, and not in fibroblasts to any
major extent. However, IL-1b protein was bound on
both macrophages and fibroblasts, indicating that
macrophages are the source of the cytokine, which
subsequently binds to both fibroblasts and macro-
phages. Our model appears to resemble this proposed
mechanism, with the response to particles occurring
predominantly in macrophages with a concomitant
increase in IL-1b production. The lack of a sustained
TNFa response to the particulate stimulation in our
study was unexpected, particularly since a TNFa
response has been observed in response to ceramic
particle injection into the rodent air pouch [7]. However,
this report also noted that synthetic monosodium urate
crystals did not elicit a TNFa response, suggested that
the composition of the inflammatory stimulus is critical
in the generation of a specific cytokine reaction. Gelb
et al. [6] suggested that the cellular mechanism for the
P.H. Wooley et al. / Biomaterials 23 (2002) 517–526
524

TNF component of the inflammatory response is
different from the mechanism resulting in cell accumula-
tion and other mediators of inflammation; a hypothesis
that
our
data
strongly
supports.
Although
the
cellular component of the membrane is implicated
for the production of the inflammatory cytokines, the
contribution of the fluid cells should be considered. Not
all pouch fluids were analyzed for cell content
in our study, but an inflammatory exudate was
usually
seen.
Control
pouch
fluids
contained
PMNs (57%), lymphocytes (41%) and monocytes
(2%). Fluid cells from PMMA pouches contained
11% eosinophils, 56% PMNs and 33% lympho-
cytes, while fluid cells from UHMWPE pouches
contained 53% lymphocytes, 44% PMNs, 3% eosino-
phils and 1% monocytes. The relative contribution of
these cells to the inflammatory cytokine levels remains
to be elucidated.
The unusual pattern of cytokine elicitation using Ti–
6Al–4V, with high IL-6 production and lower IL-1b
production is intriguing, since the presence of Ti–6Al–
4V particles seems to accelerate bone loss and loosening
[30]; however, Ti–6Al–4V particles have been observed
to specifically up-regulate IL-6 production in vitro using
a variety of cell types [31,32], which may imply a specific
cytokine profile associated with the response to this
metallic alloy. Variations in cytokine stimulation among
metals has been demonstrated in vitro by Haynes et al.
[33] indicating that while Co–Cr, stainless steel and
titanium alloy all induced TNFa and IL-1b, stainless
steel particles were the most potent stimulators of IL-1b
and Ti–6Al–4V particles were the strongest stimulators
of IL-6 and PGE2. However, these findings were not
confirmed by Liu et al. [34], who demonstrated that
exposure of leukocytes to Co
2+
ion increased the release
of TNFa, IL-6, and PGE2, but leukocyte exposure to
Ti
3+
ions was associated with a decreased TNFa and
PGE2 release and a minimal change in IL-6 release.
Overall, our findings suggest that the murine air
pouch provides an accurate model for the biological
response to particulate biomaterials, with a marked
macrophage accumulation and strong elicitation of the
inflammatory cytokines IL-1b and IL-6. Our data
suggests that the variations in the material composition
of the particulate stimulus may result in differences in
the tissue response, and that combinations of different
biomaterials may elicit synergy in the level of the
inflammatory response. In order to accurately assess the
osteolytic component of debris-induced aseptic loosen-
ing, it is desirable to include bone tissue within the
model. To accomplish this, we are currently investigat-
ing particle-stimulated air pouches with femoral head
implantation. These models may prove useful in the
development of novel biomaterials for orthopaedic
applications, and therapeutic strategies for the treatment
of aseptic loosening.
Acknowledgements
The authors gratefully acknowledge the contributions
of W. Dwayne Lawrence, M.D. and the Pathology
Image Analysis laboratory at Hutzel Hospital in
establishing the image techniques used in this study,
and the grant support from the Veterans Administration
(Rehabilitation Study Section) and the Arthritis Foun-
dation.
References
[1] Wooley PH, Nasser S, Fitzgerald Jr RH. The immune response
to implant materials in humans. Clin Orthop Relat Res 1996;326:
63–70.
[2] Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT. Cellular
mediators secreted by interfacial membranes obtained at revision
total hip arthroplasty. J Arthroplasty 1995;10:498–506.
[3] Kaufman RL, Tong I, Beardmore TD. Prosthetic synovitis:
clinical and histologic characteristics. J Rheumatol 1985;12:
1066–74.
[4] Santavirta S, Konttinen YT, Bergroth V, Eskola A, Tallroth K,
Lindholm TS. Aggressive granulomatous lesions associated with
hip arthroplasty. Immunopathological studies. J Bone Jt Surg-
FAm Vol 1990;72:252–8.
[5] Eftekhar NS, Doty SB, Johnston AD, Parisien MV. Prosthetic
synovitis. Hip 1985;169–83.
[6] Gelb H, Schumacher HR, Cuckler J, Ducheyne P, Baker DG. In
vivo inflammatory response to polymethylmethacrylate particu-
late debris: effect of size, morphology, and surface area. J Orthop
Res 1994;12:83–92.
[7] Nagase M, Udagawa E, Schumacher HR, Baker DG. Prolonged
inflammatory reactions induced by ceramic powders in the rat air
pouch model. Nippon Seikeigeka Gakkai Zasshi
FJ Jpn Orthop
Assoc 1990;64:602–11.
[8] Sedgwick AD, Sin YM, Edwards JC, Willoughby DA. Increased
inflammatory reactivity in newly formed lining tissue. J Pathol
1983;141:483–95.
[9] Bi Y, van de Motter RR, Ragab AA, Goldberg VM, Greenfield
EM. The role of adherent endotoxin in stimulation of osteoclast
differentiation by orthopaedic wear particles. Trans Orthop Res
Soc 1999;45:5.
[10] Chadha HS, Wooley PH, Fitzgerald RH. Cellular proliferation
and cytokine responses to polymethylmethacrylate (PMMA)
following joint replacement. Inflammat Res 1995;44:145–51.
[11] Wooley PH, Sud S, Langendorfer A, Calkins C, Christner PJ,
Peters J, Jimenez SA. T cells infiltrating the skin of Tsk2
scleroderma-like mice exhibit T cell receptor bias. Autoimmunity
1997;27:91–8.
[12] Schaefer CJ, Whalen JD, Knapp T, Wooley PH. The influence of
silicone implantation on type II collagen-induced arthritis in mice.
Arthritis Rheum 1997;40:1064–72.
[13] Nagase M, Baker DG, Schumacher HR. Prolonged inflammatory
reactions induced by artificial ceramics in the rat air pouch model.
J Rheumatol 1988;15:1334–8.
[14] Wooley PH, Morren R, Andary J, Sud S, Song Z, Mayton L,
Fitzgerald RH, Nasser S. A model of cellular responses in aseptic
loosening: UHMWPE and PMMA particles provoke different
responses in the murine air pouch. Orthop Trans 1996;21:500.
[15] Wooley PH, Mayton L, Sud S, Nasser S. Synergistic inflamma-
tory responses to metallic and polymeric particles in the murine
air
pouch
model
of
aseptic
loosening.
Arthritis
Rheum
1996;39:175.
P.H. Wooley et al. / Biomaterials 23 (2002) 517–526
525

[16] Bos I, Fredebold D, Diebold J, Lohrs U. Tissue reactions to
cemented hip sockets. Histologic and morphometric autopsy
study of 25 acetabula. Acta Orthop Scand 1995;66:1–8.
[17] Boss JH, Shajrawi I, Soudry M, Mendes DG. Histological
features of the interface membrane of failed isoelastic cementless
prostheses. Int Orthop 1990;14:399–403.
[18] Akagawa Y, Hashimoto M, Kondo N, Yamasaki A, Tsuru H.
Tissue reaction to implanted biomaterials. J Prosthet Dent 1985;
53:681–6.
[19] Gonzales JB, Purdon MA, Horowitz SM. In vitro studies on the
role of titanium in aseptic loosening. Clin Orthop Relat Res
1996;330:244–50.
[20] Agins HJ, Alcock NW, Bansal M, Salvati EA, Wilson Jr. PD,
Pellicci PM, Bullough PG. Metallic wear in failed titanium-alloy
total hip replacements. A histological and quantitative analysis.
J Bone Jt Surg
FAm Vol 1988;70:347–56.
[21] Korovessis P, Repanti M. Evolution of aggressive granulomatous
periprosthetic lesions in cemented hip arthroplasties. Clin Orthop
Relat Res 1994;300:155–61.
[22] Lombardi Jr. AV, Mallory TH, Vaughn BK, Drouillard P.
Aseptic loosening in total hip arthroplasty secondary to osteolysis
induced by wear debris from titanium-alloy modular femoral
heads. J Bone Jt Surg
FAm Vol 1989;71:1337–42.
[23] Witt JD, Swann M. Metal wear and tissue response in failed
titanium alloy total hip replacements. J Bone Jt Surg
FBr Vol
1991;73:559–63.
[24] Goodman SB, Fornasier VL, Lee J, Kei J. The histological effects
of the implantation of different sizes of polyethylene particles in
the rabbit tibia. J Biomed Mater Res 1990;24:517–24.
[25] Isaji M, Momose Y, Naito J. Enhancement of inflammatory
reactions in a nonimmunological air pouch model in rats. British
J Exp Pathol 1989;70:705–16.
[26] Dawson J, Sedgwick AD, Edwards JC, Lees P. Lymphocyte
kinetics in a murine model of chronic inflammation. Agents
Actions 1989;27:461–4.
[27] al
Saffar
N,
Revell
PA.
Interleukin-1
production
by
activated macrophages surrounding loosened orthopaedic im-
plants: a potential role in osteolysis. Br J Rheumatol 1994;33:
309–16.
[28] Perry MJ, Mortuza FY, Ponsford FM, Elson CJ, Atkins RM.
Analysis of cell types and mediator production from tissues
around loosening joint implants. Br J Rheumatol 1995;34:
1127–34.
[29] Jiranek WA, Machado M, Jasty M, Jevsevar D, Wolfe HJ,
Goldring SR, Goldberg MJ, Harris WH. Production of cytokines
around loosened cemented acetabular components. Analysis with
immunohistochemical techniques and in situ hybridization.
J Bone Jt Surg
FAm Vol 1993;75:863–79.
[30] Buly RL, Huo MH, Salvati E, Brien W, Bansal M. Titanium wear
debris in failed cemented total hip arthroplasty. An analysis of
71 cases. J Arthroplasty 1992;7:315–23.
[31] Haynes DR, Rogers SD, Howie DW, Pearcy MJ, Vernon-Roberts
B. Drug inhibition of the macrophage response to metal wear
particles in vitro. Clin Orthop Relat Res 1996;323:316–26.
[32] Morris CJ, Blake DR, Hewitt SD, Lunec J. Macrophage ferritin
and iron deposition in the rat air pouch model of inflammatory
synovitis. Ann Rheum Dis 1987;46:334–8.
[33] Haynes DR, Boyle SJ, Rogers SD, Howie DW, Vernon-Roberts
B. Variation in cytokines induced by particles from different
prosthetic materials. Clin Orthop 1998;352:223–30.
[34] Liu HC, Chang WH, Lin FH, Lu KH, Tsuang YH, Sun JS.
Cytokine and prostaglandin E2 release from leukocytes in
response to metal ions derived from different prosthetic materials:
an in vitro study. Artif Organs 1999;23:1099–106.
P.H. Wooley et al. / Biomaterials 23 (2002) 517–526
526
Wyszukiwarka
Podobne podstrony:
Transient TLR Activation Restores Inflammatory Response and Ability To Control Pulmonary Bacterial I
ibt writing sample responses
Komunikacja rynkowa direct response marketing
Total Phisical Responce
Inflammatio?tarrhalis chronica hypertrophica
TOTAL PHYSICAL RESPONSE
wyklad metody kontaktu z respondentem
Glucocorticoids alter fever and IL 6 responses to psychological
Zapalenia (inflammatio)
planning guidance for response to nuclear detonation 2 edition final
Day 1 L9 Inflammatory lesions
Kopelmann, Rosette Cultural variation in response to strategic emotions
Response Cries
response
SHSBC386 COMMUNICATION OVERTS AND RESPONSIBILITY
mDNS Responder FOSS notices
więcej podobnych podstron